Induction of immunologic tolerance to alloantigens is a major goal in the field of transplantation. Here, we demonstrate that efficient transduction and expression of a retrovirally transduced major histocompatibility complex (MHC) class I gene(H-2Kb) in bone marrow (BM)–derived cells, resulting in a permanent state of hematopoietic molecular chimerism, induces stable tolerance to the transduced gene product. Reconstitution of lethally irradiated syngeneic recipients with BM transduced with virus encoding H-2Kb resulted in life-long expression of the retroviral gene product on the surface of BM-derived hematopoietic lineages including Sca-1+, lineage negative, hematopoietic progenitors. T cells from mice receiving MHC-transduced BM were unable to kill targets expressing H-2Kbbut were able to respond to third-party controls. Mice reconstituted with H-2Kb-transduced BM exhibited long-term acceptance of H-2Kb mismatched skin grafts but were able to rapidly reject third-party control grafts. Thus, gene therapy approaches may be used to induce T-cell tolerance.
Introduction
The ability to induce immunologic tolerance to transplantation antigens remains a major goal in transplantation because of its promise to allow replacement of host organs without the need for immunosuppression. The most stable way of establishing tolerance to transplantation antigens is to induce lymphohematopoietic chimerism through allogeneic bone marrow transplantation (BMT).1 The establishment of mixed cellular chimerism through BMT in adult animals leads to specific tolerance in an otherwise fully immunocompetent host (for a review, see Sykes2), and even low levels of chimerism are sufficient to induce transplantation tolerance.3 However, BMT across major histocompatibility complex (MHC) barriers has drawbacks as a means of inducing tolerance to allogeneic organs because of the severity of the preparative regimen required, the potential for inducing severe graft-versus-host disease (GVHD), the high rates of engraftment failure, and the difficulty in obtaining suitable matched bone marrow (BM) donors.
It may be possible to use gene therapy to establish tolerance by inducing molecular rather than cellular chimerism. Reconstitution of lethally irradiated mice with autologous BM cells infected with retroviruses carrying the allogeneic MHC class I geneH-2Kb induced hyporesponsiveness to the transduced MHC gene product resulting in the prolonged survival ofH-2Kb disparate skin grafts (for a review, see Bagley and Iacomini4). However, challenging mice reconstituted with H-2Kb–transduced BM with skin grafts expressing additional allogeneic determinants together withH-2Kb restored rapid rejection ofH-2Kb–expressing skin grafts.5,6In addition, cytotoxic T-cell precursors capable of lysing H-2Kb targets were detectable in mice receivingH-2Kb–transduced BM, suggesting that prolongation of skin graft survival induced by gene therapy was the result of a relatively unstable peripheral mechanism, rather than central deletion of H-2Kb alloreactive T cells.6
The failure to induce stable long-term tolerance to alloantigens by gene engineering of BM could have been due to a failure to efficiently express the retrovirally encoded alloantigen on cell types capable of inducing central tolerance, loss of gene expression, or a low level of BM transduction or initial expression. Because multilineage long-term survival of allogeneic BM cells is required to maintain stable tolerance induced by allogeneic BMT,7 we hypothesized that to achieve stable T-cell tolerance by gene therapy, it would be necessary to achieve long-term multilineage expression of the retrovirally transduced alloantigen on BM-derived cells. Here, we demonstrate that efficient long-term expression of retrovirally transduced MHC genes in BM-derived cells results in stable tolerance to alloantigens and indefinite survival of allogeneic skin grafts. Thus, a genetic engineering approach targeted at re-educating the host immune system by the induction of molecular chimerism can be used to induce T-cell tolerance and overcome allograft rejection.
Materials and methods
Mice
Female B10.AKM/SnJ (H-2Kk, Ik, Dq), B10.MBR/Sx (H-2Kb, Ik, Dq), and B10.BR (H-2k) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed using microisolator conditions in autoclaved cages and maintained on irradiated feed and autoclaved acidified drinking water. All sentinel mice housed in the same colony were viral antibody–free. Six- to 12-week-old female mice were used in all experiments.
Retroviral vector construction and packaging
A strategy based on the polymerase chain reaction was used to clone the complementary DNA (cDNA) encodingH-2Kb (Kb) into the MMP retroviral vector kindly provided by Dr Richard C. Mulligan (Children's Hospital, Boston, MA) to generate pMMP-Kb. The MMP retroviral vector is a derivative of MFG.8 No drug selectable marker is present in the MMP vector. DNA sequencing was used to confirm the integrity of the resulting clones. Vesicular stomatitis virus G (VSV-G) envelope protein pseudotyped viruses were prepared by packaging the pMMP-Kb retroviral vector in 293T cells by transient transfection as previously described9 10 to generate VSV-Kb virus. Functional titers of VSV-Kb retroviral supernatants were determined by analyzing expression of Kb on NIH3T3 cells by cell surface staining and flow cytometry following infection. The monoclonal anti-Kb antibody AF6-88.5 was used to analyze Kb expression 48 hours after infection. All virus preparations were made in affiliation with the Harvard Institute for Human Genetics' Gene Therapy Initiative. The viral titer obtained for the preparation of VSV-Kb used in this report was 2 × 106 infectious particles/mL. MMP-based VSV-G pseudotyped viruses carrying the gene encoding enhanced green fluorescence protein, hereafter referred to as VSV-GFP, were provided by Dr Richard C. Mulligan and used as a control.
BM transduction and BMT
The BM were harvested and transduced as described previously.11 Briefly, BM cells from mice treated 7 days previously with 5-fluorouracil (5-FU; 150 mg/kg) were cultured in tissue culture plates coated with Retronectin (Takara Biomedicals, Shiga, Japan) and transduced in Dulbecco minimum essential medium containing 15% lot-tested fetal calf serum and cytokines to achieve a final concentration of 100 ng/mL human interleukin 6 (IL-6; R & D Systems, Minneapolis, MN), 100 ng/mL recombinant mouse stem cell factor (SCF; Biosource International, Camarillo, CA), 50 ng/mL recombinant mouse thrombopoietin (TPO; R & D Systems), and 50 ng/mL recombinant mouse Flt-3 ligand (R & D Systems). All transductions were performed at 37°C with 5% CO2 for 96 hours. Retronectin-coated plates were prepared according to the manufacturer's instructions. BM cells were cultured at a density of 2 × 106 cells/mL in the presence of 2 × 106 viral particles to achieve a multiplicity of infection (MOI) of 1. After 24 hours of culture, an additional 2 × 106 virus particles were added. Viral supernatants and transduction media were replaced at 72 hours after the start of the transduction. Then 24 hours later, the cells were harvested, washed twice in Hanks balanced salt solution and counted. Cells (4 × 106) were injected into preconditioned B10.AKM recipients. To precondition recipients, mice were injected with 1 mg anti-CD8 (2.43) and 0.3 mg anti-CD4 (GK1.5) intraperitoneally on days −7 and −1 prior to BMT and lethally irradiated (10.25 Gy) on day −1.
Skin grafting
Four weeks after BMT, reconstituted mice were bled and reconstitution of CD4 and CD8 T cells was analyzed by flow cytometry following cell surface staining. When the percentages of CD4 and CD8 cells in the peripheral blood mononuclear cells (PBMCs) had reached levels similar to those observed in untreated controls (generally 8-10 weeks), tail skin grafting was performed and evaluated as previously described.12
Cytotoxic T-lymphocyte assay
B10.AKM mice reconstituted with transduced BM were primed in vivo with B10.MBR skin grafts. In some experiments, mice were also immunized intraperitoneally with 4 × 106 irradiated B10.MBR splenocytes. For all cytotoxic T-lymphocyte (CTL) assays, splenocytes were harvested and restimulated for 5 days in vitro with irradiated splenocytes at a 10:1 ratio as described in the text. The cells were then harvested and used in a standard CTL assay as described.6
Generation of Abelson transformed pre–B-cell lines
The B10.AKM Abelson virus transformed pre–B-cells line TBA-2 was established as described by Iacomini et al.13
Flow cytometry
To determine if VSV-Kb could transduce early progenitor cells, BM transduced with VSV-Kb was stained with monoclonal antibodies specific for Mac-1, CD11c, CD5, B220, Ter119, Gr-1, Sca-1, and Kb as described.14 Cy-chrome–conjugated anti-CD4 (RM4-5), phycoerythrin (PE)–conjugated anti-CD8 (53-6.7), fluorescein isothiocyanate (FITC)–conjugated anti-H-2Kb (AF6-88.5), PE-conjugated anti-B220 (RA3-6B2), and biotinylated CD11c (HL3) were obtained from Pharmingen (San Diego, CA). CD117/c-Kit 2B8 and Ter119 were generously provided by Dr I. Weissman of Stanford University.
Statistics
All statistical calculations were performed using GraphPad Prism 2.01 software (Graphpad Software, San Diego CA). The Kaplan and Meier method with a 95% CI was used for the calculation of survival curves. Comparison of survival curves was performed using the log-rank test.
Results
Retroviral transduction of BM with retrovirus carrying an MHC class I gene
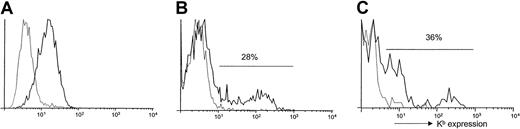
Because we were previously unable to consistently detect transduced MHC class I gene products on the surface of BM-derived cells,4,5,15 we hypothesized that expression of retrovirally transduced MHC class I genes in BM-derived cells resulted in hyporesponsiveness rather than tolerance in our previous studies because of inefficient BM transduction and cell surface expression of the transduced MHC gene. Our preliminary results indicated that using the improved BM transduction conditions that we have recently described11 was not sufficient to allow for detectable levels of long-term expression of Kb on the surface of BM-derived cells using the vector systems described previously.16 Therefore, to achieve long-term multilineage expression of the retrovirally transduced alloantigen on BM-derived cells we improved the retroviral vector used. The full-length cDNA encoding H-2Kb was cloned into the pMMP retroviral vector in which the myeloproliferative sarcoma virus (MPSV) long terminal repeat promoter-enhancer elements drive expression ofH-2Kb. MPSV transcriptional elements have been shown previously to allow expression in hematopoietic cell lineages.17 VSV-G protein–enveloped retroviruses carrying the gene encoding H-2Kb, hereafter referred to as VSV-Kb, were produced in 293T cells by transient transfection.9 10 Infection of NIH3T3 cells with VSV-Kb revealed that the virus is capable of transferring Kbexpression on the surface of infected cells (Figure1A).
Expression of Kb on transduced cells.
(A) Expression of Kb on the surface of VSV-Kb–transduced NIH3T3 cells. The 5 × 105 NIH3T3 cells were transduced overnight at an MOI of 5 with VSV-Kb (solid line) and examined for Kb expression by cell surface staining and flow cytometry 48 hours later. Mock-transduced cells (dashed line) were used as a control. (B) Expression of Kb on the surface of VSV-Kb (solid line) or mock-transduced (dashed line) B10.AKM BM. Expression of Kb was analyzed following cell surface staining by flow cytometry. (C) Expression of Kb on the surface of BM hematopoietic progenitor cells. Following transduction, B10.AKM BM cells were stained with antibodies specific for the lineage markers indicated in “Materials and methods,” Sca-1 and Kb. The cells were then analyzed by flow cytometry to examine expression of Kb. Shown are cells gated on the Lin−Sca-1+ fraction, which is approximately 3% of total BM after 5 days in culture. Thirty-six percent of Lin−Sca-1+ cells express Kbfollowing infection of 5-FU–treated BM with VSV-Kb (solid line). Mock-transduced controls (dashed line) are shown for comparison purposes. Shown is a representative experiment.
Expression of Kb on transduced cells.
(A) Expression of Kb on the surface of VSV-Kb–transduced NIH3T3 cells. The 5 × 105 NIH3T3 cells were transduced overnight at an MOI of 5 with VSV-Kb (solid line) and examined for Kb expression by cell surface staining and flow cytometry 48 hours later. Mock-transduced cells (dashed line) were used as a control. (B) Expression of Kb on the surface of VSV-Kb (solid line) or mock-transduced (dashed line) B10.AKM BM. Expression of Kb was analyzed following cell surface staining by flow cytometry. (C) Expression of Kb on the surface of BM hematopoietic progenitor cells. Following transduction, B10.AKM BM cells were stained with antibodies specific for the lineage markers indicated in “Materials and methods,” Sca-1 and Kb. The cells were then analyzed by flow cytometry to examine expression of Kb. Shown are cells gated on the Lin−Sca-1+ fraction, which is approximately 3% of total BM after 5 days in culture. Thirty-six percent of Lin−Sca-1+ cells express Kbfollowing infection of 5-FU–treated BM with VSV-Kb (solid line). Mock-transduced controls (dashed line) are shown for comparison purposes. Shown is a representative experiment.
To examine the ability of VSV-Kb to transfer Kb gene expression to primary murine BM cells, BM from B10.AKM mice treated with 150 mg/kg 5-FU 7 days previously was harvested and transduced as described.11 Immediately after transduction, approximately 28% of VSV-Kb–transduced BM cells expressed Kb on the cell surface, whereas mock-transduced B10.AKM BM cells remained negative (Figure 1B), reflecting a substantial increase over the levels of expression observed previously.5 Following infection with VSV-Kb, cell surface expression of Kb was detectable on approximately 42% of Mac-1+ cells (macrophages, granulocytes, and natural killer [NK] cells), 9% of B220+ cells (B-lineage cells), 45% of neutrophils, and less than 1% of CD3+ cells (T cells; Table1). As shown in Table 1, expression levels were generally lower in mature T and B cells when compared with Mac-1+ cells. Following transduction, approximately 3% of BM cells were lineage marker negative and Sca-1+(not shown), a population shown to be enriched for hematopoietic progenitors.18 In BM transduced with VSV-Kb, 36% of the lineage marker negative (Lin−), Sca-1+ cells expressed Kb on the cell surface (Figure 1C). These data suggest that VSV-Kb is able to transduce early hematopoietic progenitor cells.
BM subsets expressing Kb following transduction
| Antibody . | Target . | BM cell type . | % Lineage+* cells . | % Lin+† cells expressing Kb . |
|---|---|---|---|---|
| M1/70 | CD11b, Mac-1 | Macrophages, NK cells, granulocytes | 67 ± 7 | 42 ± 8 |
| RA3-6B2 | CD45R, B220 | B lineage | 18 ± 4 | 9 ± 4 |
| CT-CD3 | CD3 | T-cell receptor | 1 ± 1 | < 1 |
| RB6-8C5 | Ly-6G (Gr-1) | Granulocytes | 7 ± 3 | 49 ± 10 |
| 7/4 | Unknown | Neutrophils | 15 ± 4 | 45 ± 11 |
| Total BM | 20 ± 7 |
| Antibody . | Target . | BM cell type . | % Lineage+* cells . | % Lin+† cells expressing Kb . |
|---|---|---|---|---|
| M1/70 | CD11b, Mac-1 | Macrophages, NK cells, granulocytes | 67 ± 7 | 42 ± 8 |
| RA3-6B2 | CD45R, B220 | B lineage | 18 ± 4 | 9 ± 4 |
| CT-CD3 | CD3 | T-cell receptor | 1 ± 1 | < 1 |
| RB6-8C5 | Ly-6G (Gr-1) | Granulocytes | 7 ± 3 | 49 ± 10 |
| 7/4 | Unknown | Neutrophils | 15 ± 4 | 45 ± 11 |
| Total BM | 20 ± 7 |
Shown are the results of two independent experiments.
Percentage of cells in BM expressing the lineage marker.
Percentage of cells expressing the lineage marker that are expressing Kb.
Long-term expression of retrovirally encoded Kb in BM-derived cells in vivo
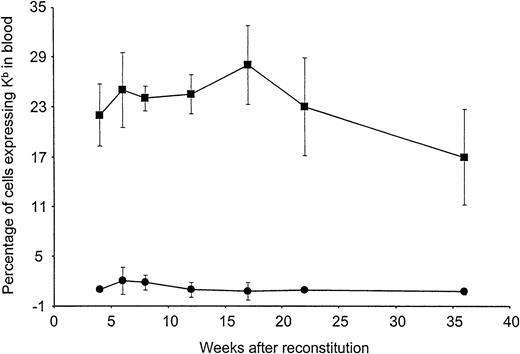
To examine long-term expression of Kb in vivo, preconditioned B10.AKM recipients were reconstituted with either 4 × 106 VSV-Kb or mock-transduced B10.AKM BM cells. BM-derived cells expressing Kb on their surface were detectable by flow cytometry in the blood of mice receiving VSV-Kb–transduced BM at all time points analyzed over the 36-week follow-up period (Figure 2). The percentage of cells in the blood expressing Kb appeared to remain stable, suggesting that early progenitor cells had been transduced. Expression of Kb on PBMCs was not significantly different at week 14 after reconstitution when compared with week 32 after reconstitution (P > .05). At 22 weeks after reconstitution, groups of animals were killed and cells from BM, thymus, spleen, lymph nodes, and peritoneal lavage were analyzed for Kb expression by cell surface staining and flow cytometry. Cells expressing Kb were detected in all lymphoid tissues analyzed from mice reconstituted with VSV-Kb–transduced BM (Table2). Expression of Kb was observed in all cell lineages examined at levels proportional to overall expression of Kb. As expected, cells expressing Kb were not detected in the blood of mice receiving mock-transduced BM or BM that had been transduced with a VSV-G pseudotyped Moloney murine leukemia virus encoding the gene for enhanced green fluorescent protein (VSV-GFP).
Long-term expression of H-2Kb in peripheral blood leukocytes of mice reconstituted with VSV-Kb–transduced BM.
B10.AKM mice were preconditioned with anti-CD4 and anti-CD8 antibodies, lethally irradiated, and reconstituted with 4 × 106VSV-Kb–transduced (squares) or mock-transduced (circles) syngeneic BM. At 4, 6, 8, 12, 16, 22, and 36 weeks after reconstitution, peripheral blood from these animals was examined for Kb expression by flow cytometry following cell surface staining. Shown are the percentages of Kb expressing cells. The values provided represent the mean and SD of a representative experiment containing a total of 11 VSV-Kb–transduced mice and 8 mock-transduced mice. Flow cytometry gates were set using cells from mice receiving mock-transduced BM. Dead cells were excluded from this analysis.
Long-term expression of H-2Kb in peripheral blood leukocytes of mice reconstituted with VSV-Kb–transduced BM.
B10.AKM mice were preconditioned with anti-CD4 and anti-CD8 antibodies, lethally irradiated, and reconstituted with 4 × 106VSV-Kb–transduced (squares) or mock-transduced (circles) syngeneic BM. At 4, 6, 8, 12, 16, 22, and 36 weeks after reconstitution, peripheral blood from these animals was examined for Kb expression by flow cytometry following cell surface staining. Shown are the percentages of Kb expressing cells. The values provided represent the mean and SD of a representative experiment containing a total of 11 VSV-Kb–transduced mice and 8 mock-transduced mice. Flow cytometry gates were set using cells from mice receiving mock-transduced BM. Dead cells were excluded from this analysis.
Lineage distribution of Kb-expressing cells in reconstituted mice
| . | Spleen . | Lymph node . | BM . | Thymus . | Peritoneum . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Lin+* . | % Kb+† . | % Lin+ . | % Kb+ . | % Lin+ . | % Kb+ . | % Lin+ . | %Kb+ . | % Lin+ . | % Kb+ . | |
| Total‡ | N/A | 22 ± 8 | N/A | 21 ± 10 | N/A | 22 ± 6 | N/A | 19 ± 6 | N/A | 18 ± 6 |
| Mac-1 | 41 ± 6 | 7 ± 3 | 30 ± 10 | 5 ± 3 | 30 ± 10 | 60 ± 9 | 37 ± 6 | < 1 | 17 ± 6 | 10 ± 4 |
| B220 | 23 ± 4 | 62 ± 10 | 23 ± 6 | 43 ± 9 | 21 ± 6 | 20 ± 6 | 27 ± 7 | 2 ± 1 | 21 ± 8 | 44 ± 69 |
| CD3 | 18 ± 7 | 16 ± 6 | 31 ± 8 | 40 ± 8 | 43 ± 7 | 4 ± 2 | 18 ± 8 | 61 ± 12 | 28 ± 5 | 29 ± 69 |
| CD11c | 50 ± 10 | 4 ± 2 | 66 ± 11 | 2 ± 1 | 60 ± 9 | 2 ± 1 | 46 ± 10 | < 1 | 10 ± 6 | 6 ± 3 |
| . | Spleen . | Lymph node . | BM . | Thymus . | Peritoneum . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Lin+* . | % Kb+† . | % Lin+ . | % Kb+ . | % Lin+ . | % Kb+ . | % Lin+ . | %Kb+ . | % Lin+ . | % Kb+ . | |
| Total‡ | N/A | 22 ± 8 | N/A | 21 ± 10 | N/A | 22 ± 6 | N/A | 19 ± 6 | N/A | 18 ± 6 |
| Mac-1 | 41 ± 6 | 7 ± 3 | 30 ± 10 | 5 ± 3 | 30 ± 10 | 60 ± 9 | 37 ± 6 | < 1 | 17 ± 6 | 10 ± 4 |
| B220 | 23 ± 4 | 62 ± 10 | 23 ± 6 | 43 ± 9 | 21 ± 6 | 20 ± 6 | 27 ± 7 | 2 ± 1 | 21 ± 8 | 44 ± 69 |
| CD3 | 18 ± 7 | 16 ± 6 | 31 ± 8 | 40 ± 8 | 43 ± 7 | 4 ± 2 | 18 ± 8 | 61 ± 12 | 28 ± 5 | 29 ± 69 |
| CD11c | 50 ± 10 | 4 ± 2 | 66 ± 11 | 2 ± 1 | 60 ± 9 | 2 ± 1 | 46 ± 10 | < 1 | 10 ± 6 | 6 ± 3 |
N/A indicates not applicable.
Percentage of lineage marker positive cells that express Kb.
Percentage of Kb+ cells expressing each lineage marker.
Total percentage of Kb+ cells in each tissue.
Expression of retrovirally transduced Kb on BM-derived cells induces stable long-term tolerance to Kb
To determine whether expression of Kb on BM-derived cells was sufficient to induce immunologic tolerance to Kb, we assessed the ability of splenocytes from mice reconstituted with VSV-Kb– or VSV-GFP–transduced BM to kill Kb-expressing target cells. To generate Kb+ target cells, a transformed Abelson pre-B cell line (TBA-2) was generated from B10.AKM BM. These cells were then transduced with VSV-Kb and sorted by flow cytometry based on cell surface expression of Kb. The resulting cell line, TBA-Kb, was 99% Kb+ by cell surface staining and flow cytometry (data not shown).
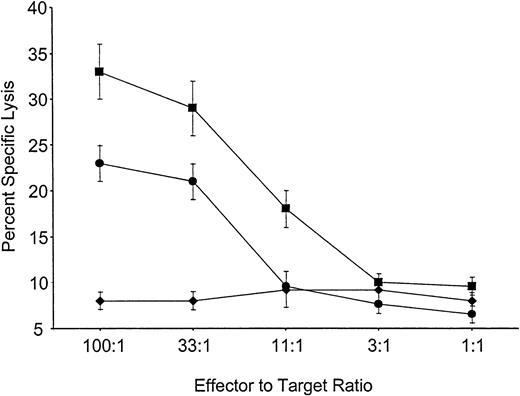
Mice reconstituted with VSV-Kb or VSV-GFP–transduced BM were primed in vivo by grafting both Kb-disparate B10.MBR and third-party B10.BR skin 8 weeks after BMT. Eight weeks later the mice were killed, and splenocytes from these animals were cultured for 5 days in the presence of either B10.MBR- or B10.BR-irradiated splenocytes. After 5 days, cells were harvested and their ability to lyse Kb expressing B10.MBR or third-party B10.BR targets in a standard CTL assay was determined. Splenocytes from mice reconstituted with VSV-Kb–transduced BM were able to generate a vigorous CTL response against third-party B10.BR concanavalin A (ConA) blasts (Figure 3). However, splenocytes from the same mice were unable to kill TBA-Kb targets (Figure 3). Control mice receiving VSV-GFP–transduced BM made vigorous CTL responses to TBA-Kb cells (Figure 3). Splenocytes from mice receiving either VSV-Kb or VSV-GFP–transduced BM reconstituted animals failed to lyse parental TBA-2 targets (data not shown). These data suggest that mice reconstituted with VSV-Kb–transduced BM were tolerant to Kb.
Mice reconstituted with VSV-Kb–transduced BM fail to kill Kb-bearing targets.
Shown is killing of TBA-Kb targets (diamonds) or third-party B10.BR ConA blasts (squares) by splenocytes from mice reconstituted with VSV-Kb–transduced BM. Killing of TBA-Kb cells by splenocytes harvested from a control mouse reconstituted with VSV-GFP–transduced BM is shown in circles. Shown is a representative experiment.
Mice reconstituted with VSV-Kb–transduced BM fail to kill Kb-bearing targets.
Shown is killing of TBA-Kb targets (diamonds) or third-party B10.BR ConA blasts (squares) by splenocytes from mice reconstituted with VSV-Kb–transduced BM. Killing of TBA-Kb cells by splenocytes harvested from a control mouse reconstituted with VSV-GFP–transduced BM is shown in circles. Shown is a representative experiment.
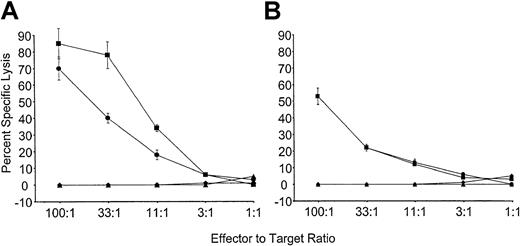
To determine if tolerance observed to Kb was stable, mice reconstituted with VSV-Kb or VSV-GFP–transduced BM were grafted with B10.MBR and third-party B10.BR skin as described above. Twenty-eight weeks after reconstitution, mice from both groups were immunized with 5 × 106 irradiated B10.MBR splenocytes. Ten days after immunization, the mice were killed, and splenocytes restimulated in vitro with irradiated B10.MBR cells. T cells from control mice receiving VSV-GFP–transduced BM were able to lyse both TBA-Kb cells and B10.MBR ConA blasts (Figure4). In contrast, mice reconstituted with VSV-Kb–transduced BM were unable to lyse either TBA-Kb cells or B10.MBR ConA blasts (Figure 4). To determine if this effect could be overcome by the addition of exogenous IL-2, as had been described previously,6 splenocytes from immunized VSV-GFP and VSV-Kb mice were also cultured with irradiated B10.MBR stimulator splenocytes in the presence of 20 U/mL exogenous IL-2. Whereas splenocytes from mice receiving VSV-GFP–transduced BM were able to lyse TBA-Kb cells and B10.MBR ConA blasts, mice reconstituted with VSV-Kb–transduced BM were unable to lyse either target. These data strongly suggest that gene therapy can be used to induce stable tolerance to alloantigens.
Mice reconstituted with VSV-Kb–transduced BM are tolerant to Kb.
B10.AKM mice reconstituted with VSV-Kb– or VSV-GFP–transduced BM received a B10.MBR and B10.BR skin graft 8 weeks after reconstitution and were immunized with 5 × 106 irradiated B10.MBR splenocytes 28 weeks after reconstitution. Ten days after immunization, splenocytes from VSV-GFP and VSV-Kb mice were harvested and cultured for 5 days with irradiated B10.MBR stimulator cells. Splenocytes from control mice reconstituted with VSV-GFP–transduced BM were tested for their ability to kill ConA-treated B10.MBR splenocytes (squares) or Abelson transformed pre-B cells lines expressing Kb(TBA-Kb; circles). Splenocytes from mice reconstituted with VSV-Kb–transduced BM were tested for their ability to kill ConA-treated B10.MBR splenocytes (triangles) or TBA-Kb cells (diamonds). Results of CTL-mediated lysis after restimulation in vitro in the presence (A) or absence (B) of 20 IL-2 U/mL is shown from a representative experiment.
Mice reconstituted with VSV-Kb–transduced BM are tolerant to Kb.
B10.AKM mice reconstituted with VSV-Kb– or VSV-GFP–transduced BM received a B10.MBR and B10.BR skin graft 8 weeks after reconstitution and were immunized with 5 × 106 irradiated B10.MBR splenocytes 28 weeks after reconstitution. Ten days after immunization, splenocytes from VSV-GFP and VSV-Kb mice were harvested and cultured for 5 days with irradiated B10.MBR stimulator cells. Splenocytes from control mice reconstituted with VSV-GFP–transduced BM were tested for their ability to kill ConA-treated B10.MBR splenocytes (squares) or Abelson transformed pre-B cells lines expressing Kb(TBA-Kb; circles). Splenocytes from mice reconstituted with VSV-Kb–transduced BM were tested for their ability to kill ConA-treated B10.MBR splenocytes (triangles) or TBA-Kb cells (diamonds). Results of CTL-mediated lysis after restimulation in vitro in the presence (A) or absence (B) of 20 IL-2 U/mL is shown from a representative experiment.
Long-term acceptance of Kb mismatched skin grafts in molecular chimeras
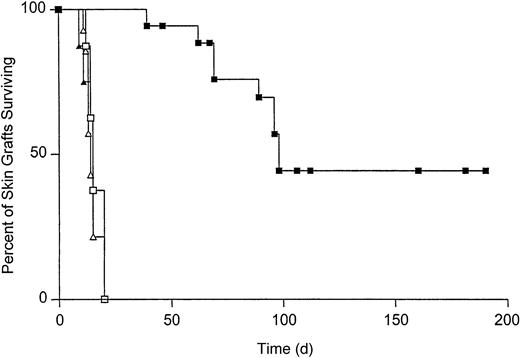
To determine the effect of T-cell tolerance on the survival of Kb-disparate grafts, B10.AKM mice were reconstituted with VSV-Kb or mock-transduced BM. Starting at 4 weeks after reconstitution, T-cell reconstitution was analyzed by staining peripheral blood cells with monoclonal antibodies specific for CD4 and CD8 followed by flow cytometry. T-cell recovery was complete by 8 weeks after reconstitution (data not shown). When T-cell reconstitution was complete, mice received both a B10.MBR (Kb mismatched) and B10.BR (third-party, H-2k) skin graft. Skin grafts were inspected daily for signs of rejection. Third-party B10.BR skin grafts were rejected rapidly from mice reconstituted with either mock-transduced BM (median survival time [MST] = 14, n = 14) or VSV-Kb–transduced BM (MST = 15, n = 19, P = .3 between groups; Figure 5). B10.MBR grafts were also rejected rapidly from mice reconstituted with mock-transduced BM (MST = 15, n = 14, P = .8; Figure 5). In contrast, survival of B10.MBR skin grafts on B10.AKM mice reconstituted with VSV-Kb–transduced BM was significantly prolonged (MST = 98, n = 18, P = .0001; Figure 5). Forty percent of B10.AKM mice reconstituted with VSV-Kb–transduced BM accepted their B10.MBR skin grafts indefinitely (> 150 days). Splenocytes from animals that had rejected their B10.MBR skin grafts also failed to lyse TBA-Kb targets in a standard CTL assay after reimmunization with 5 × 106 irradiated B10.MBR splenocytes, and restimulation for 5 days in vitro (data not shown). Skin graft rejection did not appear to be caused by alloantibody production because serum from mice reconstituted with VSV-Kb– or mock-transduced BM did not contain IgG specific for B10.MBR splenocytes (data not shown).
Survival of B10.MBR and B10.BR skin grafts on recipients reconstituted with either VSV-Kb– or mock-transduced BM.
Survival of B10.MBR (closed symbols) and third-party B10.BR (open symbols) skin grafts on mice receiving VSV-Kb–transduced BM (squares) or mock-transduced BM (triangles). Survival of B10.MBR skin grafts was significantly prolonged on mice receiving VSV-Kb–transduced BM (MST = 98 days, n = 18) when compared to survival on controls receiving mock-transduced BM (MST = 15 days, n = 14,P = .0001 between groups). No difference was observed in survival of third-party B10.BR skin on mice receiving VSV-Kb– or mock-transduced BM (MST = 14 and 15 days, respectively;P = .3). Shown are the combined the results of 2 independent experiments.
Survival of B10.MBR and B10.BR skin grafts on recipients reconstituted with either VSV-Kb– or mock-transduced BM.
Survival of B10.MBR (closed symbols) and third-party B10.BR (open symbols) skin grafts on mice receiving VSV-Kb–transduced BM (squares) or mock-transduced BM (triangles). Survival of B10.MBR skin grafts was significantly prolonged on mice receiving VSV-Kb–transduced BM (MST = 98 days, n = 18) when compared to survival on controls receiving mock-transduced BM (MST = 15 days, n = 14,P = .0001 between groups). No difference was observed in survival of third-party B10.BR skin on mice receiving VSV-Kb– or mock-transduced BM (MST = 14 and 15 days, respectively;P = .3). Shown are the combined the results of 2 independent experiments.
Discussion
Previous studies using genetic engineering of BM demonstrated that expression of the retrovirally encoded MHC class I geneH-2Kb in BM-derived cells induced hyporesponsiveness rather than tolerance to Kb. Hyporesponsiveness to Kb appeared to be regulated peripherally because the effect of gene therapy could be broken in vivo or in vitro by providing sufficient T-cell help.5,6 In these previous studies, we could not reliably detect Kb on the surface of BM-derived cells by cell surface staining and flow cytometry, and in some studies it became apparent that expression of the retrovirally encoded MHC class I antigen was lost over time.5 15 Based on these data, we hypothesized that the level of cell surface expression of Kb was inadequate or the efficiency of BM transduction too low to allow for the generation of a significant number of Kb-expressing cells able to mediate negative selection of alloreactive T cells. Here we demonstrate that efficient transduction and expression of a retrovirally encoded MHC class I gene in BM-derived cells can be used to induce stable long-term tolerance to the retrovirally encoded alloantigen.
To improve BM transduction efficiency, we examined the effect of changing the cytokine cocktail used during infection of BM.19 Cytokine cocktails have been shown in other studies11 to greatly increase the efficiency of BM transduction, and in our case led to a substantial increase in frequency of BM cells expressing Kb following transduction. However, this modification alone did not allow for efficient expression of Kb on BM-derived cells in vivo. We therefore reasoned that expression of retrovirally encoded Kb would have to be improved. Using the MMP retroviral vector, we were able to achieve expression of Kb of a significant fraction of BM cells following transduction, including BM cells with cell surface characteristics of hematopoietic progenitors. We suggest that the MPSV transcriptional elements used in this construct as well as the VSV-G envelope allowed for improved infection of early progenitors and expression in hematopoietic cell lineages. (A detailed analysis of transduction conditions and vectors is in preparation.) Analysis of mice reconstituted with VSV-Kb demonstrated long-term multilineage cell surface expression of Kb. Importantly, expression was also detected on myeloid lineages long-term. Because myeloid lineage progenitors are short-lived,18 20 these data support the notion that we were able to infect relatively early hematopoietic progenitors.
Efficient expression of Kb on BM-derived cells was sufficient to significantly prolong survival of B10.MBR skin grafts expressing Kb. Approximately 40% of mice accepted their B10.MBR grafts long-term (> 100 days) while rapidly rejecting third-party control grafts. In vitro analysis of CTLs suggested that T cells in mice receiving VSV-Kb–transduced BM were unable to lyse targets expressing Kb. Importantly, in vivo sensitization by skin grafting and subsequent re-exposure to antigen by immunization followed by restimulation in vitro in the presence of IL-2 did not abrogate the effect of gene therapy. This is in sharp contrast to previous studies in which T-cell hyporesponsiveness to Kbinduced by genetic engineering of BM could be broken by provision of T-cell help.5 6 We suggest that improved expression of Kb on the surface of BM-derived cells induced stable T-cell tolerance, rather than hyporesponsiveness. Because the effect of gene therapy in this study was not broken following rigorous restimulation, we suggest that the tolerance induced to Kb was through a central thymic rather than peripheral mechanism. We suggest that gene therapy can be used to reshape the T-cell repertoire leading to negative selection of alloreactive T cells in the thymus.
Although our studies demonstrated specific tolerance to Kb, approximately 60% of mice receiving VSV-Kb–transduced BM eventually rejected B10.MBR skin grafts. Mice that had rejected their B10.MBR grafts maintained stable expression of Kb on BM-derived cells that was indistinguishable from mice that accepted their skin grafts, and we were unable to detect Kb-reactive cells in these animals following subsequent reimmunization and stimulation in vitro in the presence of IL-2 (not shown). Therefore, skin graft rejection did not correlate with T-cell responsiveness to Kb. Interestingly, late rejection of B10.MBR grafts appeared to be visually distinct from acute cellular rejection of B10.MBR grafts placed on control mice and occurred over several days rather than acutely (not shown). One possible explanation for this may be related to expression of tissue-specific minor antigens expressed on B10.MBR skin, or the existence of unknown minor antigen disparities between B10.AKM and B10.MBR mice. The region centromeric to I-A is of B10.A origin in B10.AKM mice and of C57BL/10 origin in B10.MBR.21 Thus, although B10.AKM and B10.MBR are congenic lines, these lines may carry additional minor antigen disparities in addition to being mismatched at the H-2K locus. Furthermore, these lines have been have been maintained separately by Jackson Laboratories for more that 20 generations, opening up the possibility that new polymorphisms between the strains have become fixed. We are currently testing this hypothesis genetically to analyze segregation of minor antigens in B10.AKM and B10.MBR mice.
To our knowledge, this is the first example in which gene therapy has been successfully applied to induce stable T-cell tolerance to an alloantigen without any additional immunosuppression. Although others have shown that gene therapy can be used to induce T-cell hyporesponsiveness, these studies have fallen short of demonstrating true tolerance.22-25 Based on our studies, we suggest that the level of antigen expression achieved can greatly affect whether gene therapy results in hyporesponsiveness or tolerance. Therefore in these previous studies as well as our own, the level of expression was not sufficient to induce true immunologic tolerance, which is stable after antigen challenge. Indeed, Schumacher and coworkers reported very low transduction efficiencies.22 It is also possible that differences in the cell types expressing the retrovirally transduced genes could affect the ability of BM-derived cells to induce tolerance. The transduction conditions used by Kang et al25 included the growth factor IL-7, which is known to favor survival and proliferation of B-cell lineages. Interestingly, it has been reported that B cells can only tolerize virgin T cells.26 Lastly, it is possible that the ability to induce tolerance by gene therapy depends on the type of antigen being examined. Nevertheless, our data strongly suggest that efficient expression of retrovirally transduced alloantigens on BM-derived cells is sufficient to induce specific immunologic tolerance that remains stable following rigorous antigen challenge. Thus, gene therapy approaches can be used to fundamentally reshape the immunologic repertoire.
Using genetically modified autologous BM to establish molecular chimerism eliminates many of the complications associated with allogeneic BMT to establish mixed cellular chimerism. Most importantly, establishing molecular chimerism results in the same stable and specific tolerance to Kb established following induction of mixed cellular chimerism. We suggest that the use of autologous BM mitigates the potential for GVHD and engraftment failure and may permit the use of less toxic host conditioning regimens. For these reasons our approach may be clinically applicable. Similar approaches may eventually be applicable for the induction of tolerance in immunologic disorders such as autoimmune disease.
The authors wish to thank Drs Richard C. Mulligan and Jeng-Shin Lee for providing the MMP retroviral vector, packaging system, and technical advice as well as the Harvard Gene Therapy Initiative Vector Core, in part supported by the Association Francaise contre les Myopathies (AFM), for production of viral vectors. In addition, we thank Drs Joren Madsen and Shiv Pillai for critical review of the manuscript, Yin Wu for expert technical assistance, and members of the Iacomini Laboratory for helpful suggestions and comments.
Supported in part by National Institutes of Health grant RO1 AI43619 to J.I. J.B. is supported by NIH training grant T32 AI07529, and in part by a grant from the Children's A-T Project.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John Iacomini, Transplantation Biology Research Center, Massachusetts General Hospital, 149-5210, 13th St, Boston, MA 02129; e-mail: john.iacomini@tbrc.mgh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal