Polymorphonuclear leukocytes (PMNs) mediate antibody-dependent cellular cytotoxicity (ADCC), which is increased by the addition of granulocyte-macrophage colony-stimulating factor (GM-CSF). We sought to determine whether PMN ADCC also would be increased by the addition of an antibody/GM-CSF fusion protein and whether this would be associated with the up-regulation and activation of Mac-1 (CD11b/CD18) and with azurophil granule exocytosis. ADCC against LA-N-1 human neuroblastoma cells was evaluated with 4-hour calcein acetoxymethyl ester (calcein-AM) microcytotoxicity assay, electron microscopy, and multi-parameter flow cytometry. With the calcein-AM assay, LA-N-1 cell survival was 10%, 55%, and 75% when PMN ADCC was mediated by the antidisialoganglioside (anti-GD2) immunocytokine hu14.18/GM-CSF, by monoclonal antibody (mAb) hu14.18 mixed with GM-CSF, and by hu14.18 alone. Function-blocking mAbs demonstrated that FcγRII and FcγRIII were required for ADCC with hu14.18 alone or mixed with GM-CSF, but that only FcγRII was required for ADCC with hu14.18/GM-CSF. ADCC mediated by hu14.18 and hu14.18/GM-CSF was Mac-1 dependent. Electron microscopy demonstrated the greatest PMN adhesion, spreading, and lysis of targets with hu14.18/GM-CSF. Monoclonal antibodies blocking Mac-1 function allowed the tethering of PMN to targets with hu14.18/GM-CSF but prevented adhesion, spreading, and cytolysis. Flow cytometry showed that hu14.18 with or without GM-CSF and hu14.18/GM-CSF all mediated Mac-1–dependent PMN–target cell conjugate formation but that GM-CSF was required for the highest expression and activation of Mac-1, as evidenced by the mAb24-defined β2-integrin activation epitope. Hu14.18/GM-CSF induced the highest sustained azurophil granule exocytosis, almost exclusively in PMNs with activated Mac-1. Thus, hu14.18/GM-CSF facilitates PMN ADCC against neuroblastoma cells associated with FcγRII and Mac-1–dependent enhanced adhesion and degranulation.
Introduction
Antibody-cytokine fusion proteins combine the targeting ability of antibodies with the immune stimulation of cytokines for cancer immunotherapy.1 Studies in mice of antidisialoganglioside (anti-GD2)/interleukin-2 and anti-GD2/lymphotoxin-α fusion proteins (immunocytokines) demonstrated the eradication of hepatic metastases of neuroblastoma2,3and the induction of protective immunity against melanoma.4 These and other immunocytokine strategies have been based on the stimulation of NK-cell– or T-cell–mediated responses. Polymorphonuclear leukocytes (PMNs) constitute the largest population of white blood cells, and they can mediate antibody-dependent cellular cytotoxicity (ADCC) against tumor cells.5-9 In vitro studies demonstrated the strong facilitation of PMN ADCC against neuroblastoma cells by mixing granulocyte- macrophage colony-stimulating factor (GM-CSF) with an anti-GD2 antibody and by a human–mouse chimeric anti-GD2/GM-CSF immunocytokine.5,10 Fc receptors (FcR) involved in PMN ADCC may include FcγRII (CD32) and FcγRIII (CD16), which are constitutively expressed, and FcγRI (CD64), which is induced by interferon-γ and G-CSF.11,12 In addition, FcαRI of PMNs may be activated by constructing bispecific monoclonal antibodies (mAbs) that recognize a target cell molecule and FcαRI.13 Binding of an antibody-cytokine fusion protein to FcR may be affected by the physical presence of the cytokine or by the cytokine-modulating FcR expression.14 15 FcRs used by PMNs in ADCC with anti-GD2/GM-CSF immunocytokines have not yet been determined.
β2-Integrin receptors, particularly Mac-1 (αmβ2, CD11b/CD18, and complement receptor type 3), have been reported to have an essential role in PMN ADCC against neuroblastoma, lymphoma, and breast cancer cell lines.5,13,16 Mac-1 is a major PMN β2-integrin receptor that, on activation, acquires the ability to bind multiple ligands and to mediate several adhesion-dependent processes, including phagocytosis, superoxide production, and degranulation.17 It recently has been demonstrated that Mac-1 function is necessary for PMN spreading on antibody-coated targets and for tumor cytolysis.13 FcR and GM-CSF receptors activate Mac-1 through distinct signaling pathways.18 Therefore, we hypothesized that a GM-CSF immunocytokine may mediate greater Mac-1 activation than the antibody component alone and that this would be associated with greater PMN ADCC.
In the present study, we tested the humanized anti-GD2 mAb hu14.18 and fusion protein hu14.18/GM-CSF in PMN ADCC against neuroblastoma cells. Our data demonstrate that hu14.18/GM-CSF significantly increases PMN ADCC compared to hu14.18 alone or mixed with GM-CSF and that ADCC with both hu14.18/GM-CSF and hu14.18 depends on Mac-1 function. Hu14.18/GM-CSF increased ADCC by increasing the expression and activation of Mac-1, adhesion and spreading of neutrophils onto neuroblastoma cells, and azurophil (primary) granule exocytosis.
Materials and methods
Cell culture and reagents
The LA-N-1 neuroblastoma cell line19 was maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Gemini-Bio-Products, Calabasas, CA), and 2 mmoll-glutamine (Irvine Scientific, Santa Ana, CA) (complete RPMI 1640 medium). Hanks balanced salt solution was from Irvine Scientific. Histopaque-1077 was from Sigma Diagnostics (St Louis, MO). Calcein acetoxymethyl ester (calcein-AM), propidium iodide, and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DiD) were from Molecular Probes (Eugene, OR). Sargramostim leukine (GM-CSF; 250 μg/mL reconstituted with sterile water) was purchased from Immunex (Seattle, WA).
Humanized anti-GD2 hu14.18 mAb and hu14.18/GM-CSF immunocytokine
Humanized anti-GD2 mAb hu14.18 and hu14.18/GM-CSF fusion proteins (immunocytokine) were prepared at Lexigen Pharmaceuticals (Lexington, MA). Briefly, the original 14.18 V regions were humanized by CDR grafting into human frameworks with extensive homology with the murine sequences. Engineered sequences were introduced as cDNAs into an antibody expression vector already containing the human constant region genes for the light and the heavy chains or for heavy chain fused in-frame with the cDNA encoding human GM-CSF.20 The vector was introduced into mouse NS/0 myeloma cells, and transfectants were selected in medium containing methotrexate. Enzyme-linked immunosorbent assay was used to identify clones expressing the antibody or fusion protein. These proteins were purified with a Sepharose–protein A column and were stored in 100 mM Na2HPO4/150 mM NaCl, pH 7, at 4°C.
Binding of hu14.18 and hu14.18/GM-CSF to LA-N-1 neuroblastoma cells, which express GD2, was measured with secondary goat antihuman–fluorescein isothiocyanate (FITC) antibodies using flow cytometry. With doses of 0.1, 1, and 10 μg/mL, hu14.18 and hu14.18/GM-CSF bound equally well, and 100% of LA-N-1 cells were stained by 1 μg/mL. However, fluorescence intensity for hu14.18 and hu14.18/GM-CSF did not reach a plateau, even at 10 μg/mL.
Functional activity of the GM-CSF component of hu14.18/GM-CSF was measured with a standard CFU-GM assay21 and was compared with rhGM-CSF. Bone marrow mononuclear cells were cultured in 35 × 10 mm dishes (2 × 105 cells/dish) in 0.5% agar Iscoves modified Dulbecco medium supplemented with 0.4 μg/mL rhGM-CSF (Immunex), hu14.18/GM-CSF (0.1, 0.5, 5, 25 μg/mL), or an equal volume of phosphate-buffered saline (PBS). After 14 days of incubation in a humidified chamber with 5% CO2, granulocyte macrophage colonies (CFU-GM) with more than 50 cells were counted using an inverted phase-contrast microscope in 3 replicate dishes per condition, and the average numbers were compared. Stimulation of CFU-GM colony formation by hu14.18/GM-CSF (0.5 to 5 μg/mL) was 80% to 87% as effective as an optimal dose of rhGM-CSF (0.4 μg/mL), demonstrating retention of GM-CSF biologic activity.
Monoclonal antibodies
The following mAbs were used: anti-CD32 IV.3 Fab, anti-CD16 3G8 F(ab)2 (Medarex, West Lebanon, NH); anti-CD18 R3.3, anti-CD11a R7.1, anti-CD11b LM2/1, anti-CD11c CBR-p150/4G1, anti-CD11c 3.9 (Biosource International, Camarillo, CA); anti-CD18 7E4, anti-CD11a 25.3, anti-CD11c BU15, anti–CD11b-FITC (or -phycoerythrin [PE]) Bear1, anti–CD63-FITC (or -PE) CLBGran/12 (Immunotech, Miami, FL); anti-CD11b 2LMP19c (Dr K. Pulford, Oxford, United Kingdom); and anti–β2-activation reporter epitope mAb24 (Dr N. Hogg, London, United Kingdom).
PMN preparation
Venous blood was obtained from healthy adult volunteers and was anticoagulated with heparin, 100 U/mL (SoloPak Laboratories, Elk Grove Village, IL). Informed consent and procedures approved by the Children's Hospital Los Angeles Committee on Clinical Investigations were followed. As previously described,22 erythrocytes were removed by sedimentation with dextran (United States Biomedical, Cleveland, OH), and leukocytes in the supernatant were subjected to density separation using Histopaque-1077 (Sigma Diagnostics) and centrifugation. PMNs were collected from the pellet, and contaminating erythrocytes were removed by hypotonic lysis with ammonium chloride. Isolation was performed using sterile conditions. PMNs were 95% to 99% pure and 97% to 99% viable, as determined by flow cytometry forward and side scatter and propidium iodide exclusion.
ADCC assay
ADCC was quantified by measuring retained calcein fluorescence in target cells with Digital Image Microscopy Scanning (DIMSCAN) as previously described.23,24 Neuroblastoma cells were detached with Puck saline A and 1 mM EDTA (Puck EDTA),25washed, and resuspended in complete RPMI 1640 medium. Calcein-AM was added to produce a final concentration of 5 μg/mL, and cells were incubated at 37°C for 30 minutes in the dark. After incubation, cells were washed and resuspended in complete RPMI 1640 medium, counted using trypan blue to identify viable cells, and added to microwells (4000 cells in 100 μL per well; 96-well Falcon 3072 plates, Becton Dickinson, Franklin Lakes, NJ). Appropriate concentrations of effector cells, hu14.18 antibody, GM-CSF, and hu14.18/GMCSF were prepared in complete RPMI 1640 medium just before plating, and 6 replicate wells for each condition were set up with a final volume of 200 μL. Each plate included 6 wells of neuroblastoma cells alone as a control. Plates were incubated at 37°C for 4 hours, and retained fluorescence was quantified by DIMSCAN. Cytotoxicity was expressed as a tumor cell viability index [(tumor cell viability index, % = fluorescence intensity of experimental well/mean fluorescence intensity of control wells) × 100]. Mean tumor cell viability index ± SD was calculated for each condition from 6 replicate wells.
Flow cytometry
DiD (5 μM) was added to cultured LA-N-1 neuroblastoma cells for 12 hours and was followed by the detachment of cells with Puck EDTA. PMNs and neuroblastoma cells were placed in 96-well plates with an effector-target ratio of 20:1 (200 000 PMNs and 10 000 neuroblastoma cells in 100 μL complete RPMI 1640 medium per well). ADCC was initiated by adding hu14.18 or hu14.18/GM-CSF to a final concentration of 5 μg/mL. After desired periods of incubation at 37°C, anti–CD11b-FITC, anti–CD63-PE, IgG1-FITC (isotype control), or IgG1-PE (isotype control) were added in 100 μL at a final concentration of 10 μg/mL, and plates were placed at room temperature for 20 minutes. Then cells from each microwell were gently transferred to flow cytometry tubes, diluted to 600 μL with PBS solution (PBS + 0.1% sodium azide + 0.1% bovine serum albumin), and analyzed within 15 minutes. The anti-CD11b mAb Bear-1 used for immunostaining does not block Mac-1 function.26
When mAb24 was used to detect activated Mac-1, mAb24 (10 μg/mL final concentration) or IgG1 isotype control mAb in 100 μL was added to cells in microwells for 20 minutes at room temperature. Then cells from each microwell were gently transferred to 1.5-mL micro-centrifuge tubes, diluted with PBS solution (1 mL), centrifuged for 5 minutes (2500 rpm; Eppendorf 5415 C microcentrifuge, Brinkmann Instruments, Westbury, NY), and resuspended in PBS solution (100 μL) with secondary goat antimouse PE-conjugated F(ab′)2 (10 μg/mL final concentration; Immunotech). After 20-minute incubation at room temperature, cells were washed and resuspended in 100 μL PBS solution with FITC-conjugated anti-CD11b or anti-CD63 mAb (10 μg/mL final concentration) for 20 minutes at room temperature. Finally, cells were diluted to 600 μL with PBS solution, gently transferred to flow cytometry tubes, and analyzed within 15 minutes.
Argon 488-nm and HeNe 633-nm lasers were colinearly aligned and used to excite FITC, PE, and DiD, respectively. Samples stained with each dye separately were run to determine the level of electronic compensation required with a Beckman-Coulter (Hialeah, FL) band-pass filter set (525 ± 20 nm; 575 ± 20 nm, and 675 ± 20 nm) to achieve separation of emitted signals from the 3 dyes. Ten thousand events were analyzed for each specimen, which was derived from 4 replicate microwells. Each set of experiments was performed 3 times.
Electron microscopy
Neuroblastoma, antibody, and PMN mixtures were set up in 96-well plates as described above. Cell pellets were prepared after indicated time intervals by combining cells from 4 replicate wells per condition and centrifuging them for 10 minutes (3000 rpm; Beckman Microfuge E). Cells then were fixed with 2% glutaraldehyde in PBS, postfixed with 1% osmium tetroxide in PBS, dehydrated by a graded series of ethanol, and embedded in Epon-812. Polymerization was carried out in a 60°C oven for 48 hours. After examining semithin sections by light microscopy, ultrathin sections were cut, mounted on collodion one-hole grids, and stained with uranyl acetate and lead citrate. Ultrastructural images were examined and photographed using a Philips CM12 electron microscope. Semiquantitative analysis of PMN spreading was performed by counting electron microscopy images of 10 tumor cells and identifying those with a single PMN covering at least 30% of target cell surface.
Data analyses
Flow cytometry data were analyzed with EXPO Analysis software (Beckman-Coulter). We used Excel (Microsoft, Redmond, WA) to analyze DIMSCAN ADCC data to calculate the mean ± SD for 6 replicate wells and the tumor cell viability index (see above). SigmaPlot 4.0 (Jandell Scientific, San Rafael, CA) was used to create graphs, and GraphPad Prism (GraphPad Software, San Diego, CA) was used to perform t tests or one-way analysis of variance (ANOVA) with the Tukey-Kramer posttest comparison of group means. Significance was accepted when P < .05.
Results
PMN ADCC is greater with hu14.18/GM-CSF than with hu14.18 alone or mixed with GM-CSF
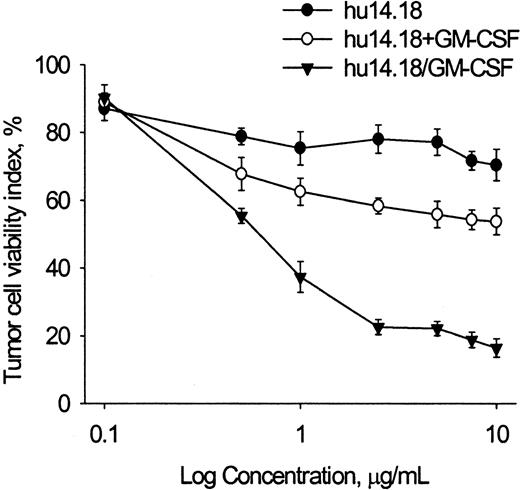
PMN ADCC activity of hu14.18, hu14.18 mixed with GM-CSF, and hu14.18/GM-CSF was determined using LA-N-1 neuroblastoma cell targets at levels achieved in serum in phase 1 studies of the closely related human–mouse chimeric 14.18 antibody (Figure1).27-30 Pilot experiments showed that GM-CSF alone (1-1000 ng/mL) did not induce PMN cytotoxicity but did increase PMN ADCC when added to hu14.18 (5 μg/mL), with the increase reaching a plateau at 10 ng/mL GM-CSF (data not shown). Hu14.18 alone induced maximal cytotoxicity at 1 μg/mL, with 75.3% ± 4.9% tumor cells remaining viable at 4 hours. Addition of soluble GM-CSF (100 ng/mL) significantly increased ADCC by hu14.18, with 58.3% ± 2.2% tumor cells viable at 2.5 μg/mL (P < .001, t test). Hu14.18/GM-CSF mediated greater ADCC than hu14.18 alone (P < .001, ttest) or mixed with GM-CSF (P < .001, t test), with only 22.6% ± 2.3% of cells viable at 2.5 μg/mL. Thus, though hu14.18 and hu14.18/GM-CSF bind equally well to neuroblastoma cells (see “Materials and methods”), the latter is more effective in mediating PMN ADCC.
PMN ADCC against LA-N-1 neuroblastoma cells with hu14.18 alone, hu14.18 mixed with GM-CSF, and hu14.18/GM-CSF.
Calcein-AM–labeled neuroblastoma cells (10 000 cells per well) were incubated with PMN (20:1 E/T ratio) and with anti-GD2 mAb hu14.18 alone, hu14.18 mixed with GM-CSF (100 ng/mL), or hu14.18/GM-CSF. Retained calcein fluorescence was quantified after 4 hours with DIMSCAN, and results are expressed as the tumor cell viability index (percent) calculated as described in “Materials and methods.” Each point is the mean ± SD for 6 replicate wells. Data are from 1 of 4 experiments that gave similar results.
PMN ADCC against LA-N-1 neuroblastoma cells with hu14.18 alone, hu14.18 mixed with GM-CSF, and hu14.18/GM-CSF.
Calcein-AM–labeled neuroblastoma cells (10 000 cells per well) were incubated with PMN (20:1 E/T ratio) and with anti-GD2 mAb hu14.18 alone, hu14.18 mixed with GM-CSF (100 ng/mL), or hu14.18/GM-CSF. Retained calcein fluorescence was quantified after 4 hours with DIMSCAN, and results are expressed as the tumor cell viability index (percent) calculated as described in “Materials and methods.” Each point is the mean ± SD for 6 replicate wells. Data are from 1 of 4 experiments that gave similar results.
FcγRII and Mac-1 are required for PMN ADCC with hu14.18/GM-CSF
To identify Fcγ receptors required for PMN ADCC mediated by hu14.18 and hu14.18/GM-CSF, we used IV.3 anti-FcγRII Fab and 3G8 anti-FcγRIII F(ab)2 blocking mAbs (Figure2A). ADCC mediated by hu14.18 alone or with added GM-CSF (100 ng/mL) was dependent on FcγRII (CD32) and FcγRIII (CD16). In contrast, hu14.18/GM-CSF, which mediated the strongest ADCC, required only CD32. FcγRI (CD64) was not detectable on freshly isolated PMNs or after 4-hour incubation with GM-CSF or hu14.18/GM-CSF (data not shown).
Fcγ and β2 integrin receptor requirement for PMN ADCC with hu14.18 alone, hu14.18 mixed with GM-CSF, and hu14.18/GM-CSF.
(A) ADCC was mediated by hu14.18 alone (5 μg/mL), hu14.18 (5 μg/mL) mixed with GM-CSF (100 ng/mL) (hu14.18 + GM-CSF), or hu14.18/GM-CSF (5 μg/mL). Anti-FcγRII (CD32) IV.3 Fab, anti-FcγRIII (CD16) 3G8 F(ab) 2 (10 μg/mL of each), or the same volume of PBS was added to the cell mixture just before the initiation of ADCC. (B) ADCC with hu14.18/GM-CSF (control; 5 μg/mL) and with the addition of anti-CD18 7E4, anti-CD11a R7.1, anti-CD11b 2LPM19C, anti-CD11c 3.9, or mouse IgG1 isotype control antibody (10 μg/mL of each) to the cell mixture just before the initiation of ADCC. Retained calcein fluorescence was quantified after 4 hours with DIMSCAN, and results are expressed as tumor cell viability index (mean ± SD for 6 replicate wells). ***Significant difference (P < .001, ANOVA) compared to controls. Data are from 1 of 4 experiments that gave similar results.
Fcγ and β2 integrin receptor requirement for PMN ADCC with hu14.18 alone, hu14.18 mixed with GM-CSF, and hu14.18/GM-CSF.
(A) ADCC was mediated by hu14.18 alone (5 μg/mL), hu14.18 (5 μg/mL) mixed with GM-CSF (100 ng/mL) (hu14.18 + GM-CSF), or hu14.18/GM-CSF (5 μg/mL). Anti-FcγRII (CD32) IV.3 Fab, anti-FcγRIII (CD16) 3G8 F(ab) 2 (10 μg/mL of each), or the same volume of PBS was added to the cell mixture just before the initiation of ADCC. (B) ADCC with hu14.18/GM-CSF (control; 5 μg/mL) and with the addition of anti-CD18 7E4, anti-CD11a R7.1, anti-CD11b 2LPM19C, anti-CD11c 3.9, or mouse IgG1 isotype control antibody (10 μg/mL of each) to the cell mixture just before the initiation of ADCC. Retained calcein fluorescence was quantified after 4 hours with DIMSCAN, and results are expressed as tumor cell viability index (mean ± SD for 6 replicate wells). ***Significant difference (P < .001, ANOVA) compared to controls. Data are from 1 of 4 experiments that gave similar results.
Function-blocking mAbs against the common β-subunit (CD18) and against each α-subunit (CD11a, CD11b, and CD11c) were used to examine the requirement for β2-integrin receptors in PMN ADCC. The blocking mAbs 7E4 against CD18 and 2LPM19C against CD11b completely abrogated ADCC mediated by hu14.18/GM-CSF (Figure 2B) and hu14.18, with or without GM-CSF (data not shown). Blocking antibodies against other α-subunits (CD11a and CD11c) did not inhibit cytotoxicity. Thus, the increased ADCC with hu14.18/GM-CSF compared with hu14.18 does not require CD16 but is absolutely dependent on CD32 and Mac-1 (CD11b/CD18).
Spreading and adhesion by PMN on target cells and cytolysis is greatest with hu14.18/GM-CSF and requires Mac-1
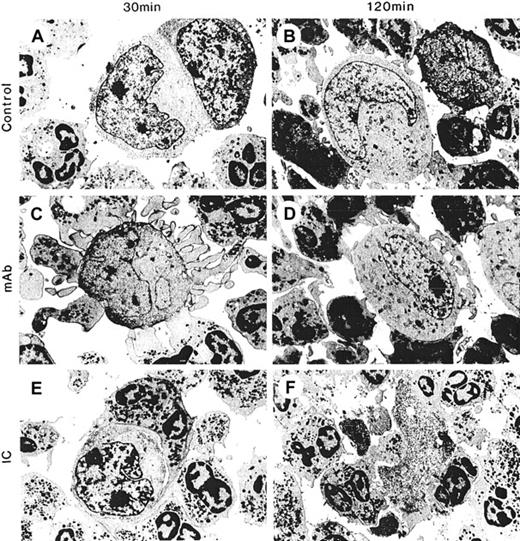
Cell-cell interactions between PMNs and tumor cells in ADCC were visualized with electron microscopy at 30 minutes and 2 hours after the start of ADCC. There was no or minimal spontaneous interaction between PMN and neuroblastoma cells in the absence of hu14.18 and hu14.18/GM-CSF (Figure 3A-B). In the presence of hu14.18 (Figure 3C-D) and hu14.18/GM-CSF (Figure 3E-F), PMN pseudopodia adhered to the surfaces of neuroblastoma cells. However, spreading and tight adhesion was evident with hu14.18/GM-CSF but not with hu14.18. In nearly all conjugates formed at 30 and 120 minutes with hu14.18/GM-CSF, PMNs covered 30% or more of the target cell surface, whereas in those formed with hu14.18, PMNs did not do so (Table 1). At 2 hours, most neuroblastoma cells in conjugates with hu14.18/GM-CSF were destroyed (Figure 3F), whereas those with hu14.18 remained intact (Figure 3D). Other electron micrographs of ADCC with hu14.18/GM-CSF revealed focal and global lysis of target cell membranes, spreading, and adhesion of PMN onto nuclear membranes, lysis of nuclear membranes, and phagocytosis of nuclei (data not shown). Treatment with anti-CD18 7E4 and anti-CD11b 2LPM19C blocking mAbs allowed tethering of PMN to neuroblastoma cells with hu14.18/GM-CSF at 30 minutes and 2 hours, but it completely prevented spreading, adhesion, and cytolysis (Figure4, Table 1). Tethering was mediated by Fc-FcγR interaction because all contacts were prevented when anti-FcγRII blocking Fab IV.3 was added (data not shown).
Spreading and adhesion of PMN onto tumor cells and cytolysis with hu14.18 and hu14.18/GM-CSF.
PMNs (200 000) were incubated with LA-N-1 neuroblastoma cells (10 000) alone (control) for (A) 30 minutes and (B) 120 minutes; with hu14.18 (mAb) for (C) 30 minutes and (D) 120 minutes; or with hu14.18/GM-CSF (IC) for (E) 30 minutes and (F) 120 minutes. Electron micrographs were taken with an original magnification of × 5400.
Spreading and adhesion of PMN onto tumor cells and cytolysis with hu14.18 and hu14.18/GM-CSF.
PMNs (200 000) were incubated with LA-N-1 neuroblastoma cells (10 000) alone (control) for (A) 30 minutes and (B) 120 minutes; with hu14.18 (mAb) for (C) 30 minutes and (D) 120 minutes; or with hu14.18/GM-CSF (IC) for (E) 30 minutes and (F) 120 minutes. Electron micrographs were taken with an original magnification of × 5400.
Semiquantitative analysis of PMN spreading over neuroblastoma cells during ADCC
| Condition . | Tumor cells with attached and spread PMNs (%) . | |
|---|---|---|
| 30 min . | 120 min . | |
| Control | 0 | 0 |
| mAb hu14.18 | 0 | 0 |
| IC hu14.18/GM-CSF | 80 | 90 |
| IC + anti-CD18 | 0 | 0 |
| IC + anti-CD11b | 0 | 0 |
| Condition . | Tumor cells with attached and spread PMNs (%) . | |
|---|---|---|
| 30 min . | 120 min . | |
| Control | 0 | 0 |
| mAb hu14.18 | 0 | 0 |
| IC hu14.18/GM-CSF | 80 | 90 |
| IC + anti-CD18 | 0 | 0 |
| IC + anti-CD11b | 0 | 0 |
Effect of function-blocking mAbs against Mac-1 (CD11b/CD18) on spreading, adhesion, and cytolysis by PMN with hu14.18/GM-CSF.
ADCC with hu14.18/GM-CSF was as described in Figure 3 except that function-blocking mAbs were added just before the addition of hu14.18/GM-CSF. ADCC was performed in the presence of anti-CD18 7E4mAb (10 μg/mL) for (A) 30 minutes and (B) 120 minutes or anti-CD11b 2LPM19C mAb (10 μg/mL) for (C) 30 minutes and (D) 120 minutes. Electron micrographs were taken with an original magnification of × 5400.
Effect of function-blocking mAbs against Mac-1 (CD11b/CD18) on spreading, adhesion, and cytolysis by PMN with hu14.18/GM-CSF.
ADCC with hu14.18/GM-CSF was as described in Figure 3 except that function-blocking mAbs were added just before the addition of hu14.18/GM-CSF. ADCC was performed in the presence of anti-CD18 7E4mAb (10 μg/mL) for (A) 30 minutes and (B) 120 minutes or anti-CD11b 2LPM19C mAb (10 μg/mL) for (C) 30 minutes and (D) 120 minutes. Electron micrographs were taken with an original magnification of × 5400.
GM-CSF increases expression and activation of Mac-1 during PMN ADCC
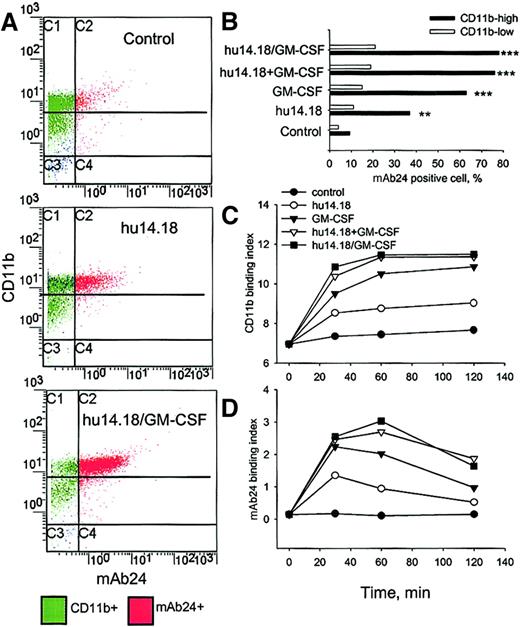
Because spreading and adhesion of PMNs on target cells and ADCC were Mac-1 dependent, we examined cell surface expression and activation of Mac-1 during ADCC with flow cytometry. Mac-1 was evaluated with a nonblocking anti-CD11b mAb and with mAb24, which recognizes an activation-specific epitope of CD11b.31 32Three major subpopulations of PMN were distinguished on the CD11b/mAb24 histograms after 60 minutes of ADCC (Figure5A): CD11blowmAb24−, CD11bhighmAb24−, and CD11bhighmAb24+. The CD11bhighmAb24+ subpopulation was increased by hu14.18 (P < .01, ANOVA) and even more so by GM-CSF alone, GM-CSF with hu14.18, and hu14.18/GM-CSF (P < .001, ANOVA) (Figure 5B). Neither GM-CSF with hu14.18 nor hu14.18/GM-CSF significantly up-regulated and activated Mac-1 in comparison to GM-CSF alone (P > .05, ANOVA). mA624 up-regulation in the CD11blow subpopulation was minimal and did not significantly differ for hu14.18, GM-CSF, hu14.18 with GM-CSF, and hu14.18/GM-CSF (P > .05, ANOVA). Figure 5C demonstrates that hu14.18/GM-CSF up-regulated CD11b more than hu14.18 (P < .01, ANOVA for 30, 60, and 120 minutes) but that GM-CSF alone or combined with hu14.18 similarly up-regulated CD11b (P > .05). Figure 5D shows that the activation of Mac-1 by hu14.18/GM-CSF, hu14.18 with GM-CSF, or GM-CSF alone reached a high level and remained so for up to 120 minutes, whereas activation by hu14.18 peaked at 30 minutes and then decreased (P < .01, ANOVA for 60 to 120 minutes). In the absence of target cells, CD11b was neither up-regulated nor activated by hu14.18 but was so by GM-CSF alone or mixed with hu14.18 or by hu14.18/GM-CSF (data not shown).
Expression and activation of Mac-1 during PMN ADCC.
(A) PMNs and DiD-labeled neuroblastoma cells were incubated alone (control), with hu14.18, or with hu14.18/GM-CSF, and flow cytometry analyses were performed at 30, 60, and 120 minutes. In the example shown, expression of CD11b and the β2-integrin activation epitope were analyzed 60 minutes after the initiation of ADCC with FITC–anti-CD11b (the nonblocking Bear-1 mAb) and mAb24 (+ PE–antimouse IgG), respectively. (B) The percentage of mAb24-positive cells in PMN subpopulations expressing high or low levels of CD11b after 60 minutes of ADCC was determined by gating on these distinct subsets. Binding index (% positive cells × mean fluorescence channel/100) for (C) CD11b and (D) mAb24 was calculated at indicated times. Significant differences compared to controls are indicated by ** for P < .01 and *** for P < .001 (ANOVA). Data are from 1 of 3 experiments that provided similar results.
Expression and activation of Mac-1 during PMN ADCC.
(A) PMNs and DiD-labeled neuroblastoma cells were incubated alone (control), with hu14.18, or with hu14.18/GM-CSF, and flow cytometry analyses were performed at 30, 60, and 120 minutes. In the example shown, expression of CD11b and the β2-integrin activation epitope were analyzed 60 minutes after the initiation of ADCC with FITC–anti-CD11b (the nonblocking Bear-1 mAb) and mAb24 (+ PE–antimouse IgG), respectively. (B) The percentage of mAb24-positive cells in PMN subpopulations expressing high or low levels of CD11b after 60 minutes of ADCC was determined by gating on these distinct subsets. Binding index (% positive cells × mean fluorescence channel/100) for (C) CD11b and (D) mAb24 was calculated at indicated times. Significant differences compared to controls are indicated by ** for P < .01 and *** for P < .001 (ANOVA). Data are from 1 of 3 experiments that provided similar results.
GM-CSF does not effect PMN–tumor cell conjugate formation
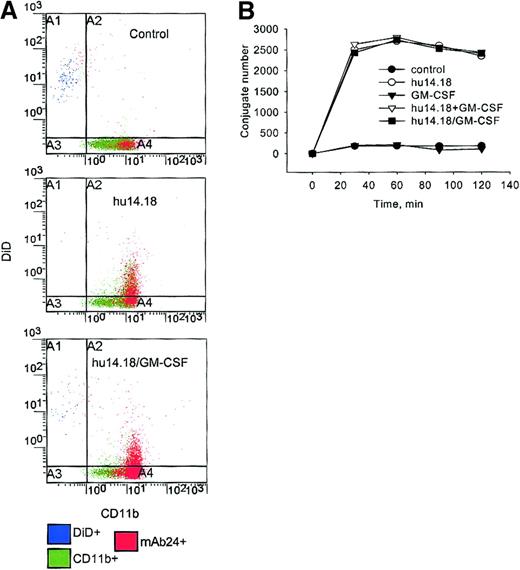
Because Mac-1 activation enables high avidity binding of the receptor to its ligands,33 the up-regulation and activation of Mac-1 could be associated with increased formation of PMN–tumor cell conjugates. CD11b-FITC–labeled PMNs that were conjugated with DiD-labeled neuroblastoma cells could be identified as DiD+CD11b+ events in the region A2 of Figure6A. There was no difference in the number of conjugates formed with hu14.18 alone or mixed with GM-CSF and hu14.18/GM-CSF when 29 time points (5 to 120 minutes) from 5 separate experiments were analyzed (P > .05, ANOVA, Figure 6B). GM-CSF alone did not increase conjugate formation at any time in comparison with control PMNs alone (P > .05, ANOVA). Similar results were obtained with 4 different effector-target ratios ranging from 1:1 to 25:1 (data not shown).
PMN–tumor cell conjugate formation.
PMNs were incubated with DiD-labeled neuroblastoma cells alone (control), GM-CSF alone, hu14.18 with or without GM-CSF, or hu14.18/GM-CSF immunocytokine for 30, 60, 90, and 120 minutes. (A) In this example, the expression of CD11b by PMNs is shown after 60 minutes with an FITC–anti-CD11b mAb, and data are presented as dual-color diagrams. (B) PMN–tumor cell conjugates were identified as CD11b+DiD+ events and are represented as the number per 10 000 events at the indicated times. Data are from 1 of 5 experiments that gave similar results.
PMN–tumor cell conjugate formation.
PMNs were incubated with DiD-labeled neuroblastoma cells alone (control), GM-CSF alone, hu14.18 with or without GM-CSF, or hu14.18/GM-CSF immunocytokine for 30, 60, 90, and 120 minutes. (A) In this example, the expression of CD11b by PMNs is shown after 60 minutes with an FITC–anti-CD11b mAb, and data are presented as dual-color diagrams. (B) PMN–tumor cell conjugates were identified as CD11b+DiD+ events and are represented as the number per 10 000 events at the indicated times. Data are from 1 of 5 experiments that gave similar results.
Degranulation is greatest with hu14.18/GM-CSF and requires FcγRII and activated Mac-1
CD63 is a marker of azurophil (primary) granules, and exocytosis results in the expression of CD63 on the cell surface because of granule fusion with the cell membrane.34 The relationship between Mac-1 activation (mAb24+ PMN) and CD63 expression during PMN ADCC was examined using 2-color flow cytometry analysis (Figure 7). Degranulation stimulated by hu14.18 with or without GM-CSF and by hu14.18/GM-CSF was associated with mAb24+ PMN and was almost absent in the mAb24− population (Figure 7A). Hu14.18/GM-CSF induced a time-dependent increase in CD63 expression by mAb24+ PMNs that was significantly higher than that induced by hu14.18 with or without GM-CSF from 30 to 120 minutes (P < .001, ANOVA) (Figure 7B). GM-CSF alone did not induce CD63 expression. Hu14.18 mAb mixed with GM-CSF induced greater CD63 expression than mAb alone, but it was significantly below the level reached with hu14.18/GM-CSF from 45 to 120 minutes (P < .001, ANOVA). Anti-FcγRII (CD32) and anti-CD11b, but not anti-FcγRIII (CD16) function-blocking mAbs, abrogated CD63 up-regulation mediated by hu14.18/GM-CSF (Figure 7B). No CD63 induction was observed in any condition in the absence of target cells (data not shown).
Azurophil granule exocytosis by mAb24-positive PMN.
(A) PMNs were incubated with neuroblastoma cells alone (control), GM-CSF alone, hu14.18 with or without GM-CSF, hu14.18/GM-CSF, or hu14.18/GM-CSF with anti-CD32, anti-CD16, or anti-CD11b for 5 to 120 minutes. (A) In this example, the expression of CD63 and the β2-integrin activation epitope (mAb24+) was evaluated at 60 minutes. (B) mAb24+CD63+cells per 10 000 events at 5 to 120 minutes were quantified. Data are from 1 of 3 experiments that provided similar results.
Azurophil granule exocytosis by mAb24-positive PMN.
(A) PMNs were incubated with neuroblastoma cells alone (control), GM-CSF alone, hu14.18 with or without GM-CSF, hu14.18/GM-CSF, or hu14.18/GM-CSF with anti-CD32, anti-CD16, or anti-CD11b for 5 to 120 minutes. (A) In this example, the expression of CD63 and the β2-integrin activation epitope (mAb24+) was evaluated at 60 minutes. (B) mAb24+CD63+cells per 10 000 events at 5 to 120 minutes were quantified. Data are from 1 of 3 experiments that provided similar results.
Discussion
This study demonstrated that a humanized anti-GD2/GM-CSF immunocytokine, hu14.18/GM-CSF, is more effective in mediating PMN ADCC than the parent anti-GD2 mAb hu14.18 alone or mixed with GM-CSF and that ADCC under all conditions required functional Mac-1 (CD11b/CD18). Electron microscopy revealed greater Mac-1–dependent PMN adhesion, spreading, and cytolysis with hu14.18/GM-CSF than with hu14.18. Flow cytometry showed that increased expression and activation of Mac-1 occurred during PMN ADCC with mAb hu14.18 alone but that this was significantly less than with GM-CSF, GM-CSF mixed with hu14.18, and hu14.18/GM-CSF, indicating the importance of GM-CSF in regulating Mac-1. Even though the latter conditions equally enhanced the expression and activation of Mac-1, hu14.18/GM-CSF caused the highest and most sustained azurophil granule exocytosis, which was Mac-1 and FcRγII dependent. These experiments demonstrate for the first time the importance of enhanced expression and activation of Mac-1 for PMN ADCC against tumor cells. Additionally, they suggest that enhancing azurophil granule exocytosis is critical for maximizing PMN ADCC.
The first step in PMN ADCC is the interaction of target-bound antibody with PMN FcR. FcγRII and FcγRIII were previously reported to be necessary for PMN ADCC against neuroblastoma cells with the 3F8 anti-GD2 murine mAb.5 However, PMN ADCC against Burkitt lymphoma cells (Raji cell line) with the Lym-1 murine mAb was shown to require only FcγRII using F(ab′)2 reagents to block FcR.6 Our experiments demonstrated that both receptors are required for PMN ADCC with hu14.18, whereas only FcγRII is required with hu14.18/GM-CSF. We used IV.3 F(ab′)2 to test FcγRII and 3G8 F(ab′) to test FcγRIII requirements, thus avoiding potential interactions of the Fc portion of the blocking mAbs with FcR. We found that the whole 3G8 anti-FcγRIII mAb used in the earlier study of FcRs in PMN ADCC5 nearly completely abrogated ADCC mediated by hu14.18/GM-CSF (data not shown), indicating that the whole 3G8 mAb has a nonspecific effect. FcγRIII use by hu14.18 with or without GM-CSF, but not by hu14.18/GM-CSF, may be the result of one or more of the following: (1) the GM-CSF component of hu14.18/GM-CSF may alter the tertiary structure of the Fc-portion, decreasing its affinity for FcγRIII; (2) simultaneous cross-linking of the FcγRII and GM-CSF receptors by hu14.18/GM-CSF may overcome a requirement for FcγRIII signaling; (3) shedding of FcγRIII from the PMN surface may be increased by GM-CSF in the hu14.18/GM-CSF-CSF immunocytokine.15
With function-blocking mAbs, we showed that Mac-1 is required for PMN ADCC mediated by both hu14.18 and hu14.18/GM-CSF. This is in agreement with other reports that Mac-1 is required for PMN ADCC against tumor cells.5,13,16 This is the first demonstration that PMN ADCC with an antibody/GM-CSF immunocytokine requires Mac-1 function. Electron microscopy of PMN ADCC with hu14.18/GM-CSF or with hu14.18 revealed the greatest adhesion, spreading, and tumor cell lysis with the immunocytokine. When anti-CD18– or anti-CD11b–blocking mAbs were included with hu14.18/GM-CSF, PMNs were tethered to target cells but did not adhere, spread, or cause cytolysis. Mac-1–mediated focal adhesion contacts also have been described recently for PMN ADCC with an anti–HER-2/neu murine mAb and with an anti–FcαRI/anti-HER-2/neu bispecific mAb.13 These experiments indicate that an essential and important function of Mac-1 is to mediate strong adhesion and spreading of PMN onto tumor cells.
Flow cytometry showed an increase in expression and activation of Mac-1 during PMN ADCC with mAb hu14.18 alone and an even greater increase with GM-CSF, GM-CSF mixed with hu14.18, and hu14.18/GM-CSF (Figure 5). This indicates the importance of antibody and GM-CSF in regulating Mac-1. Functionally active Mac-1 was identified with mAb24, which recognizes an epitope within the I domain of the β2-integrin α-subunit when it binds Mg2+.35,36 Two-color staining of PMN with mAb24 and Bear-1 anti-CD11b mAbs showed Mac-1 activation predominantly in CD11bhigh cells, though the up-regulation of CD11b was not invariably accompanied by Mac-1 activation. The subpopulation of CD11bhighmAb24+ PMN was significantly greater when GM-CSF was present. Additionally, the activation of Mac-1 induced by hu14.18/GM-CSF or hu14.18 and GM-CSF was sustained for 120 minutes, whereas that induced by hu14.18 peaked at a lower level at 30 minutes and then decreased. This higher and sustained activation is likely to contribute to greater ADCC. GM-CSF has previously been reported to up-regulate Mac-1, but activation was not examined.5Surprisingly, a similar number of effector-target cell conjugates were observed with hu14.18/GM-CSF and with hu14.18 with or without GM-CSF (Figure 6B). However, a greater percentage of PMNs were mAb24+ in conjugates formed by hu14.18 mixed with GM-CSF or by hu14.18/GM-CSF than in those formed with hu14.18 (data not shown). Thus, increased expression and activation of Mac-1 does not appear to influence the initial process of PMN–tumor cell conjugate formation.
Two-color flow cytometry with mAb24 and anti-CD63 mAbs revealed azurophil (primary) granule exocytosis almost exclusively in PMNs with activated Mac-1 (Figure 7). The number of mAb24+CD63+ PMNs increased up to 30-fold and was sustained with hu14.18/GM-CSF, whereas this subpopulation increased only 10-fold and was not sustained with hu14.18. Despite the similar effects on Mac-1 of GM-CSF, GM-CSF mixed with hu14.18, and hu14.18/GM-CSF, the latter induced greater CD63 expression than the mixture. GM-CSF alone did not cause azurophil granule exocytosis compared to control, untreated PMNs. The hu14.18/GM-CSF immunocytokine induced PMN degranulation only in the presence of the target cells, and this was FcγRII and Mac-1 dependent, indicating that azurophil granule release in ADCC requires PMN attachment, adhesion, and spreading on target cells. Sustained Mac-1–mediated PMN adhesion and spreading may also be important for ADCC by providing maximal concentrations of neutrophil granule contents at the tumor cell surface and an optimal pH for enzymatic activity of primary granule proteases.37 Thus, our data suggest that mAb24+CD63+ PMNs are the cytolytic effectors in PMN ADCC. Supporting this model is a previous report that PMN azurophil granule exocytosis is required for PMN ADCC against neuroblastoma cells with the anti-GD2 chimeric mAb ch14.18 and that this is enhanced by the addition of GM-CSF.38 In the latter study, cathepsin-G and defensins from azurophil granules were implicated in mediating cytotoxicity. Another investigation of PMN ADCC against neuroblastoma cells with the murine anti-GD2 mAb 3F8 showed that cytotoxicity was independent of reactive oxygen species because PMN from patients with chronic granulomatous disease were as cytotoxic as those from healthy donors.39
Although our data demonstrate that Mac-1 mediates adhesion, spreading, and azurophil granule exocytosis once PMN–tumor cell conjugates have been established by antibodies, it is not clear how Mac-1 contributes to these important events. Ligands for Mac-1 on neuroblastoma cells are unknown. We found that 10 neuroblastoma cell lines expressed little or no ICAM-1, a known ligand for Mac-1, by flow cytometry (data not shown), and this is in accord with other reports.40Therefore, Mac-1 may bind other unknown molecule(s) or may transmit signals from GPI-linked receptors given that cooperation with Mac-1 through the lectinlike site on CD11b has been reported for CD66b.6 Indeed, Mac-1 activation through specific binding to the lectinlike domain of the COOH-terminus of CD11b is involved in the antitumor activity of the soluble β-glucans lentinan and schizophyllan.41
We conclude that Fc and GM-CSF receptor triggering increase the expression and activation of Mac-1, which is necessary for strong adhesion and spreading of PMN on target cells and for azurophil granule exocytosis. We propose that the hu14.18/GM-CSF immunocytokine increases PMN ADCC by enhancing Mac-1–dependent azurophil exocytosis. Further studies exploring additional means of increasing Mac-1 function and identifying its ligand(s) on tumor cells will be important for the development of antibody and immunocytokine-based therapies for neuroblastoma and other tumors.
We thank Dr Nancy Hogg and Dr Karen Pulford for providing monoclonal antibodies mAb24 and 2LPM19C, respectively, Dr Thomas Coates for arranging for PMN donors, Ms Ripple Kakkar, Ms Michelle Geelhoed, Mr Peter Jordan, and Dr Mark Podberezin for technical assistance, and Drs Anat Epstein and Susan Groshen for review of the manuscript.
Supported in part by grant CA81403 from the National Cancer Institute, National Institutes of Health; by the Neil Bogart Memorial Fund of the T. J. Martell Foundation for Leukemia, Cancer, and AIDS Research; and by the Ashley Barrasso Foundation for Cancer Research.
S.D.G. and M.S. have declared a financial interest in a company whose potential products (hu14.18 and hu14.18/GM-CSF) were studied in the present work. These authors are employed by Lexigen Pharmaceuticals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert C. Seeger, Department of Pediatrics, Division of Hematology-Oncology, MS 57, Children's Hospital Los Angeles, 4650 Sunset Blvd, Los Angeles, CA 90027; e-mail:rseeger@chla.usc.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal