We treated 45 adult patients with T-lymphoblastic lymphoma (T-LBL) (age range 15-61 years) with 2 protocols designed for adult acute lymphoblastic leukemia (ALL). An encouraging cure rate of 90% was recently reported for T-LBL in children treated with a similar approach. In our study, an 8-drug standard induction was administered over 8 weeks including prophylactic cranial (24 Gy) and mediastinal irradiation (24 Gy) followed by consolidation and reinduction therapy. At diagnosis, 91% of the 45 patients showed a mediastinal tumor and 40% had pleural/pericardial effusions; 73% of the patients had stage III/IV disease. Overall, 42 patients (93%) achieved a complete remission (CR), 2 patients (4%) achieved a partial remission, and 1 patient (2%) died during induction. In patients with stage I-III disease (n = 18) the CR rate was 100% compared with 89% in stage IV (n = 27). There were 15 patients who relapsed (36%) within 12 months. The majority of relapses (47%) occurred in the mediastinum (n = 7) despite mediastinal irradiation with 24 Gy in 6 out of 7 patients. The estimates for overall survival, continuous CR, and disease-free survival at 7 years are 51%, 65%, and 62%, respectively. Stage, age, lactate dehydrogenase, and all other parameters had no influence on achievement of CR or outcome. This study demonstrates in a large cohort of patients with adult T-LBL that a high CR rate and a favorable outcome can be achieved with an ALL-type regimen. Mediastinal recurrence was the major obstacle and further improvement by intensification of chemotherapy, increased dose of mediastinal irradiation (36 Gy), and extended indications for stem cell transplantation seem to be required.
Introduction
T-lymphoblastic lymphoma (T-LBL) is a rare subtype of adult non-Hodgkin lymphoma (NHL) with an incidence of approximately 2%. Various treatment approaches have been applied to LBL in adults, including protocols for high-grade NHL such as CHOP, CHOEP, treatment according to different regimens for acute lymphoblastic leukemia (ALL), and autologous stem cell transplantation (SCT). Reports on treatment results are scarce and mostly include LBL of B- and T-cell origin without separate analysis. In a retrospective analysis of a large cohort of pediatric patients with T-LBL (n = 105), a cure rate of 90% was achieved with treatment according to a regimen for childhood ALL. No prognostic factors for achievement of CR or survival could be identified.1
Clinical and biologic features of T-LBL such as male predominance, high incidence of mediastinal tumor, and other lymphomatous manifestations are similar to those of T-ALL and different from B-LBL.2T-ALL and T-LBL are separated by an arbitrary margin of 25% bone marrow involvement; patients with higher degree of bone marrow infiltration are generally classified as T-ALL. A similar treatment approach for T-ALL and T-LBL which is oriented to disease biology therefore appears reasonable. The 2 recent protocols of the German Multicenter Study Group for adult ALL (GMALL), 04/89 (6/89-6/93) and 05/93 (7/93-6/99), had specific treatment arms for T-ALL and therefore patients with T-LBL were treated according to these protocols.
The aim of this analysis was to characterize clinical and biologic features and to evaluate outcome and prognostic factors for adult patients with T-LBL treated according to an ALL-type regimen. Further analyses should be aimed at the questions of whether this successful approach in childhood T-LBL is effective in adult patients, which differences between adult and pediatric patients can be identified, and whether specific treatment approaches for adults are needed.
Patients, materials, and methods
Patients
Between June 1989 and December 1998, 50 adult patients with T-LBL were treated according to 2 consecutive treatment protocols for adult ALL (age range 15-65 years). Patients with a confirmed diagnosis of T-LBL were included in this analysis. Exclusion criteria were demonstration of human immunodeficiency virus, pre-existing disease prohibiting intensive chemotherapy, and pretreatment with cytostatic drugs other than corticosteroids and vincristine (more than 2 weeks) or one cycle of CHOP-like regimens. There were 4 patients who have been excluded due to extensive pretreatment exceeding these criteria (6xCHOP; 4xMEV; B-NHL protocol; 4xCHOP) and 1 patient due to lacking follow-up data. There are 45 patients included in this analysis. After informed consent, 28 patients were enrolled from 13 centers of the GMALL and 17 patients were recruited at the Department of Haematology at the Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (MSCMCC) in Warsaw, Poland.
Diagnosis
A diagnosis was established by histologic or cytologic examination3 of lymph node or tumor biopsy alone in 17 patients, demonstration of T-lymphoblastic subtype by detection of T-cell–specific surface markers4 in addition to histology in 27 patients, or by immunologic classification of bone marrow blasts or blast cells from pleural effusions in 2 patients. Histologic classification was made according to the updated Kiel-Classification for NHL5 or the Revised European American Lymphoma Classification.6
Clinical diagnosis and staging included peripheral blood values and smears, bone marrow aspiration and smears, examination of cerebrospinal fluid (CSF), general laboratory tests including serum lactate dehydrogenase (LDH), ultrasonography, x-ray, and computed tomography (CT). Clinical staging was performed according to the Ann Arbor Classification.7 Central nervous system (CNS) involvement was determined by routine diagnostic lumbar puncture at diagnosis either in the case of more than 18/3 cells in the CSF and morphologic/immunologic demonstration of blast cells on cytospin preparation, or by detection of leukemic infiltration with CT or MRT. Mediastinal involvement was diagnosed by x-ray examination of the chest and additional CT evaluation in patients with mediastinal enlargement.
Treatment
Chemotherapy.
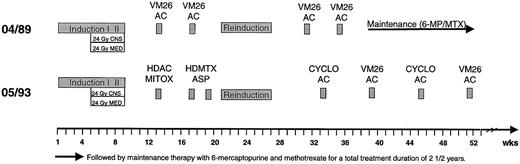
All patients received a protocol designed for the treatment of adult patients with ALL. There were 7 patients treated according to the GMALL 04/89 protocol and 38 according to the GMALL 05/93 protocol. The general treatment outline is shown in Figure1 and treatment elements are detailed in Table 1. In both studies treatment started with an 8-drug induction therapy with 2 phases (weeks 1-4 and 5-8) administered over 8 weeks. Chemotherapy further included a 6-drug reinduction therapy over 6 weeks at month 6 in both studies. Consolidation treatment in GMALL 04/89 consisted of 2 cycles of teniposide combined with cytarabine (VM26/AC) between induction and reinduction and 2 cycles VM26/AC after reinduction followed by maintenance therapy. In GMALL 05/93, consolidation was intensified with high-dose cytarabine in combination with mitoxantrone (HDAC/MI) and high-dose methotrexate with L-asparaginase (HDMTX/ASP) before reinduction and 4 alternating cycles of cyclophosphamide/cytarabine (CYCLO/AC) and VM26/AC after reinduction.
Treatment overview of GMALL studies 04/89 and 05/93.
AC indicates cytarabine; ASP, asparaginase; CYCLO, cyclophosphamide; HD, high dose; MITOX, mitoxantrone; MTX, methotrexate; VM26, teniposide.
Treatment overview of GMALL studies 04/89 and 05/93.
AC indicates cytarabine; ASP, asparaginase; CYCLO, cyclophosphamide; HD, high dose; MITOX, mitoxantrone; MTX, methotrexate; VM26, teniposide.
Treatment elements of GMALL studies 04/89 and 05/93
| Drug . | Dose . | Days of administration . |
|---|---|---|
| Induction I | ||
| Prednisone (po) | 60 mg/m2 | 1-28, then taper over 3 × 3 days |
| Vincristine (iv) | 2 mg | 1, 8, 15, 22 |
| Daunorubicin (iv, 30 min) | 45 mg/m2 | 1, 8, 15, 22 |
| Methotrexate (it) | 15 mg | 1 |
| L-Asparaginase (iv, 30 min) | 5 000 IU/m2 | 15-28, every other day |
| Induction II | ||
| Cyclophosphamide (iv) | 1 000 (650) mg/m2 | 29, 43, 57 |
| Cytarabine (iv, 1 h) | 75 mg/m2 | 31-34, 38-41, 45-48, 52-55 |
| 6-Mercaptopurine (orally) | 60 mg/m2 | 29-57 |
| Methotrexate (it) | 15 mg | 31, 38, 45, 52 |
| Consolidation I | ||
| HDAC/MITOX | ||
| Cytarabine (iv, 3 h) | 2* × 1 000 mg/m2 | 1-4 |
| Mitoxantrone (iv, 30 min) | 10 mg/m2 | 3-5 (2-5) |
| HDMTX/ASP | ||
| Methotrexate (iv, 24 h) | 1 500 mg/m2 | 1, 15 |
| Asparaginase | 10 000 U/m2 | 2, 16 |
| 6-Mercaptopurine | 25 mg/m2 | 1-5, 15-19 |
| Reinduction I | ||
| Prednisolone (orally) | 60 mg/m2 | 1-28 |
| Vincristine (iv) | 2 mg | 1, 8, 15, 22 |
| Adriamycine (iv, 30 min) | 25 mg/m2 | 1, 8, 15, 22 |
| Triple prophylaxis (it) | 1 | |
| Reinduction II | ||
| Cyclophosphamide (iv) | 1 000 (650) mg/m2 | 29 |
| Cytarabine (iv, 1 h) | 75 mg/m2 | 31-34, 38-41 |
| 6-Thioguanine (orally) | 60 mg/m2 | 29-42 |
| Triple prophylaxis (it) | 29 | |
| Consolidation II | ||
| VM26/ARAC† | ||
| Etoposide (iv, 1 h) | 100 (60) mg/m2 | 1-5 |
| Cytarabine (iv, 1 h) | 150 (75) mg/m2 | 1-5 |
| CYCLO/ARAC | ||
| Cyclophosphamide (iv) | 1 000 mg/m2 | d 1 |
| Cytarabine (iv, 24 h) | 500 mg/m2 | d 1 |
| Triple prophylaxis | ||
| Methotrexate (it) | 15 mg | |
| Cytarabine (it) | 40 mg | |
| Dexamethasone (it) | 4 mg |
| Drug . | Dose . | Days of administration . |
|---|---|---|
| Induction I | ||
| Prednisone (po) | 60 mg/m2 | 1-28, then taper over 3 × 3 days |
| Vincristine (iv) | 2 mg | 1, 8, 15, 22 |
| Daunorubicin (iv, 30 min) | 45 mg/m2 | 1, 8, 15, 22 |
| Methotrexate (it) | 15 mg | 1 |
| L-Asparaginase (iv, 30 min) | 5 000 IU/m2 | 15-28, every other day |
| Induction II | ||
| Cyclophosphamide (iv) | 1 000 (650) mg/m2 | 29, 43, 57 |
| Cytarabine (iv, 1 h) | 75 mg/m2 | 31-34, 38-41, 45-48, 52-55 |
| 6-Mercaptopurine (orally) | 60 mg/m2 | 29-57 |
| Methotrexate (it) | 15 mg | 31, 38, 45, 52 |
| Consolidation I | ||
| HDAC/MITOX | ||
| Cytarabine (iv, 3 h) | 2* × 1 000 mg/m2 | 1-4 |
| Mitoxantrone (iv, 30 min) | 10 mg/m2 | 3-5 (2-5) |
| HDMTX/ASP | ||
| Methotrexate (iv, 24 h) | 1 500 mg/m2 | 1, 15 |
| Asparaginase | 10 000 U/m2 | 2, 16 |
| 6-Mercaptopurine | 25 mg/m2 | 1-5, 15-19 |
| Reinduction I | ||
| Prednisolone (orally) | 60 mg/m2 | 1-28 |
| Vincristine (iv) | 2 mg | 1, 8, 15, 22 |
| Adriamycine (iv, 30 min) | 25 mg/m2 | 1, 8, 15, 22 |
| Triple prophylaxis (it) | 1 | |
| Reinduction II | ||
| Cyclophosphamide (iv) | 1 000 (650) mg/m2 | 29 |
| Cytarabine (iv, 1 h) | 75 mg/m2 | 31-34, 38-41 |
| 6-Thioguanine (orally) | 60 mg/m2 | 29-42 |
| Triple prophylaxis (it) | 29 | |
| Consolidation II | ||
| VM26/ARAC† | ||
| Etoposide (iv, 1 h) | 100 (60) mg/m2 | 1-5 |
| Cytarabine (iv, 1 h) | 150 (75) mg/m2 | 1-5 |
| CYCLO/ARAC | ||
| Cyclophosphamide (iv) | 1 000 mg/m2 | d 1 |
| Cytarabine (iv, 24 h) | 500 mg/m2 | d 1 |
| Triple prophylaxis | ||
| Methotrexate (it) | 15 mg | |
| Cytarabine (it) | 40 mg | |
| Dexamethasone (it) | 4 mg |
Every 12 hours.
Dosage for study 04/89 in brackets.
iv indicates intravenous; it, intrathecally; po, oral.
CNS prophylaxis included one application of intrathecal methotrexate at diagnosis and 4 additional applications during phase II of induction parallel to CNS irradiation. There were 2 doses of intrathecal triple chemotherapy with methotrexate, cytarabine, and dexamethasone scheduled during reinduction therapy.
Treatment for T-LBL was conducted according to active ALL protocols irrespective of stage of disease (I/II or III/IV) or other risk factors. Instead of 2 1/2 years total treatment duration in ALL patients, in T-LBL a total treatment duration of at least 6 months (including reinduction) and up to 12 months was recommended.
Local irradiation.
Prophylactic cranial irradiation with 24 Gy was scheduled for phase II of induction (weeks 5-8) after achievement of CR. When CR was achieved later (after phase II of induction), CNS irradiation was administered thereafter. Prophylactic mediastinal irradiation (24 Gy) was recommended for all patients during phase II of induction irrespective of mediastinal tumor response. For patients in whom the tumor was already resolved (CR), the irradiation field comprised only the anatomic mediastinum and not the original extent of mediastinal tumor; in the case of persistent tumor after phase I of induction, irradiation was administered to the residual tumor. In the case of severe cytopenia, chemotherapy was interrupted and irradiation continued whenever possible. Local irradiation of other manifestations was not part of the protocol.
Supportive care.
Supportive care apart from the usual measures for chemotherapy such as sufficient fluid uptake and antiemetic treatment included antimicrobial prophylaxis with cotrimoxazol (3 × 960 mg/d) and colistin (2 million IU/d) and antifungal prophylaxis with amphotericin B solution (4 × 500 mg/d) and amphotericin inhalation (1 × 20 mg/d). In patients with fever, a stepwise intervention program for empiric antimicrobial and antifungal treatment was initiated.
Response evaluation
Response evaluation was performed on day 28 after the end of phase I of induction therapy and at the end of induction phase II (day 56). It included clinical assessment, peripheral blood analysis, and bone marrow aspiration in patients with initial bone marrow involvement. The response of mediastinal tumor was evaluated with an x-ray of the chest and CT after phase I and II of induction. In patients with residual tumor, no biopsy or resection was performed to confirm or exclude vital lymphoma. CR was defined as disappearance of blast cells from bone marrow (< 5%), peripheral blood, CSF, and any other clinical manifestation, the latter confirmed by imaging procedures. Early achievement of CR was defined as CR after completion of the 8-week induction therapy (phase I and II). Late CR was defined as later achievement of CR after additional consolidation cycles and/or mediastinal irradiation.
Statistical analysis
The median follow-up for surviving patients was 45 months (range, 12-91 months). One patient was lost to follow-up in continuous complete remission (CCR) at day 768. Overall survival was calculated from the date of treatment initiation to death or to the date of last follow-up in surviving patients. Remission duration was calculated from the date of first remission to the date of relapse or date of last follow-up in patients with CCR. Patients with SCT in first remission were censored at the time of transplantation in the curves for remission duration. Disease-free survival was calculated from the date of first remission to the date of relapse or death in CR (events) or to the date of last follow-up. Analysis was performed according to the Kaplan-Meier method8 and differences were analyzed with the log-rank test. Differences of the frequency of patient parameters were analyzed with the χ2 test or Fisher exact test, depending on the number of groups compared. The statistical analysis was performed with the SAS program (SAS-PC, Version 8; SAS Institute, Cary, NC).
Results
Patient characteristics
The median age of the 45 evaluable patients was 25 years, with a range of 15-61 years. There were 33 male patients and 11 female patients (3:1). The median values for peripheral blood parameters were 8.4 × 109/L (range, 2.5-115) for white blood cells (WBCs); 5.9 × 109/L (range, 1.4-36) for neutrophils; 257 × 109/L (range, 48-697) for platelets; and 14.2 g/dL (range, 9.6-16.5) for hemoglobin. Thus, none of the patients had a substantial leukopenia, neutropenia, thrombocytopenia, or anemia. A bone marrow infiltration was present in 31% of the patients (n = 14). There were 3 patients with predominant mediastinal tumor who were included although bone marrow infiltration exceeded 25%, and in those patients the WBC count was increased above 50 × 109/L. LDH was increased more than 240 U/L (normal value) in 84% of the patients. In 29% (n = 11) of the patients, LDH values were above 500 U/L. B-symptoms were present in 27% (n = 10) of the evaluable patients (Table2).
Entry criteria in adult T-LBL and prognostic impact on achievement of complete remission, overall survival, and probability of continuous complete remission
| Entry criteria . | Frequency N (%) . | CR rate* N (%) . | Probability of survival* . | Probability of CCR* . |
|---|---|---|---|---|
| Age | ||||
| 15-20 yrs | 12 (27) | 11 (92) | 0.55 | 0.80 |
| 21-50 yrs | 29 (64) | 27 (93) | 0.46 | 0.63 |
| > 50 yrs | 4 (9) | 4 (100) | 0.75 | 0.50 |
| Sex | ||||
| Male | 33 (73) | 30 (91) | 0.49 | 0.61 |
| Female | 12 (27) | 12 (100) | 0.56 | 0.75 |
| WBC count | ||||
| ≤ 50 000/μL | 35 (92) | 33 (94) | 0.44 | 0.64 |
| > 50 000/μL | 3 (8) | 2 (67) | 0.33 | 0.50 |
| BM involvement | ||||
| No | 31 (69) | 29 (94) | 0.54 | 0.68 |
| Yes | 14 (31) | 13 (93) | 0.46 | 0.59 |
| Mediastinal tumor | ||||
| No | 4 (9) | 4 (100) | 0.75 | 1.00 |
| Yes | 41 (91) | 38 (93) | 0.49 | 0.61 |
| Pleural effusion | ||||
| No | 27 (60) | 26 (96) | 0.55 | 0.68 |
| Yes | 18 (40) | 16 (89) | 0.50 | 0.60 |
| Other organ involvement | ||||
| No | 41 (91) | 38 (93) | 0.51 | 0.64 |
| Yes | 4 (9) | 4 (100) | 0.33 | 0.75 |
| Lymphadenopathy | ||||
| No | 13 (29) | 12 (92) | 0.68 | 0.71 |
| Yes | 32 (71) | 30 (94) | 0.45 | 0.61 |
| B symptoms | ||||
| No | 27 (73) | 26 (96) | 0.70 | 0.76 |
| Yes | 10 (27) | 9 (90) | 0.25 | 0.44 |
| LDH | ||||
| ≤ 240 U/L | 6 (16) | 6 (100) | 0.33 | 0.44 |
| > 240 U/L | 32 (84) | 29 (91) | 0.50 | 0.67 |
| ≤ 500 U/L | 29 (76) | 28 (97) | 0.44 | 0.65 |
| > 500 U/L | 9 (24) | 7 (78) | 0.44 | 0.57 |
| Stage | ||||
| I/II | 12 (27) | 12 (100) | 0.56 | 0.64 |
| III/IV | 33 (73) | 30 (91) | 0.48 | 0.65 |
| Entry criteria . | Frequency N (%) . | CR rate* N (%) . | Probability of survival* . | Probability of CCR* . |
|---|---|---|---|---|
| Age | ||||
| 15-20 yrs | 12 (27) | 11 (92) | 0.55 | 0.80 |
| 21-50 yrs | 29 (64) | 27 (93) | 0.46 | 0.63 |
| > 50 yrs | 4 (9) | 4 (100) | 0.75 | 0.50 |
| Sex | ||||
| Male | 33 (73) | 30 (91) | 0.49 | 0.61 |
| Female | 12 (27) | 12 (100) | 0.56 | 0.75 |
| WBC count | ||||
| ≤ 50 000/μL | 35 (92) | 33 (94) | 0.44 | 0.64 |
| > 50 000/μL | 3 (8) | 2 (67) | 0.33 | 0.50 |
| BM involvement | ||||
| No | 31 (69) | 29 (94) | 0.54 | 0.68 |
| Yes | 14 (31) | 13 (93) | 0.46 | 0.59 |
| Mediastinal tumor | ||||
| No | 4 (9) | 4 (100) | 0.75 | 1.00 |
| Yes | 41 (91) | 38 (93) | 0.49 | 0.61 |
| Pleural effusion | ||||
| No | 27 (60) | 26 (96) | 0.55 | 0.68 |
| Yes | 18 (40) | 16 (89) | 0.50 | 0.60 |
| Other organ involvement | ||||
| No | 41 (91) | 38 (93) | 0.51 | 0.64 |
| Yes | 4 (9) | 4 (100) | 0.33 | 0.75 |
| Lymphadenopathy | ||||
| No | 13 (29) | 12 (92) | 0.68 | 0.71 |
| Yes | 32 (71) | 30 (94) | 0.45 | 0.61 |
| B symptoms | ||||
| No | 27 (73) | 26 (96) | 0.70 | 0.76 |
| Yes | 10 (27) | 9 (90) | 0.25 | 0.44 |
| LDH | ||||
| ≤ 240 U/L | 6 (16) | 6 (100) | 0.33 | 0.44 |
| > 240 U/L | 32 (84) | 29 (91) | 0.50 | 0.67 |
| ≤ 500 U/L | 29 (76) | 28 (97) | 0.44 | 0.65 |
| > 500 U/L | 9 (24) | 7 (78) | 0.44 | 0.57 |
| Stage | ||||
| I/II | 12 (27) | 12 (100) | 0.56 | 0.64 |
| III/IV | 33 (73) | 30 (91) | 0.48 | 0.65 |
P value > .05 for all comparisons.
T-LBL indicates T-lymphoblastic lymphoma; CR, complete remission; CCR, continuous complete remission; WBC, white blood cell; BM, bone marrow; LDH, lactate dehydrogenase.
In 91% (n = 41) of the patients a mediastinal tumor was present; in 44% (n = 18) the tumor was accompanied by concomitant pleural and/or pericardial effusions. Lymphadenopathy was seen in 71% of the patients; hepatosplenomegaly in 15% of the patients; and in 9% of the patients (n = 4) there was involvement of other organs such as Waldeyer ring (n = 1), skin (n = 1), bone (n = 1), and kidney (n = 1). There were 3 patients who had lymph node involvement only. None of the patients showed initial CNS involvement. According to clinical manifestations and entry parameters, 12 patients (27%) were classified as stage I/II (n = 4/8) and 33 patients (73%) as stage III/IV (n = 6/27). Of the patients with stage III/IV disease, 93% had increased LDH levels compared with 50% of the patients with stage I/II disease (P = .003). For other peripheral blood parameters no difference was found between patients with stage I/II disease and those with stage III/IV disease.
One patient suffered from malignant histiocytosis in addition to T-LBL but was included in the analysis. There were 6 patients who had received pretreatment prior to inclusion in the study. The pretreatment was vincristine/mitoxantrone (n = 1), one cycle of CHOP (n = 2) or CHOEP (n = 1), or mediastinal irradiation in an emergency situation (n = 2); all of these patients were included in the analysis.
Treatment results
Remission induction.
Of the 45 patients in the study, 42 achieved a complete remission (93%). The CR rate was 100% in patients with stage I-III disease (n = 18) and 89% in stage IV disease (n = 27) (P > .05). In the latter group, 2 patients showed partial remission or progressive disease (6%) and one patient (2%) died on day 7 of chemotherapy due to tumor lysis syndrome (Table3).
Overall results in adult T-LBL
| . | Total . | Stage I/II . | Stage III/IV . |
|---|---|---|---|
| Evaluable | 45 | 12 | 33 |
| CR | 42 (93%) | 12 (100%) | 30 (91%) |
| After induction | 34 (83%) | 9 (83%) | 25 (84%) |
| After salvage | 7 (17%)3-150 | 2 (17%)3-150 | 5 (16%) |
| Failure | 2 (5%) | 0 | 2 (6%) |
| Early death | 1 (2%) | 0 | 1 (3%) |
| Relapse | 15 (36%)3-151 | 4 (33%) | 11 (37%)3-151 |
| Death in CR | 2 (5%) | 0 | 2 (7%) |
| CCR | 25 (59%)3-152 | 8 (67%) | 17 (57%)3-152 |
| . | Total . | Stage I/II . | Stage III/IV . |
|---|---|---|---|
| Evaluable | 45 | 12 | 33 |
| CR | 42 (93%) | 12 (100%) | 30 (91%) |
| After induction | 34 (83%) | 9 (83%) | 25 (84%) |
| After salvage | 7 (17%)3-150 | 2 (17%)3-150 | 5 (16%) |
| Failure | 2 (5%) | 0 | 2 (6%) |
| Early death | 1 (2%) | 0 | 1 (3%) |
| Relapse | 15 (36%)3-151 | 4 (33%) | 11 (37%)3-151 |
| Death in CR | 2 (5%) | 0 | 2 (7%) |
| CCR | 25 (59%)3-152 | 8 (67%) | 17 (57%)3-152 |
One patient first evaluated before reinduction.
One relapse after stem cell transplantation (SCT).
One CCR after SCT.
T-LBL indicates T-lymphoblastic leukemia; CR, complete remission; CCR, continuous complete remission.
In 75% of the patients (n = 34), CR was achieved after 8 weeks of induction therapy. There were 7 patients with partial remission who achieved CR with additional consolidation therapy and/or mediastinal irradiation. In one patient, remission status was first evaluated before reinduction therapy. Residual mediastinal tumor was most frequently the reason for late achievement of CR.
All entry criteria (Table 2) were tested for prognostic impact on achievement of CR. Although there was a trend to lower CR rates in patients with larger tumor masses (stage III/IV, mediastinal involvement, high WBC count, LDH) none of the differences was statistically significant.
Treatment realization.
Treatment realization was analyzed in CR patients only. Since treatment duration for T-LBL according to ALL protocols was recommended to be at least 6 months (including reinduction) and up to 12 months, the majority of patients (69%) received treatment until reinduction. In 12 patients treatment was continued with consolidation II (29%) and/or maintenance therapy (24%). There were 10 patients who received early consolidation with HDAC/MITOX only (24%), 21 patients who received HDAC/MITOX and HDMTX/ASP (50%), and 6 patients who were consolidated with VM26/ARAC (14%). The median treatment duration was 8 months (range, 38 days-18 months).
Prophylactic CNS irradiation was administered in 38 patients (90%). Of the patients with initial mediastinal involvement, 84% (n = 32) received mediastinal irradiation. Of the patients without mediastinal irradiation, 2 experienced early relapse (mediastinal relapse day 134, CNS and bone marrow relapse day 58), 1 died in CR (month 2), and 3 remain in CCR. There were 2 patients who received autologous SCTs in first CR. One patient is in CCR and the other patient relapsed.
Remission duration and survival
There are 25 patients (60%) in CCR at a median of 41 months (range, 5-89 months) (Table 3). There were 2 patients (5%) who died in CR; the causes of death were Candida septicaemia after consolidation therapy with HDAC/MITOX at month 2 and liver failure after acute hepatitis B at month 17 during maintenance therapy. There are 26 patients alive at 12 to 91 months after diagnosis, and 19 patients died.
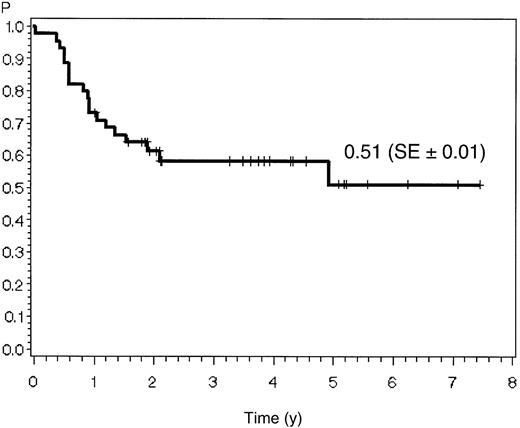
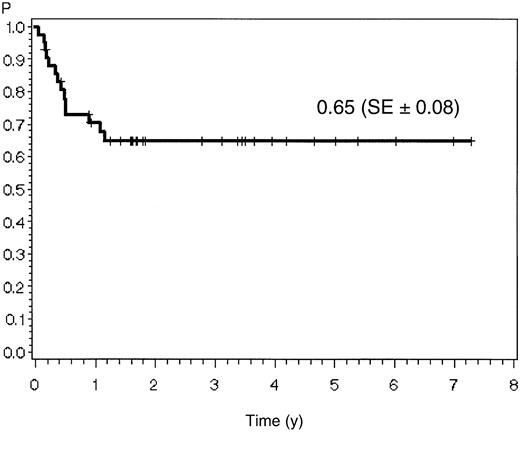
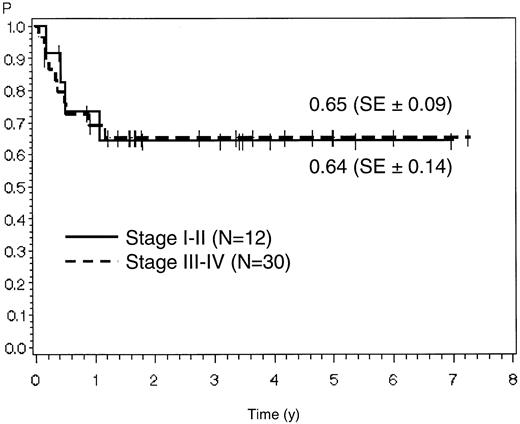
The estimate for overall survival at 7 years is 51% (SE ± 10%) for all 45 patients. Probabilities for remission duration and disease-free survival were 65% (SE ± 8%) and 62% (SE ± 8%), respectively, for the 42 CR patients (Figure2; Figure3).
Relapse localization, time to relapse, and outcome of salvage therapy.
Of the 42 CR patients, 15 (36%) experienced a relapse (Table 4). The majority of relapses (7/15) occurred in the mediastinum. All of these patients had mediastinal involvement at diagnosis and 6 had received mediastinal irradiation with 24 Gy. There were 3 patients with initial bone marrow involvement who experienced bone marrow relapse. One of them also showed CNS involvement at relapse. There were 5 patients who developed relapse at new sites, which were bone marrow (n = 2), disseminated lymph node involvement (n = 1), lung (n = 1), and breast (n = 1). Nearly all relapses (13 of 15) occurred within the first year after diagnosis or shortly thereafter. The median time to relapse was 147 days (range, 23-426 days).
Initial involvement, relapse localization, time to relapse, and survival in relapse patients
| Initial involvement . | Stage . | Relapse time . | Localization . | Survival . |
|---|---|---|---|---|
| Med, PL | I | 183 d | Lung | 1582 d+ |
| Med, LN | II | 66 d | Med, LN | 335 d |
| Med, PL, LN | II | 395 d | Mamma | 1796 d |
| Med, PL, LN | II | 159 d | Med, PL | 327 d |
| Med, LN, Wald | III | 134 d | Med | 561 d |
| LN4-150 | III | 331 d | BM | 489 d |
| Med, LN, BM, Liver | IV | 58 d | BM, CNS | 182 d |
| Med, PL, LN | IV | 23 d | Med | 185 d |
| Med, PL, LN, Liver | IV | 180 d | LN | 379 d |
| Med, BM | IV | 123 d | Med | 334 d |
| Med, PL, LN, Liver | IV | 183 d | BM, LN | 299 d |
| Med | IV | 81 d | Med, PL | 161 d |
| Med, LN, BM | IV | 426 d | Med, PL | 761 d |
| Med, LN, BM | IV | 329 d | BM | 435 d |
| Med, LN, BM | IV | 60 d | BM, LN | 215 d |
| Initial involvement . | Stage . | Relapse time . | Localization . | Survival . |
|---|---|---|---|---|
| Med, PL | I | 183 d | Lung | 1582 d+ |
| Med, LN | II | 66 d | Med, LN | 335 d |
| Med, PL, LN | II | 395 d | Mamma | 1796 d |
| Med, PL, LN | II | 159 d | Med, PL | 327 d |
| Med, LN, Wald | III | 134 d | Med | 561 d |
| LN4-150 | III | 331 d | BM | 489 d |
| Med, LN, BM, Liver | IV | 58 d | BM, CNS | 182 d |
| Med, PL, LN | IV | 23 d | Med | 185 d |
| Med, PL, LN, Liver | IV | 180 d | LN | 379 d |
| Med, BM | IV | 123 d | Med | 334 d |
| Med, PL, LN, Liver | IV | 183 d | BM, LN | 299 d |
| Med | IV | 81 d | Med, PL | 161 d |
| Med, LN, BM | IV | 426 d | Med, PL | 761 d |
| Med, LN, BM | IV | 329 d | BM | 435 d |
| Med, LN, BM | IV | 60 d | BM, LN | 215 d |
Malignant histiocytosis as secondary malignancy.
Med indicates mediastinal tumor; PL, pleura effusion; LN, lymph nodes; Wald, Waldeyer ring; BM, bone marrow; CNS, central nervous system.
Salvage therapies after relapse included DEXA-BEAM (n = 1), HDAC (n = 1), ALL induction therapy (n = 2), B-ALL regimen (n = 2), and various lymphoma-related protocols (n = 5). There were 14 relapse patients who died; 1 patient remains alive and in second CR at 53 months after DEXA-BEAM followed by allogeneic SCT.
Prognostic factors for remission duration and survival.
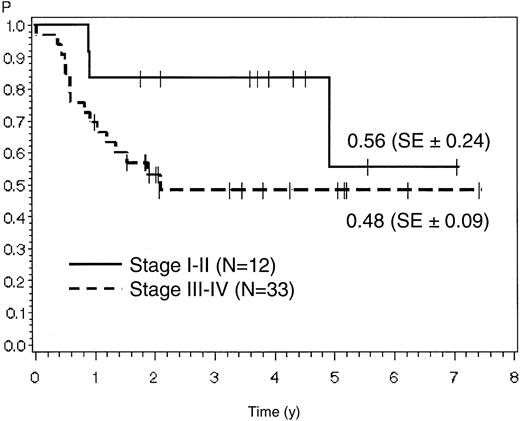
For stage I/II the estimates for overall survival and remission duration were 56% (SE ± 24%) and 64% (SE ± 14%), respectively, corresponding to 48% (SE ± 9%) and 65% (SE ± 9%) in patients with stage III/IV disease (P > .05) (Figures 4 and 5). For stage I-III compared with stage IV, the overall survival was 57% (SE ± 15%) versus 49% (SE ± 10%) and remission duration was 71% (SE ± 11%) versus 61% (SE ± 10%) (P > .05). Patients with early achievement of CR (within 8 weeks of induction therapy) showed a better outcome than patients with late achievement of CR only after additional salvage therapy. Remission duration was 67% (SE ± 8%) compared with 51% (SE ± 20%) (P > .05).
Probability of overall survival in patients with stage I/II versus stage III/IV disease.
Probability of overall survival in patients with stage I/II versus stage III/IV disease.
Probability of remission duration in patients with stage I/II versus stage III/IV disease.
Probability of remission duration in patients with stage I/II versus stage III/IV disease.
Although there was a trend toward a lower survival and remission duration in patients with high WBC counts, bone marrow involvement, mediastinal tumor, B symptoms, and LDH more than 500 U/L, none of these differences was statistically significant. Also, no difference in terms of survival and remission duration was detected between GMALL 04/89 and GMALL 05/93 with probabilities of 57% (n = 7) and 67% (n = 35) for remission duration, respectively.
The International Prognostic Index9 could not be analyzed in this patient cohort since only one patient was older than 60 years and performance status had not been assessed. A modified version including LDH (normal/elevated), stage (I/II vs III/IV), extranodal involvement (0-1 vs ≥ 2 sites), and B symptoms instead of performance status showed no difference between patients with 0-1, 2, 3, and 4 risk factors (data not shown).
If 2 unfavorable prognostic factors (LDH > 500 U/L, late CR) were combined in a prognostic model, patients with none of the risk factors showed 73% (n = 23) remission duration compared with 43% (n = 12) in patients with either or both risk factors (P = .09).
Discussion
The clinical characteristics and outcome of the largest series so far of adult patients with T-LBL treated according to a shortened protocol for adult ALL are described in this report. The treatment approach was similar to a recently reported successful regimen in childhood T-LBL.1 The large majority of patients (91%) was younger than 50 years, reflecting the generally lower median age of T-ALL/NHL patients. A mediastinal involvement, which is a typical characteristic of T-LBL, was evident in 89% of the patients, and was often (40%) associated with pleural and/or pericardial effusion. Thus, the incidence of mediastinal tumors was even higher than in T-ALL (63%).10 Most patients (72%) had advanced stage III/IV disease, with elevated LDH values (> 240 U/L) in 84% of the patients. In childhood NHL, all patients with mediastinal involvement are allocated at least to stage III disease.11 If this system was applied in our cohort the incidence of stage III/IV disease was 96%—exactly as in the childhood T-LBL study.1
Clinical picture and laboratory values of T-LBL patients were different from T-ALL in several respects. Thus, peripheral blood values were generally near to normal in T-LBL indicating a potentially better bone marrow reserve and tolerability of chemotherapy. There were 3 patients with large mediastinal tumor mass and bone marrow involvement > 25% included in this series who had inferior outcomes (2 of 3 relapsed and died). No patient showed initial CNS involvement although routine analysis of CSF was part of the protocol compared with 5% in T-ALL patients.10 Overall characteristics of adult T-LBL were rather similar to those in childhood T-LBL, including male predominance, high proportion of mediastinal tumors, stage III/IV disease, and elevated LDH. There was, however, a higher incidence of bone marrow involvement (31%) in the adult patient cohort compared with children (15%).1
A variety of therapeutic approaches has been reported for adult LBL generally not separating B- and T-cell type. Conventional NHL protocols with CHOP-like regimens yielded low CR rates ranging between 53% and 79%.12-15 With an intensified CHOP regimen (LSA2L2 regimen) with additional CNS prophylaxis, consolidation, and maintenance therapy, a CR rate more than 80% and a disease-free survival rate of 56% was achieved.16 With ALL-type regimens, CR rates between 77% and 100% and disease-free survival rates of 45% to 67% were reported for B-/T-LBL.14,17-21 These results indicate that intensive induction and consolidation therapy including CNS prophylaxis similar to treatment of ALL may be beneficial in B-/T-LBL. Separate results for adult T-LBL were, however, only rarely reported. In an overview of the Non-Hodgkin Lymphoma Classification Project, the failure-free survival for T-LBL was 24% at 6 years,22 and in a series of 10 patients with T-LBL (including children) the CR rate was only 10% with a median survival of 12 months.2Treatment regimens were not specified in both reports. Better results are only available from 2 abstract reports. In one study with 18 patients, the CR rate with an ALL-type regimen was 100% and the remission duration 67%.19 In the other study with a regimen for B-ALL/NHL, a CR rate of 72% (n = 18) was reported.23 Thus, there is some evidence that unfavorable results were achieved in adult LBL of T-cell type with the exception of intensive ALL-type regimens in small patient cohorts.
The strongest evidence for the high effectivity of ALL-type chemotherapy in T-LBL comes from the recent report on 105 children with T-LBL.1 Induction and reinduction therapy were quite similar to the regimen reported here. Consolidation consisted of HDMTX (5 g/m2 × 4) in combination with mercaptopurin. Patients also received conventional maintenance therapy for a total treatment duration of 24 months in contrast to our study. The event-free survival rate of 90% was superior to previous studies in childhood T-LBL.1
In our study, a high CR rate of 93% could be achieved in adult T-LBL. All of the patients (100%) with stage I-III disease achieved remission, and in patients with stage IV disease the CR rate was still 89%. Large tumor mass such as mediastinal tumors or high LDH had no significant influence on CR rate. In addition, 2 of 3 patients who were refractory to CHOP or CHOEP achieved CR. Thus, the induction regimen was overall highly effective in all stages of disease and in all types of involvement. Although induction therapy is generally associated with pronounced hematotoxicity in patients with ALL, we observed only one case of early death due to tumor lysis syndrome in T-LBL. Thus, early mortality in T-LBL is clearly lower compared with the 5% to 10% mainly infection-related early death rate in T-ALL. This may be due to the better bone marrow reserve in T-LBL without extensive bone marrow involvement.
The remission duration of 67% compares favorably with published results in adult B-/T-LBL particularly if it is taken into account that only patients with T-LBL were included and that 57% of the patients suffered from stage IV disease. The overall survival of 51% was achieved with chemotherapy alone since only 2 patients had received autologous SCT in first CR and the total treatment duration was significantly shorter (median 8 months) compared with the conventional ALL treatment of 2 1/2 to 3 years.
In earlier studies, particularly with NHL-type regimens without specific CNS directed treatment, CNS and combined CNS/BM relapse rates of more than 30% were reported.12 A majority (91%) of our study patients received prophylactic CNS irradiation with 24 Gy and all of them received intrathecal (i.th.) therapy. This approach proved to be effective since only one patient experienced a combined CNS and BM relapse.
One major specific issue in T-LBL is the role of mediastinal tumors as a cause for initial treatment failure and as relapse localization. There were 34 of 41 (83%) evaluable CR patients who had achieved early CR after the 8-week induction therapy. In the remaining 7 patients CR was achieved late after subsequent mediastinal irradiation and/or additional consolidation therapy; the major reason for later achievement of CR was residual mediastinal tumors after induction therapy. Also, the majority of relapses (10/15) occurred in the initially involved field with the mediastinum as the predominant relapse site (7/15). In our study, mediastinal irradiation was proposed for all patients with T-LBL—as for T-ALL—and 85% of the patients received 24 Gy mediastinal irradiation irrespective of whether the mediastinal tumor resolved or not. Local recurrence occurred in the mediastinum despite irradiation in 6 patients. Furthermore, mediastinal relapses occurred in patients with complete resolution of mediastinal tumors as well as in patients with residual tumors after induction therapy. It remains open whether relapse risk could have been predicted in these patients by additional diagnostic measures. When resection or biopsy was performed in the pediatric cohort, only necrotic tissue was found in 10 of 19 patients with residual tumor on day 33 of induction therapy.1 Thus, indications for intensification of local treatment may not be based on results of local diagnostic procedures at present.
In childhood T-LBL several strategies for prevention of mediastinal recurrence were described. Irradiation dose may have a role since low-dose irradiation with 15 Gy was apparently not effective. In one study, 31 of 34 mediastinal relapses occurred in patients who had received 15 Gy local irradiation.24 On the other hand, in the BFM study on childhood T-LBL no mediastinal irradiation was performed and the local recurrence rate (7%) was still very low.1 Intensive treatment with HDMTX during consolidation specifically designed as “extracompartment protocol” may have contributed to this result. In our series, the mediastinal relapse rate was high, despite a very similar induction therapy and prophylactic mediastinal irradiation but with less-intensive consolidation with HDMTX. One conclusion for the new GMALL protocol for T-LBL is to improve local disease control by increased dose (36 Gy instead of 24 Gy) for prophylactic mediastinal irradiation.
In addition to the improved local control of mediastinal tumors, an extension of treatment duration for more than 6 months and intensified systemic chemotherapy may be required. The majority of relapses occurred on treatment or shortly thereafter and no relapse was observed in patients with treatment continued after reinduction. Intensification of systemic chemotherapy seems possible in adults since treatment-related mortality in CR was low in our study. The focus should be placed on drugs with particular effectivity in T-ALL. From adult T-ALL it is known that cyclophosphamide and cytarabine contributed to an improvement of overall results.25 In childhood T-ALL, intensive use of asparaginase26 and methotrexate27 contributed to better outcome. Also, pretreatment with cyclophosphamide pulses may play a role in T-LBL since high remission rates in T-ALL were achieved in such studies.28 The new GMALL study for adult T-LBL will therefore include pretreatment with cyclophosphamide and intensification of HDMTX by inclusion of 3 cycles (1.5 g/m2 × 2) in combination with asparaginase. Furthermore, treatment duration for T-LBL will be extended to one year.
It is evident that all efforts for improvement of treatment strategies have to be made in frontline therapy since outcome of relapsed patients with T-LBL is extremely poor. Only one patient survived after allogeneic SCT in second CR in our study. Results of autologous SCT in LBL are also inferior beyond first CR with a 31% disease-free survival rate for patients with LBL in second CR and 15% for those with resistant disease.29 Salvage treatment should therefore aim to refer the patients to an allogeneic SCT as soon as possible. In patients without a compatible donor, autologous SCT in second remission is an option and collection of peripheral stem cells during frontline treatment appears to be reasonable. Furthermore, new cytostatic drugs such as cladribine and 506U7830 with specific activity on T-cells or immunotherapy with T-cell–specific antibodies such as anti-CD3 and anti-CD52 deserve evaluation in relapsed and refractory patients with T-LBL to improve the chance of second remission.
Prognostic factors for adult T-LBL are so far not identified. Also, in our patient cohort no single prognostic factor for remission rate, survival, or remission duration could be defined. Particularly, the stage of disease did not show a significant impact on outcome although the probability of survival at 4 years was clearly inferior in stage III/IV disease (48%) compared with stage I/II disease (83%). Similarly, no prognostic factors were identified in the largest series in childhood T-LBL. Neither age, stage, LDH level, nor immunophenotype had an impact on event-free survival.1 Our data provide evidence that patients with late achievement of CR or elevated LDH (> 500 U/L) had an inferior prognosis in the context of this treatment regimen.
New prognostic factors are therefore required to define indications for SCT in first remission. The better characterization of biologic markers (eg, immunophenotype of T-LBL) may contribute to this aim. Rational assessment of individual treatment response and relapse risk may be based on evaluation of minimal residual disease from bone marrow or peripheral blood.
In conclusion, it can be stated that this study demonstrates in the largest cohort so far of adult T-LBL a high CR rate and a favorable outcome with an ALL-type regimen derived from pediatric studies. The regimen provided effective prophylaxis of CNS relapse with i.th. therapy, CNS irradiation, and systemic high-dose chemotherapy. The major problem of local disease control in T-LBL is the effective treatment of mediastinal tumors. This may be achieved by increased local irradiation and intensified systemic chemotherapy. One major focus of future studies is the identification of prognostic factors, and minimal residual disease evaluation may be the most attractive approach.
GMALL study committee: R. Arnold, Berlin; C. R. Bartram, Heidelberg; A. Böhme, Frankfurt; M. Freund, Rostock; A. Ganser, Hannover; N. Gökbuget, Frankfurt; D. Hoelzer, Frankfurt; H. Horst, Kiel; M. Kneba, Kiel; T. Lipp, München; W. D. Ludwig, Berlin; G. Maschmeyer, Berlin; D. Messerer, München; H. Rieder, Marburg; E. Thiel, Berlin; A. Weiss, Mannheim, Germany.
Contributing principal investigators: T. Faak, Hamburg; W. Digel, Freiburg; M. Kneba, Kiel; K. Kolbe, Mainz; R. Forstpointner, München; C. v. Streit, Augsburg; I. Strohscheer, Berlin; R. Ratei, Berlin; K. Jentsch-Ullrich, Magdeburg; T. Geer, Schwäbisch-Hall; M. Schwenke, Greifswald; C. Rudolph, Cottbus; K. Stahlhut, Potsdam; J. Walewski, J. Romejko-Jarosinska, J. Zwolinski, Warsaw, Poland.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-01-0110.
Supported by the Deutsche Krebshilfe, Federal Republic of Germany (contract no. M84/92Hol), and the Federal Ministry of Education and Research, Federal Republic of Germany (grant no. 01GI 9971).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
D. Hoelzer, Medical Clinic III, Department of Hematology, University of Frankfurt, Theodor Stern Kai 7, 60590 Frankfurt, Germany; e-mail: hoelzer@em.uni-frankfurt.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal