Exposure of tissue factor (TF) present in the vascular wall to blood is considered to initiate arterial thrombus formation. Moreover, antibody-mediated inhibition of TF function has been shown to prevent thrombus growth in human ex vivo perfusion experiments.1 TF-bearing monocytes and neutrophils were identified in human ex vivo–formed thrombi and in circulating blood. Based on in vitro perfusion experiments and flow cytometry, it was also reported that TF-positive microparticles originating from monocytes and possibly polymorphonuclear (PMN) leukocytes are transferred to platelets within the thrombus; thereby, TF-positive platelets trigger and propagate thrombosis.2 But the cell types that are associated with TF in thrombi remain unclear and could be related to the particular experimental conditions used by the investigators.3
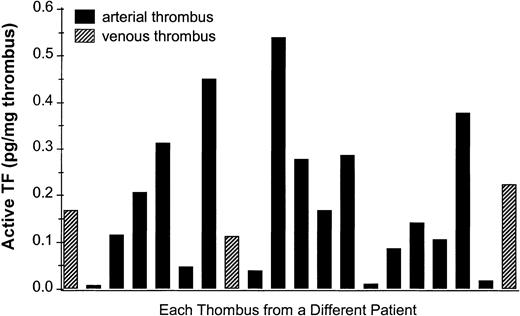
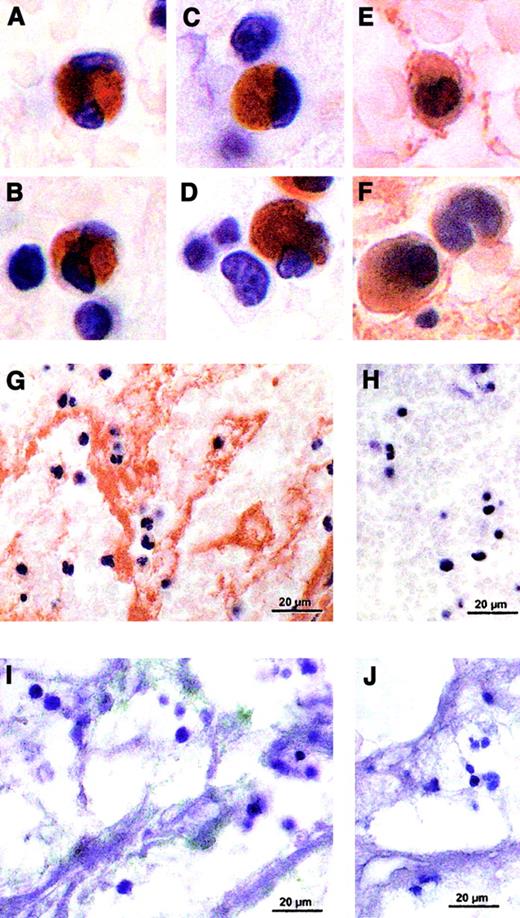
In order to circumvent the ex vivo and in vitro test systems, we have further characterized in situ TF in human arterial and venous thrombi obtained from patients undergoing thrombectomy. Informed consent was obtained from all patients. TF activity was measured in human thrombi by a 2-stage clotting assay4 and localized by binding of digoxigenin-labeled FVIIa5 or immunostained with anti-TF antibodies.5 6 TF activity was found in all 20 analyzed human thrombi (Figure1), and staining of TF revealed TF-positive monocytes and PMN leukocytes (Figure2A-F) in the arterial and the venous thrombi. Interestingly, only about 6% of total leukocyte number are TF-positive, suggesting that subtypes of leukocytes are able to express or transport TF. The presence of positive and negative cells in the same sections argues against nonspecific staining. In addition, TF is also observed extracellularly in small vesicles associated with platelets and fibrin (Figure 2G,I).
TF activity in human thrombi.
Procoagulant activity was measured in a 2-stage clotting assay. Human thrombi obtained from thrombectomy were homogenized, preincubated with FVIIa and FX for 3 minutes in presence or absence of the murine monoclonal anti-human TF antibody (HTF1-7B8). Then, human plasma containing a phospholipid mix (PC/PS = 70/30) was added and the clotting assay started by addition of Ca++. The amount of TF was calculated from a standard curve obtained using relipidated full-length TF.
TF activity in human thrombi.
Procoagulant activity was measured in a 2-stage clotting assay. Human thrombi obtained from thrombectomy were homogenized, preincubated with FVIIa and FX for 3 minutes in presence or absence of the murine monoclonal anti-human TF antibody (HTF1-7B8). Then, human plasma containing a phospholipid mix (PC/PS = 70/30) was added and the clotting assay started by addition of Ca++. The amount of TF was calculated from a standard curve obtained using relipidated full-length TF.
In situ TF staining of representative human thrombi.
Human arterial or venous thrombi were fixed overnight in 4% paraformaldehyde immediately after thrombectomy and were paraffin-embedded. Five-micrometer sections were deparaffinized and rehydrated, and TF was stained by using digoxigenin-labeled FVIIa (100 nM, 2 h, 37°C) in Tris-buffered saline (TBS) containing 5 mM Ca++, followed by incubation with sheep Fab antidigoxigenin antibody conjugated with horseradish peroxidase (1:1000 dilution, 1 h, 37°C). Color was developed using diaminobenzidine tetrahydrochloride (DAB) (A-D). Immunohistochemical staining was performed on 5-μm sections blocked with 1% peroxyde in methanol and appropriate normal serum and incubated with rabbit polyclonal antihuman TF antibody (1 μg/mL, 2 h, 37°C), stained by a biotin streptavidin–amplified detection system, developed with DAB, and counterstained with hematoxylin (E-G). Another series of nonfixed human arterial or venous thrombi were maintained immediately after thrombectomy in 15% sucrose overnight at 4°C. Then cryosections (8 μm) were blocked with bovine serum albumin (BSA) and incubated with HTF1-7B8, a murine monoclonal anti-TF antibody (20 μg/mL, 1 h, room temperature) followed by a gold-labeled goat antimouse IgG and silver enhancement of the colloidal gold. The sections were embedded in fluorescent mounting medium (I). An irrelevant IgG rabbit mAb was used as a control at equivalent concentration of the specific antibodies (H,J).
In situ TF staining of representative human thrombi.
Human arterial or venous thrombi were fixed overnight in 4% paraformaldehyde immediately after thrombectomy and were paraffin-embedded. Five-micrometer sections were deparaffinized and rehydrated, and TF was stained by using digoxigenin-labeled FVIIa (100 nM, 2 h, 37°C) in Tris-buffered saline (TBS) containing 5 mM Ca++, followed by incubation with sheep Fab antidigoxigenin antibody conjugated with horseradish peroxidase (1:1000 dilution, 1 h, 37°C). Color was developed using diaminobenzidine tetrahydrochloride (DAB) (A-D). Immunohistochemical staining was performed on 5-μm sections blocked with 1% peroxyde in methanol and appropriate normal serum and incubated with rabbit polyclonal antihuman TF antibody (1 μg/mL, 2 h, 37°C), stained by a biotin streptavidin–amplified detection system, developed with DAB, and counterstained with hematoxylin (E-G). Another series of nonfixed human arterial or venous thrombi were maintained immediately after thrombectomy in 15% sucrose overnight at 4°C. Then cryosections (8 μm) were blocked with bovine serum albumin (BSA) and incubated with HTF1-7B8, a murine monoclonal anti-TF antibody (20 μg/mL, 1 h, room temperature) followed by a gold-labeled goat antimouse IgG and silver enhancement of the colloidal gold. The sections were embedded in fluorescent mounting medium (I). An irrelevant IgG rabbit mAb was used as a control at equivalent concentration of the specific antibodies (H,J).
In summary, we have shown that human in vivo–formed arterial and venous thrombi contain TF activity and TF antigen. The observed TF protein, revealed by 3 different markers, was found to be associated with platelets, fibrin structures, monocytes, and PMN leukocytes. The observation of TF associated with monocytes and PMN leukocytes is in good agreement with previous observations obtained in ex vivo–formed thrombi.1 Therefore, these data further support the hypothesis that leukocyte-associated TF is involved during the pathophysiologic process of in vivo thrombus growth.
For providing the human thrombi, we thank Dr Dolf Brunner, Department of Surgery, Kantonsspital Luzern, Switzerland.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal