Impaired T-cell function after T-cell– depleting (TCD) therapy has been hypothesized to be related to a transient predominance of extrathymically expanding memory T cells. To test whether after TCD therapy the naive T-helper cell population is functionally intact, the in vitro immune response of CD4+CD45RA+ (naive) and of CD4+CD45RA− (memory) cells to polyclonal mitogens (immobilized anti-CD3, phytohemagglutinin) was analyzed by flow cytometry in 22 pediatric patients after high-dose chemotherapy (including 5 after autologous and 5 after allogeneic stem cell support). At 1 to 3 months after TCD therapy, patient samples showing decreased lymphoproliferative responses also showed a reduced induction of the early activation marker CD69 by CD4+ T cells from 4 to 72 hours after stimulation even when supplemented with exogenous interleukin-2. This defect affected CD4+CD45RA− cells, but, strikingly, also CD4+CD45RA+ cells, including samples in which CD4+CD45RA+ cells were more than 90/μL, thus indicating ongoing thymopoiesis. Histogram analyses showed the median peak channel of CD69 in control CD4+CD45RA+cells rising 98-fold (median) but only 28-fold in patient cells (P < .0001). Apoptosis as detected by annexin V staining was increased in resting patient CD4+ T cells (25% versus 6%) and also affected CD4+CD45RA+ cells (12% versus 5%, P < .01). When peripheral blood mononuclear cells (PBMCs) were enriched for T cells, stimulatory responses of CD4+ cells and of CD4+CD45RA+ cells markedly improved. Thus, after TCD therapy suppressor factors contained in the non–T-cell fraction of PBMCs may affect T-helper cells irrespective of their naive or memory phenotype thus extending T-cell dysfunction to the presumably thymus-dependently regenerated T cells.
Introduction
Severe immune dysfunction is a predictable consequence of high-dose anticancer chemotherapy with or without autologous or allogeneic stem cell support and results in significant morbidity and mortality in potentially curable diseases.1-3 One obvious explanation for such immune dysfunction is a depletion of immune cells by the cytotoxic effects of anticancer drugs.4,5 However, even after quantitative regeneration immune cells do not regain full immune competence for a prolonged period of time.6-10
T cells play a key role in multiple immune responses. Therefore, both a depletion and a disturbed function caused by cytotoxic anticancer treatment are potentially harmful to the immune surveillance of the host, including an increased susceptibility to infections with otherwise less harmful pathogens.11-15 T-cell reconstitution after cytotoxic therapy does not repeat fetal T-cell ontogeny.16-19 In fact, the first T cells that repopulate the host after T-cell depleting (TCD) therapy expand extrathymically from peripheral residual T cells whereas a thymus-dependent T-cell regeneration is delayed and may be even incomplete in adult patients in whom thymic function has declined.20-22 Extrathymically expanded T cells bear a memory phenotype,23 as their expansion is determined by antigenic stimulation.24 Their capacity to restore full T-cell function is believed to be limited, because of (1) skewing of the T-cell repertoire25-27 and (2) an increased susceptibility to apoptosis, including activation-induced apoptosis.28 In contrast, T cells with a naive phenotype presumably represent recent thymic emigrants,23 29-31 having matured from precursors via thymopoiesis, and thus may be spared from the postchemotherapy immune dysfunction.
To test this hypothesis we investigated by flow cytometry the in vitro immune function of distinct naive (CD4+CD45RA+) and memory (CD4+CD45RA−) T-helper cells. Examinations were performed at one to 3 months after completion of TCD therapy, a time point at which CD4+CD45RA+ T cells were readily detectable but not yet fully recovered.
Upon stimulation with polyclonal mitogens in vitro we found a reduced up-regulation of the early activation-induced cell surface molecule, CD69, detectable in CD4+ T cells even in the presence of exogenous interleukin 2 (IL-2). Strikingly, CD4+CD45RA+ cells were similarly affected as CD4+CD45RA− cells by decreased stimulatory responses and an increased susceptibility to apoptosis as detected by annexin V staining.32 33 When peripheral blood mononuclear cell (PBMC) populations were enriched for T cells, the stimulatory responses improved markedly. Together, the findings are consistent with a concept that factors external to T cells, eventually affecting T-helper cells irrespective of a naive or memory phenotype, contribute to the prevailing T-cell dysfunction in the early post–TCD therapy period.
Patients, materials, and methods
Patients
T-cell analyses in pediatric patients with different underlying diseases (Table 1) were performed after patients recovered from a chemotherapy-induced phase of severe leukopenia (white blood count < 500/μL). Approval for these studies was obtained from the University Children's Hospital Innsbruck institutional review board. Informed consent was obtained according to the Declaration of Helsinki. The chemotherapy consisted of repeated cycles of high-dose conventional chemotherapy (except for patients 8 and 22), which was followed by autologous stem cell rescue or allogeneic bone marrow transplantation (BMT) in 10 patients. These treatment modalities uniformly result in severe T-cell depletion,5 including a depletion of T-helper cells with a naive phenotype.21 Notably, all patients were in complete remission and without complications (eg, infections, graft-versus-host disease [GVHD]) when investigated. There were 4 patients who were followed sequentially until quantitative T-cell reconstitution was observed.
Demographic data of pediatric patients examined after T-cell depleting therapy
| Patient no. . | Sex, age (y) . | Diagnosis . | Treatment modality . | Time of examination after therapy, mo . |
|---|---|---|---|---|
| 1 | Female, 8 | NB IV | HD-CTX/ASCR | 3, 9 |
| 2 | Female, 5 | RMS, relapsed | HD-CTX/ASCR | 3 |
| 3 | Male, 12 | MB, relapsed | HD-CTX/ASCR | 6 |
| 4 | Male, 11 | B-NHL IV | CTX | 3 |
| 5 | Female, 3 | Ependymoma | CTX | 1 |
| 6 | Male, 12 | ALL | CTX | 1 |
| 7 | Male, 11 | ALL | CTX | 1 |
| 8 | Female, 11 | SAA | allo-BMT (sibling) | 3 |
| 9 | Female, 16 | Osteosarcoma | CTX | 1 |
| 10 | Female, 12 | Ewing sarcoma | CTX | 1, 3, 6 |
| 11 | Male, 7 | ALL | allo-BMT (sibling) | 3 |
| 12 | Male, 15 | HD | CTX | 1 |
| 13 | Male, 14 | Osteosarcoma | CTX | 1 |
| 14 | Female, 6 | B-NHL IV | CTX | 3 |
| 15 | Male, 5 | Ewing sarcoma IV | HD-CTX/ASCR | 3, 6 |
| 16 | Female, 15 | Ovarian carcinoma | CTX | 3, 6 |
| 17 | Male, 8 | ALL, relapsed | allo-BMT (MUD) | 3 |
| 18 | Female, 12 | ALL | allo-BMT (MUD) | 3 |
| 19 | Female, 7 | AML | CTX | 1 |
| 20 | Female, 18 | Ewing sarcoma* | CTX | 1 |
| 21 | Male, 0.5 | NB IV | HD-CTX/ASCR | 1 |
| 22 | Male, 8 | β-thalassemia major | allo-BMT (MUD) | 3, 6 |
| Patient no. . | Sex, age (y) . | Diagnosis . | Treatment modality . | Time of examination after therapy, mo . |
|---|---|---|---|---|
| 1 | Female, 8 | NB IV | HD-CTX/ASCR | 3, 9 |
| 2 | Female, 5 | RMS, relapsed | HD-CTX/ASCR | 3 |
| 3 | Male, 12 | MB, relapsed | HD-CTX/ASCR | 6 |
| 4 | Male, 11 | B-NHL IV | CTX | 3 |
| 5 | Female, 3 | Ependymoma | CTX | 1 |
| 6 | Male, 12 | ALL | CTX | 1 |
| 7 | Male, 11 | ALL | CTX | 1 |
| 8 | Female, 11 | SAA | allo-BMT (sibling) | 3 |
| 9 | Female, 16 | Osteosarcoma | CTX | 1 |
| 10 | Female, 12 | Ewing sarcoma | CTX | 1, 3, 6 |
| 11 | Male, 7 | ALL | allo-BMT (sibling) | 3 |
| 12 | Male, 15 | HD | CTX | 1 |
| 13 | Male, 14 | Osteosarcoma | CTX | 1 |
| 14 | Female, 6 | B-NHL IV | CTX | 3 |
| 15 | Male, 5 | Ewing sarcoma IV | HD-CTX/ASCR | 3, 6 |
| 16 | Female, 15 | Ovarian carcinoma | CTX | 3, 6 |
| 17 | Male, 8 | ALL, relapsed | allo-BMT (MUD) | 3 |
| 18 | Female, 12 | ALL | allo-BMT (MUD) | 3 |
| 19 | Female, 7 | AML | CTX | 1 |
| 20 | Female, 18 | Ewing sarcoma* | CTX | 1 |
| 21 | Male, 0.5 | NB IV | HD-CTX/ASCR | 1 |
| 22 | Male, 8 | β-thalassemia major | allo-BMT (MUD) | 3, 6 |
NB IV indicates neuroblastoma, stage four; RMS, rhabdomyosarcoma; MB, medulloblastoma; B-NHL, B-cell non-Hodgkin lymphoma; ALL, acute lymphoblastic leukemia; SAA, severe aplastic anemia; HD, Hodgkin disease; AML, acute myelogenous leukemia; CTX, conventional high-dose chemotherapy; HD-CTX, high-dose chemotherapy; ASCR, autologous stem cell rescue; allo-BMT, allogeneic bone marrow transplantation; MUD, matched unrelated donor.
Second malignancy.
Lymphocyte culture
Heparinized peripheral blood was drawn upon routine examinations. PBMCs were separated from whole blood by density centrifugation (Lymphoprep; Nycomed, Oslo, Norway). PBMCs were cultured in RPMI (PAA Laboratories, Linz, Austria) supplemented with 10% fetal calf serum, 2mM L-glutamine, and 50μg/mL gentamycin (all from PAA Laboratories). In selected experiments, PBMCs were separated into a highly enriched T-cell population (> 95% CD3+) and a monocyte-enriched population (> 80% CD14+cells). T cells were prepared by a depletion of monocytes by plastic adherence (1 × 107 PBMCs per 5 mL culture medium placed in 10 cm Ø polystyrene petri dishes and cultured for 2 hours at 37°C) followed by magnetic cell sorting (MACS; Miltenyi Biotech, Bergisch-Gladbach, Germany) according to the manufacturer's protocol. Monocytes were enriched from PBMCs by MACS as recommended by the manufacturer. T-cell stimulants were used at the previously tested optimal stimulatory concentrations, which were 1 μg/mL for phytohemagglutinin (PHA; Sigma, St Louis, MO) and 250 ng/mL for anti-CD3 (Sigma). Anti-CD3 was immobilized by precoating the plates with goat anti–mouse IgG (Sigma). Recombinant human IL-2 was purchased from Eubio (Vienna, Austria).
Lymphocyte proliferation assays
PBMCs (1 × 105) were cultured in 100 μL medium per well in 96-well flat-bottom tissue culture plates (Falcon, Becton Dickinson Labware, Franklin Lakes, NJ) in humidified air containing 5% CO2 at 37°C in the absence (negative control) or presence of PHA or anti-CD3. The lymphoproliferative response was measured by adding 1 μL [3H]thymidine (1 μCi = 37 kBq/well; ICN Biomedicals, Costa Mesa, CA) per well for the last 18 hours of culture. Cells were then harvested on glass fiber filters (Skatron Instruments, Lier, Norway) and analyzed on a liquid scintillation counter. Net stimulation of triplicate cultures (mean ± SEM) was calculated by subtraction of the counts per minute of nonstimulated cultures from that of stimulated cultures. The in vitro proliferative responses of patients after TCD therapy were compared with that of a healthy control in each individual experiment. Initial studies (data not shown) showed that the in vitro proliferative response and the up-regulation of cell surface activation molecules (see below) as induced by polyclonal mitogens of healthy children were similar to healthy adults, thus allowing the use of adult volunteers of the hospital staff as controls.
Cell surface marker analysis
PBMCs (1 × 106) were cultured in 12-well plates (Falcon) in 1 mL medium with and without the above-mentioned polyclonal T-cell stimulants and were stained after time periods of 4 to 72 hours, as indicated. The following monoclonal antibodies (mabs) all purchased from Becton Dickinson (Mountain View, CA) were used: simultest control (IgG fluorescein isothiocyanate [FITC; FL1], IgG2a phycoerythrin [PE; FL2], IgG1 peridinin chlorophyll protein [PerCP; FL3]), anti-CD4 (PerCP), anti-CD45 (FITC), anti-CD14 (PE), anti-CD45RA (FITC), anti-CD45RO (FITC), anti-CD8 (PerCP), anti-CD69 (PE), anti-CD25 (PE), anti–HLA-DR (PE). The binding of cell surface phosphatidyl-serine structures by annexin V (PE; Pharmingen, San Diego CA) was used to detect apoptosis. Aliquots of 3 × 105 PBMCs were first incubated with MOPC 21 (mouse IgG1κ; Sigma) on ice for 20 minutes to block nonspecific binding. The cells were then incubated with the appropriate mab on ice for 20 minutes in the dark, and washed and immediately analyzed on a FACScan flow cytometer (Becton Dickinson). List mode data were analyzed using Cell Quest software (Becton Dickinson).
To examine stimulation-induced activation of T cells and of individual T-cell subsets by flow cytometry, the following algorithm was devised: lymphocytes were identified by forward scatter (FSC) versus side scatter (SSC) and gated. To exclude a contamination with residual monocytes, the lymphocytes as gated were examined for the expression of CD45 (FITC) versus CD14 (PE). The proportion of CD45+CD14+ cells contained in the lymphocyte gate was less than 1%. CD4+ or CD8+ cells were quantified by quadrant analysis (FL3 versus SSC). To detect cellular activation, cells as gated were examined for the expression of the activation markers CD69 or CD25 or HLA-DR. In experiments exploring potential differences of naive and memory T-helper cells, CD4+ cells (FL3 versus SSC) were gated and assessed for the expression of CD45RA (FL1). Both the CD45RA+ and CD45RA− subsets were then gated and examined individually for the expression of cell-surface activation markers (FL1 versus FL2). The median peak channel (mpc) on an arbitrary logarithmic scale in histogram plots was used to estimate the density of cell-surface expression of activation markers. Experiments examining CD4+ populations stained with a CD45RO mab showed similar results as CD4+CD45RA− populations. To detect apoptotis, CD4+ cells or CD4+CD45RA+ or CD4+ CD45RA− T cells were examined for the binding of annexin V in a similar manner as described above. Cell counts (cells/μL) of individual T-cell subsets identified by flow cytometry were calculated from the differential count of the total white blood cell count (WBC).
Statistical analysis
Statistical analyses were performed using SSPS for Windows software. The Student t test (2-tailed) was used to compare results obtained from patient and control samples. Differences atP < .05 were considered significant. Linear regressions (Pearson correlation coefficient) were used to determine a correlation between the lymphoproliferative response ([3H]thymidine incorporation) and the density of expression of CD69+ cells upon in vitro stimulation.
Results
Impaired induction of cell-surface activation molecules in T cells after TCD therapy
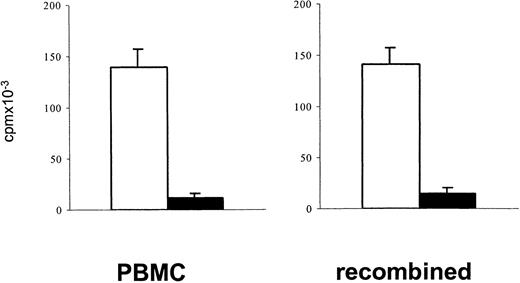
Measuring lymphoproliferative responses to polyclonal stimulation has been a standard approach to estimate a depressed T-cell function after cytotoxic chemotherapy.1 34 In the present study, PBMCs of patients after TCD therapy showed reduced in vitro lymphoproliferative responses to polyclonal stimulation with immobilized anti-CD3 or PHA (mean reduction 65%). To exclude the possibility that a decreased lymphoproliferative response to stimulation through the TCR/CD3 complex simply reflects a reduced number of proliferating T cells contained in PBMCs after TCD therapy, the [3H]thymidine incorporation upon stimulation with immobilized anti-CD3 of 1 × 105 PBMCs was compared with that of a cell population in which 7.5 × 104 purified T cells were recombined with 2.5 × 104 monocytes. A similarly reduced response of patient samples in both cell preparations (Figure 1) indicated that in fact the responsiveness of T cells to stimulation after TCD therapy was reduced.
A reduced lymphoproliferative response of PBMCs in the post–T-cell depletion period results from a reduced proliferative capacity of T cells.
To exclude that after T-cell–depleting therapy depressed lymphoproliferative responses of PBMCs simply reflect a reduced number of proliferating T cells, the proliferative responses to stimulation with immobilized anti-CD3 of 1 × 105 PBMCs were compared with that of an enriched T-cell population (7.5 × 104) recombined with enriched monocytes (2.5 × 104). Blank bars, control cells; black bars, patient cells. The bars represent the cpm (mean ± SEM) of triplicate cultures. Patient cells were obtained from an 8-year-old boy with ALL (patient 17) 3 months after matched unrelated donor (MUD)–BMT.
A reduced lymphoproliferative response of PBMCs in the post–T-cell depletion period results from a reduced proliferative capacity of T cells.
To exclude that after T-cell–depleting therapy depressed lymphoproliferative responses of PBMCs simply reflect a reduced number of proliferating T cells, the proliferative responses to stimulation with immobilized anti-CD3 of 1 × 105 PBMCs were compared with that of an enriched T-cell population (7.5 × 104) recombined with enriched monocytes (2.5 × 104). Blank bars, control cells; black bars, patient cells. The bars represent the cpm (mean ± SEM) of triplicate cultures. Patient cells were obtained from an 8-year-old boy with ALL (patient 17) 3 months after matched unrelated donor (MUD)–BMT.
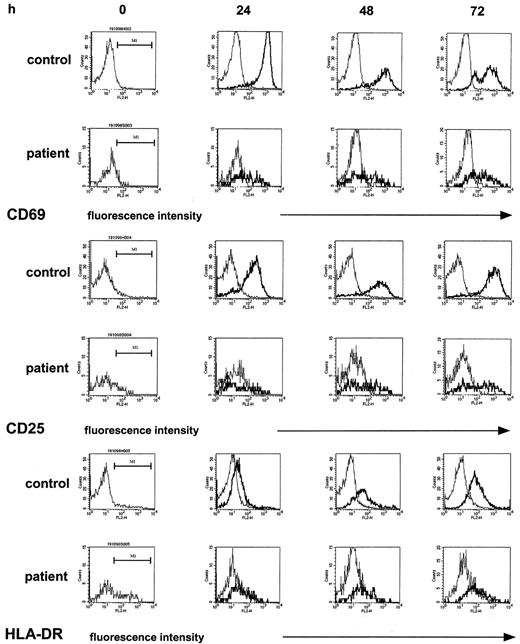
To attempt to identify, on a molecular level, a correlate to the defective T-cell proliferative response, we examined by flow cytometry the induction of the cell-surface markers CD69, CD25, and HLA-DR on T cells, all of which are essentially absent on normal, unstimulated T cells but are readily induced upon T-cell activation in a time-dependent fashion.35 In these studies, immobilized anti-CD3 or PHA were selected as stimulants as they most strongly induced the expression of the above cell-surface molecules and gave similar results. The monitoring of the expression of the respective cell-surface markers by T-helper cells for 72 hours of culture (the time period for determination of the proliferative response measured by [3H]thymidine incorporation) showed marked differences between patient and control samples (5 experiments; patients 3, 10, 18, 19, and 20). First, patient samples (Figure2, left column) contained higher proportions of CD4+CD25+ cells (9% median [range, 8%-14%] versus 3% median [range, 2%-5%], patient versus control) and of CD4+HLA-DR+ cells (24% median [range, 21%-25%] versus 7% median [range, 3%-8%], patient versus control) but not of CD4+CD69+cells (2% median [range, 3%-8%] versus 6% median [range, 1%-11%], patient versus control), a finding compatible with an increased proportion of activated T cells in patients having received high-dose chemotherapy, as previously described.28 In cultures stimulated with anti-CD3, however, the increase of expression of the above activation markers, given as the mpc on an arbitrary log scale in histogram plots—thus estimating the density of expression—was clearly superior in controls as compared with patient samples. In the control samples, the expression of CD69+ on CD4+ T cells reached a maximum at 24 hours, the mpc rising from a median of 11 in resting CD4+ T cells to a median of 1004 in stimulated CD4+ T cells. In contrast, in patient samples at 24 hours after stimulation the expression of CD69+ on CD4+ T cells was only 38%, median, of control (range, 3%-69%) and remained reduced at 48 hours and 72 hours (17% median; range, 3%-46%). Likewise, although to a lesser extent, the induction of CD25 was reduced, by 40%, median, and 30%, median, at 24 hours and 48 hours, respectively. The percent CD4+HLA-DR+ cells in control samples increased 11-fold, from 7% median, to 77% median, whereas in patient samples the increase was only 2.2-fold (from 24% to 54%). In a total of 26 experiments the assessment of the mpc of CD69 expression of CD4+ T cells at 24 hours after stimulation (expressed as percent of control) showed a correlation with the lymphoproliferative response (expressed as percent of control; r2 = 0.51). In the patient samples, as expected, the proportion of CD4+ T cells contained in total lymphocytes was significantly reduced (18% median versus 54% median, patients versus controls;P < .0001) accounting for a reverse CD4/CD8 ratio (0.5 median). In 19 experiments comparing histograms of CD4+ and CD8+ T cells, a similarly decreased up-regulation of CD69 was found (not shown).
The induction of cell-surface activation molecules in T-helper cells upon nonspecific stimulation is reduced in the post–T-cell depletion period.
PBMCs were stimulated with immobilized anti-CD3 as described. At the indicated time points, T-helper cells of control samples and patient samples were identified by staining with anti-CD4 and gated; the density of expression of CD69 and of CD25 and of HLA-DR in unstimulated cultures (thin line) and stimulated cultures (thick line) was estimated by the mpc in histogram plots. Patient samples were obtained from a 12-year-old girl with Ewing sarcoma (patient 10) 1 month after completion of conventional high-dose chemotherapy.
The induction of cell-surface activation molecules in T-helper cells upon nonspecific stimulation is reduced in the post–T-cell depletion period.
PBMCs were stimulated with immobilized anti-CD3 as described. At the indicated time points, T-helper cells of control samples and patient samples were identified by staining with anti-CD4 and gated; the density of expression of CD69 and of CD25 and of HLA-DR in unstimulated cultures (thin line) and stimulated cultures (thick line) was estimated by the mpc in histogram plots. Patient samples were obtained from a 12-year-old girl with Ewing sarcoma (patient 10) 1 month after completion of conventional high-dose chemotherapy.
Inability of exogenous IL-2 to overcome the impaired up-regulation of cellular activation markers induced by in vitro stimulation
One possible explanation for a defective T-cell activation is a lack of T-cell stimulating cytokines, particularly of IL-2, the production of which has been consistently observed to be reduced in patients having received TCD therapy.36,37 To examine a role of IL-2 on the impaired induction of cell-surface activation molecules, we explored the potential of exogenous IL-2 to restore the capability of T-helper cells to up-regulate CD69 and CD25 upon polyclonal stimulation. In an initial control experiment, we showed that, in line with previous experimental evidence,38 IL-2 induced CD69 on natural killer (NK) cells (CD3−CD56+) in a dose-dependent manner, the percent of CD69+ cells rising from 2% to 50% in the presence of 25 U/mL to 200 U/mL IL-2 at 24 hours of culture. However, essentially no change in CD69 cell-surface expression by CD4+ T cells was observed (Figure3A) comparing cultures stimulated with immobilized anti-CD3 only to stimulated cultures supplemented with 25 U/mL exogenous IL-2 (the increase of the mpc being 1.2-fold, 1.1-fold, and 0.9-fold at 4, 24, and 48 hours of culture, respectively; results obtained from patients 18-21). IL-2 (25 U/mL) slightly enhanced the density of expression of CD25 (Figure 3B) as indicated by a 1.6-fold (range, 1.2-fold to 1.6-fold) and 1.5 fold (range, 1.0-fold to 1.9 fold) increase of the mpc at 24 hours and 48 hours of culture, respectively.36 Increasing the dose of IL-2 from 25 U/mL to 100 U/mL did not further enhance CD25 expression nor affect CD69 expression by stimulated or unstimulated CD4+ T cells (not shown). Further, similar to a previous experience,37IL-2 did not alter the proliferative responses to immobilized anti-CD3 (the change of cpm ranging from 0.9-fold to 1.2-fold in both controls and patients, not shown).
A reduced induction of CD69 expression cannot be overcome by exogenous IL-2.
PBMCs were stimulated with immobilized anti-CD3 in the absence (dashed line) or presence (solid line) of exogenous IL-2 (25 U/mL) and analyzed by flow cytometry. The density of expression of CD69 (A) and of CD25 (B) was estimated by the mpc in histogram plots in control (○) and patient (■) CD4+ T cells from 4 to 48 hours of culture. Patient cells were obtained from a 12-year-old girl with ALL (patient 18) 3 months after MUD-BMT.
A reduced induction of CD69 expression cannot be overcome by exogenous IL-2.
PBMCs were stimulated with immobilized anti-CD3 in the absence (dashed line) or presence (solid line) of exogenous IL-2 (25 U/mL) and analyzed by flow cytometry. The density of expression of CD69 (A) and of CD25 (B) was estimated by the mpc in histogram plots in control (○) and patient (■) CD4+ T cells from 4 to 48 hours of culture. Patient cells were obtained from a 12-year-old girl with ALL (patient 18) 3 months after MUD-BMT.
Defective induction of CD69 in naive and memory T-helper cells
To determine whether the impaired induction of CD69 in T-helper cells differently affects the naive and memory T-helper cell subpopulation, CD4+CD45RA+ and CD4+CD45RA− populations were separately analyzed for their capability to up-regulate CD69. As the expression of the CD45RA and CD45RO epitope is mutually exclusive with the exception of a small double-positive population,18CD4+CD45RA− cells were classified to largely contain the CD4+CD45RO+ (memory) cell population. Initial studies confirmed that a stimulation with immobilized anti-CD3 or PHA for 24 hours did not change the proportions of CD4+CD45RA+ cells (not shown). As expected, in the patient samples tested within 3 months after TCD therapy, the proportions of CD4+CD45RA+ cells contained in CD4+ T cells were significantly smaller than in controls (20% median versus 68% median, patients versus controls;P < .0001, median 43/μL). Notably, however, the experiments contained samples from patients having undergone a total leukocyte depletion (WBC < 0.1/μL) as part of their recent treatment in whom the absolute count of CD4+CD45RA+ cells was more than 90/μL (patient 4, 109/μL; patient 14, 154/μL; patient 15, 126/μL; patient 16, 91/μL; patient 22, 138/μL), which indicates ongoing thymopoiesis.39
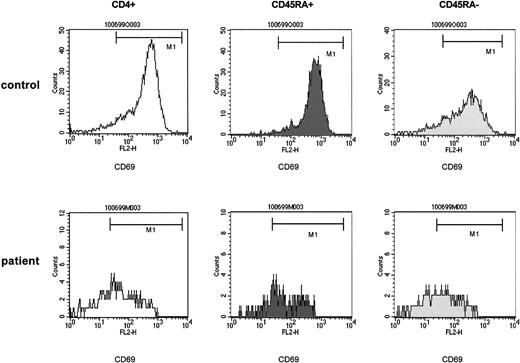
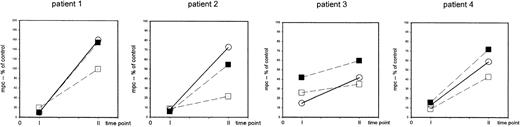
The striking finding of these analyses was that a defective induction of CD69 detectable in total CD4+ cells affected both the CD4+CD45RA+ and the CD4+CD45RA− populations to a similar extent (Figure 4). In fact, in 21 experiments (patients 1-17, 22), which comprised either T-cell–depleting treatment modality, the expression of CD69 by CD4+CD45RA+cells, assessed by the mpc in histograms at 24 hours after stimulation, showed a 98-fold (median) increase in control cells but only a 28-fold increase, median, in patient cells (P < .0001). Similarly, in the CD4+CD45RA− population the median increase in control versus patient cultures was 32-fold versus 10-fold, respectively (P < .001). Sequential analyses (Figure 5) performed in 4 patients before 3 months (time point 1) and between 6 and 9 months after TCD therapy (time point 2) showed that a recovery of the CD69 induction occurred in both the CD4+CD45RA+ and the CD4+CD45RA− populations consistent with the notion that naive and memory T cells were affected similarly.
The impaired capability of T-helper cells to be induced to express CD69 upon nonspecific stimulation affects both the naive (CD4+CD45RA+) and the memory (CD4+CD45RA−) population after T-cell depletion.
PBMCs of control samples (upper panels) and of patient samples (lower panels) were stimulated with immobilized anti-CD3. At 24 hours of culture, T-helper cells were identified by anti-CD4 and gated and assessed for expression of CD45RA. The CD45RA+ and the CD45RA− populations were gated and separately analyzed for the expression of CD69. The density of expression of CD69 was estimated by the mpc in histogram plots. Patient samples were obtained from a 5-year-old boy with stage IV Ewing sarcoma (patient 15) 3 months after high-dose chemotherapy with autologous stem cell rescue. CD4+CD45RA+ cells, 126/μL.
The impaired capability of T-helper cells to be induced to express CD69 upon nonspecific stimulation affects both the naive (CD4+CD45RA+) and the memory (CD4+CD45RA−) population after T-cell depletion.
PBMCs of control samples (upper panels) and of patient samples (lower panels) were stimulated with immobilized anti-CD3. At 24 hours of culture, T-helper cells were identified by anti-CD4 and gated and assessed for expression of CD45RA. The CD45RA+ and the CD45RA− populations were gated and separately analyzed for the expression of CD69. The density of expression of CD69 was estimated by the mpc in histogram plots. Patient samples were obtained from a 5-year-old boy with stage IV Ewing sarcoma (patient 15) 3 months after high-dose chemotherapy with autologous stem cell rescue. CD4+CD45RA+ cells, 126/μL.
The recovery of the capability of T-helper cells to be induced to express CD69 upon nonspecific stimulation occurs in both the naive (CD4+CD45RA+) and the memory (CD4+CD45RA−) population at 6 to 9 months after completion of TCD therapy.
PBMCs were stimulated with immobilized anti-CD3 or PHA (patient 1) and the density of expression of CD69 was assessed in CD4+cells (○) and in CD4+CD45RA+ cells (▪) and in CD4+CD45RA− cells (■) at 24 hours of culture. The mpc of CD69 expression in histogram plots is expressed as percent of control at 1 to 3 months (time point 1) and at 6 to 9 months (time point 2) after completion of TCD therapy. Patient 1: 8-year-old girl with stage IV neuroblastoma (patient 1), high-dose chemotherapy with autologous stem cell rescue; patient 2: 5-year-old boy with stage IV Ewing sarcoma (patient 15), high-dose chemotherapy with autologous stem cell rescue; patient 3: 12-year-old girl with Ewing sarcoma (patient 10), conventional high-dose chemotherapy; patient 4: 15-year-old girl with germ-cell tumor (patient 16), conventional high-dose chemotherapy.
The recovery of the capability of T-helper cells to be induced to express CD69 upon nonspecific stimulation occurs in both the naive (CD4+CD45RA+) and the memory (CD4+CD45RA−) population at 6 to 9 months after completion of TCD therapy.
PBMCs were stimulated with immobilized anti-CD3 or PHA (patient 1) and the density of expression of CD69 was assessed in CD4+cells (○) and in CD4+CD45RA+ cells (▪) and in CD4+CD45RA− cells (■) at 24 hours of culture. The mpc of CD69 expression in histogram plots is expressed as percent of control at 1 to 3 months (time point 1) and at 6 to 9 months (time point 2) after completion of TCD therapy. Patient 1: 8-year-old girl with stage IV neuroblastoma (patient 1), high-dose chemotherapy with autologous stem cell rescue; patient 2: 5-year-old boy with stage IV Ewing sarcoma (patient 15), high-dose chemotherapy with autologous stem cell rescue; patient 3: 12-year-old girl with Ewing sarcoma (patient 10), conventional high-dose chemotherapy; patient 4: 15-year-old girl with germ-cell tumor (patient 16), conventional high-dose chemotherapy.
Increased binding of annexin V by naive and memory T-helper cells
A recently proposed mechanism of the impaired immune function after chemotherapy is an increased susceptibility to apoptosis of T cells preferentially detectable in cells with an activated/memory phenotype.28,40 41 To test whether such increased susceptibility to apoptosis similar to an impaired induction of cellular activation also affects T-helper cells with a naive phenotype, a series of 12 experiments (patients 9-17) examined the binding of annexin V by flow cytometry to CD4+CD45RA+ and to CD4+CD45RA− cell populations after TCD therapy, in addition to the above-described assessment of (1) [3H]thymidine incorporation (reduced by 71% median) and (2) CD69 up-regulation by CD4+ T cells (reduced by 88% median) upon anti-CD3 stimulation. The proportion of CD4+ T cells was reduced (22% median versus 51% median, patients versus controls).
The proportion of annexin V binding cells in resting control CD4+ T cells at 24 hours of culture was 6% median (range, 4%-10%), and in patient cells it was 25% median (range, 6%-56%;P < .0001). A similar difference was found in anti-CD3–stimulated CD4+ T cells (P < .001). To determine annexin V binding of the naive T-helper cell population, CD4+ T cells were separated into a CD45RA+ and a CD45RA− population by fluorescence-activated cell sorting (FACS) as described in “Patients, materials, and methods.” In the patient samples the proportion of CD4+CD45RA+ cells was reduced (17% median) as compared with controls (58% median). Strikingly, resting CD4+CD45RA+ cells (Figure6) of patient samples showed an increased binding of annexin V (13% median [range, 12%-74%]), as compared with control samples42 (5% median [range, 3%-8%]) (P < .01). In stimulated cultures, patient samples contained 36% median (range, 8%-78%) and control samples only 11% median (range, 6%-27%) annexin V binding CD4+CD45RA+ cells (P < .007). The increase of annexin V binding cells upon stimulation was similar in patient cultures versus control cultures (3.6-fold median and 3.5-fold median, respectively). As well, patient CD4+CD45RA− populations (Figure 6) showed a significantly higher proportion of annexin V binding cells in both unstimulated and stimulated cultures (P < .016 andP < .035, respectively). In 2 patients examined sequentially (patients 15 and 16) the proportion of annexin V binding CD4+ T cells and CD4+CD45RA+ cells tended to normalize at 6 months after completion of therapy (not shown). Exogenous IL-2 (25 U/mL to 100 U/mL) did not modulate the proportion of annexin V binding cells in patient cultures (Table 2).
In the post–T-cell depletion period the increased susceptibility to apoptosis affects both the naive (CD4+ CD45RA+) and the memory (CD4+ CD45RA−) T-helper cell population.
PBMCs of control samples (upper panels) and of patient samples (lower panels) were cultured in medium without the presence of T-cell stimulants (resting cultures) as described. At 24 hours of culture, T-helper cells were identified by anti-CD4 and gated and assessed for expression of CD45RA. The CD4+CD45RA+ and the CD4+CD45RA− populations were gated and separately analyzed for binding of annexin V. The percent annexin V binding cells were as follows: control CD4+ cells, 4%; control CD4+ CD45RA+ cells, 1%; control CD4+CD45RA− cells, 7%; patient CD4+ cells, 19%; patient CD4+CD45RA+ cells, 18%; patient CD4+ CD45RA− cells, 19%. The patient cells were obtained from a 12-year-old girl with Ewing sarcoma (patient 10) 3 months after completion of conventional high-dose chemotherapy. CD4+CD45RA+ cells, 60/μL.
In the post–T-cell depletion period the increased susceptibility to apoptosis affects both the naive (CD4+ CD45RA+) and the memory (CD4+ CD45RA−) T-helper cell population.
PBMCs of control samples (upper panels) and of patient samples (lower panels) were cultured in medium without the presence of T-cell stimulants (resting cultures) as described. At 24 hours of culture, T-helper cells were identified by anti-CD4 and gated and assessed for expression of CD45RA. The CD4+CD45RA+ and the CD4+CD45RA− populations were gated and separately analyzed for binding of annexin V. The percent annexin V binding cells were as follows: control CD4+ cells, 4%; control CD4+ CD45RA+ cells, 1%; control CD4+CD45RA− cells, 7%; patient CD4+ cells, 19%; patient CD4+CD45RA+ cells, 18%; patient CD4+ CD45RA− cells, 19%. The patient cells were obtained from a 12-year-old girl with Ewing sarcoma (patient 10) 3 months after completion of conventional high-dose chemotherapy. CD4+CD45RA+ cells, 60/μL.
Effect of exogenous interleukin 2 on the susceptibility of CD4+ cells to apoptosis detected by annexin V staining
| Time of culture . | Annexin V–positive CD4+ cells (%) by time of culture . | ||
|---|---|---|---|
| 24 h . | 48 h . | 72 h . | |
| Control | |||
| No antigen | 6 | 2 | 2 |
| Anti-CD3 | 2 | 10 | 6 |
| Anti-CD3/IL-2 | 2 | 8 | 6 |
| Patient 1 | |||
| No antigen | 10 | 5 | 11 |
| Anti-CD3 | 22 | 20 | 21 |
| Anti-CD3/IL-2 | 24 | 16 | 20 |
| Patient 2 | |||
| No antigen | 2 | 3 | nd |
| Anti-CD3 | 2 | 9 | nd |
| Anti-CD3/IL-2 | 3 | 10 | nd |
| Patient 3 | |||
| No antigen | 2 | 4 | nd |
| Anti-CD3 | 3 | 18 | nd |
| Anti-CD3/IL-2 | 8 | 25 | nd |
| Time of culture . | Annexin V–positive CD4+ cells (%) by time of culture . | ||
|---|---|---|---|
| 24 h . | 48 h . | 72 h . | |
| Control | |||
| No antigen | 6 | 2 | 2 |
| Anti-CD3 | 2 | 10 | 6 |
| Anti-CD3/IL-2 | 2 | 8 | 6 |
| Patient 1 | |||
| No antigen | 10 | 5 | 11 |
| Anti-CD3 | 22 | 20 | 21 |
| Anti-CD3/IL-2 | 24 | 16 | 20 |
| Patient 2 | |||
| No antigen | 2 | 3 | nd |
| Anti-CD3 | 2 | 9 | nd |
| Anti-CD3/IL-2 | 3 | 10 | nd |
| Patient 3 | |||
| No antigen | 2 | 4 | nd |
| Anti-CD3 | 3 | 18 | nd |
| Anti-CD3/IL-2 | 8 | 25 | nd |
Patient 1: 12-year-old girl, 1 month after MUD-BMT; patient 2: 18-year-old girl, 1 month after CTX; patient 3: 6-month-old boy, 1 month after HD-CTX/ASCR. PBMCs were cultured and analyzed by FACS as described. Results are shown for interleukin 2 added to stimulated cultures at 25 U/mL.
nd indicates not done. See Table 1 for other abbreviations.
Improved stimulatory response of highly enriched T-cell populations
Based on the above findings it was tempting to speculate that the suppressed stimulatory response affecting naive and memory T-cell populations examined in bulk PBMCs might be mediated by T-cell extrinsic factors. To address this question, we compared the stimulatory responses to immobilized anti-CD3 of PBMCs to those of highly enriched T cells. Indeed, whereas the in vitro response to anti-CD3 examined in total PBMCs obtained from patients after TCD therapy was reduced (patients 17, 20, and 22), highly enriched T cells showed a markedly improved response evident by both an improved proliferative response and an improved up-regulation of CD69 of total CD4+ T cells including CD4+CD45RA+cells (Figure 7).
Enriching T cells from PBMCs results in a substantially improved stimulatory response to anti-CD3, evident by an increased proliferative response and improved up-regulation of CD69 by CD4+ T cells and CD4+CD45RA+ cells.
PBMCs and highly enriched T cells (see “Patients, materials, and methods”) of control samples and patient samples were stimulated with immobilized anti-CD3 comparing (A) the proliferative response measured by [3H]thymidine uptake (1.0 × 105cells/well) (left panel); blank bars, control cells; black bars, patient cells; the bars represent the cpm (mean ± SEM) of triplicate cultures; (B) the capacity to up-regulate CD69 after 24 hours of culture (right panels). CD4+ T cells were identified by anti-CD4 and gated and assessed for expression of CD45RA. The density of expression of CD69 was estimated by the mpc in histogram plots. Thin line indicates control cells; thick line, patient cells. Samples were obtained from an 8-year-old boy with thalassemia major (patient 22) 6 months after allo-BMT. CD4+CD45RA+ cells, 138/μL.
Enriching T cells from PBMCs results in a substantially improved stimulatory response to anti-CD3, evident by an increased proliferative response and improved up-regulation of CD69 by CD4+ T cells and CD4+CD45RA+ cells.
PBMCs and highly enriched T cells (see “Patients, materials, and methods”) of control samples and patient samples were stimulated with immobilized anti-CD3 comparing (A) the proliferative response measured by [3H]thymidine uptake (1.0 × 105cells/well) (left panel); blank bars, control cells; black bars, patient cells; the bars represent the cpm (mean ± SEM) of triplicate cultures; (B) the capacity to up-regulate CD69 after 24 hours of culture (right panels). CD4+ T cells were identified by anti-CD4 and gated and assessed for expression of CD45RA. The density of expression of CD69 was estimated by the mpc in histogram plots. Thin line indicates control cells; thick line, patient cells. Samples were obtained from an 8-year-old boy with thalassemia major (patient 22) 6 months after allo-BMT. CD4+CD45RA+ cells, 138/μL.
Discussion
The present findings of an impaired induction of CD69 upon in vitro stimulation and an increased binding of annexin V affecting T-helper cells, including the naive (CD4+CD45RA+) subset after TCD therapy, bear several implications relevant to the as-yet-incomplete understanding of an impaired T-cell function after cytotoxic anticancer therapy. A reduced up-regulation of CD69 was detectable as early as 4 hours of culture and was more pronounced as the concomitantly observed reduced up-regulation of CD2543 or of HLA-DR. The extent of CD69 induction, similar to observations in healthy controls35,44 and individuals with HIV infection,45,46 also in our study correlated with lymphoproliferation, which has been routinely used to measure T-cell function after TCD therapy.34 In normal human T cells stimulated via the TCR/CD3 complex, the expression of CD69 is rapidly induced; transcripts of CD69 can be identified even 30 minutes after stimulation.47 Activated Ras plays a central role for cell-surface expression,48 which reaches a maximum at 18 to 24 hours after stimulation.49,50 Although the function of CD69 has not been ultimately defined, cross-linking studies have demonstrated a role for CD69 in regulating the production of T-cell activating cytokines such as IL-251 as well as the IL-2 receptor (CD25),52 interferon γ, and tumor necrosis factor-α.53
Among other alterations of the cytokine network observed after TCD therapy,3,54 a decrease of IL-2 production36,37,55 as well as of transcription examined on a single-cell basis56 may be of particular relevance to the disturbed T-cell function. In the present study, however, exogenous IL-2 when added to anti-CD3–stimulated cultures (1) failed to improve the reduced proliferative response similar to a previous observation,37 and (2) failed to restore the impaired capability of post–TCD therapy CD4+ T cells to generate normal levels of CD69 cell-surface expression. This finding is in agreement with recent experimental evidence showing that even high doses (1000 U/mL) of exogenous IL-2 were not able to induce CD69 in naive human T cells.57 Thus, since after TCD therapy the induction of CD69 expression—which is regulated by early T-cell signaling events—after stimulation with immobilized anti-CD3 is decreased and inaccessible to a rescue by exogeneous IL-2, it is conceivable that the functional T-cell defect involves early steps of the T-cell activation cascade. Support for this concept comes from a recent study showing a reduced activation of the mitogen-activated protein (MAP) kinase in T cells after BMT.58
Of note, immobilized anti-CD3, which was preferentially used as a stimulant, activates T-helper cells independently of their antigenic determination. Thus, a reduced responsiveness of T cells reflects a functional deficit independent of the repertoire, which has been shown to be frequently limited in T-helper cells in the post–TCD therapy period.25-27 Further, because of the heterogeneity of the patients (Table 1), it appears unlikely that the observed effects are related to the underlying disease or the treatment. Likewise, a recent or concurrent treatment with cyclosporine A or granulocyte colony-stimulating factor (G-CSF), both drugs being used in patients undergoing transplantation (Table 1) and known to be correlated with an altered T-cell function,59-62 apparently was not the cause of the observed T-cell defect, per se, as the defect was similar in patients having received high-dose chemotherapy alone.
Most striking was the finding that the T-cell dysfunction of the early post–TCD therapy period did not spare T cells bearing a naive phenotype. A recent exposure to the toxic effects of anticancer chemotherapy as an explanation would implicate that naive T cells had expanded by peripheral pathways of regeneration. Yet, as to the accumulated evidence, such thymus-independent expansion of CD4+CD45RA+ T cells after high-dose chemotherapy or BMT is extremely limited.22,39 Whereas such a thymus-independent regeneration of naive T cells cannot be definitively excluded in patients in whom at the time point of examination the number of circulating CD4+CD45RA+ cells was low, a cell count of more than 90/μL of circulating CD4+CD45RA+ cells, as was found in 5 patients in the present study, conceivably indicated an expansion by use of a thymopoietic pathway.39 As these patients also had an impaired in vitro T-cell immune function, the observed effects extended to the thymus-dependently regenerated T-helper cell population. Thus, while recent evidence suggests that an effective reconstitution of naive T cells coincided with (1) a normalization of the apoptotic decline,41,63 (2) the reconstitution of immune reactivity and responses to revaccinations,64 and (3) the restoration of a diverse T-cell repertoire,65,66 the present study provides evidence that an impaired T-cell function after TCD therapy is not simply explained by a lack or a delay of thymus-dependent T-cell reconstitution. This view is in line with the findings that (1) a decreased bcl-2 level, which is supposed to reflect an increased susceptibility to apoptosis of post–TCD therapy T cells, was demonstrated even in CD4+CD45RA+cells,40 and (2) a sufficient immune function can be restored even in case of an extremely limited regeneration of naive T-helper cells,22 possibly by transfer of long-lived antigen-experienced cells.67 Rather, our findings suggest that the immunocompetence of regenerated T cells is impaired independently of the source of recruitment. Support for this conclusion comes from the finding that highly enriched T cells showed a markedly improved response of total CD4+ as well as of CD4+CD45RA+ cells to anti-CD3–induced stimulation as compared with bulk PBMCs. This finding warrants future research to explore potential suppressor effects of cell populations contained in the non–T-cell fraction of PBMCs. To this point, recent studies demonstrated a suppressor capacity of monocytes derived from G-CSF–stimulated leukapheresis products68-70 that might involve Fas-Fas ligand–mediated apoptosis.71 Similarly, initial experiments of our own continuing research has shown that a coculture of highly enriched T cells with autologous monocytes reduced T-cell proliferative responses to immobilized anti-CD3 in a dose-dependent fashion in patient samples while having no effect in control cultures (study in progress).
Regardless of the final mechanism, the finding that a T-cell dysfunction in the post–TCD therapy period may be mediated by T-cell extrinsic factors, thus eventually involving multiple T-cell populations, might have implications in the setting of adoptive cell therapy to treat viral infections after BMT72 or to eradicate minimal residual disease in cancer patients73 as the efficacy of transferred T cells may be inhibited by the same suppressive factor(s) that inhibit the function of T cells generated by the host. Such a phenomenon has been described in HIV disease in which transferred T cells became susceptible to apoptosis, similar to host T cells.74 Alternatively, however, a modulation of immunosuppression generated by the host may be useful in the management of graft-versus-host disease and tolerance induction in transplant settings.68
Together, future efforts to overcome or manipulate the functional impairment of T cells after TCD therapy will have to not only attempt to accelerate thymopoiesis (eg, by interleukin-7),75 76 but to identify the factor(s) that affect T-helper cells even when presumably being regenerated through a thymus-dependent pathway.
The authors wish to thank Hannelore Kern and Doris Vergeiner for excellent technical assistance.
Supported by a research grant of the Children's Cancer Research Institute, St Anna Children's Hospital, Vienna, Austria (A.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas Heitger, St Anna Children's Hospital, Kinderspitalgasse 6, A-1090 Vienna, Austria; e-mail:andreas.heitger@uibk.ac.at.



![Fig. 7. Enriching T cells from PBMCs results in a substantially improved stimulatory response to anti-CD3, evident by an increased proliferative response and improved up-regulation of CD69 by CD4+ T cells and CD4+CD45RA+ cells. / PBMCs and highly enriched T cells (see “Patients, materials, and methods”) of control samples and patient samples were stimulated with immobilized anti-CD3 comparing (A) the proliferative response measured by [3H]thymidine uptake (1.0 × 105cells/well) (left panel); blank bars, control cells; black bars, patient cells; the bars represent the cpm (mean ± SEM) of triplicate cultures; (B) the capacity to up-regulate CD69 after 24 hours of culture (right panels). CD4+ T cells were identified by anti-CD4 and gated and assessed for expression of CD45RA. The density of expression of CD69 was estimated by the mpc in histogram plots. Thin line indicates control cells; thick line, patient cells. Samples were obtained from an 8-year-old boy with thalassemia major (patient 22) 6 months after allo-BMT. CD4+CD45RA+ cells, 138/μL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/11/10.1182_blood.v99.11.4053/6/m_h81122607007.jpeg?Expires=1769110044&Signature=4md3HZQ3MqNBRgJe1q0tOwTO-GpwUVajfVt9g2zstCH1Sd7jXdlAij7hUjqeTsnGLTwM06X9d2hhjMCIfnq~tWprjewTuXU~PIGeWDmKLCgRJdzYnEaEGijTgazwZ1FuQgk5yDViiNba98UjGwAvLprQdfTU~RKizOTbKf5CaFt952qChd59H0pifec5vlQYOM-AoQPgonxDMyy3KN1A1ON-9UK0rs9HLoqdqs2hWIgdLwMnQ-WTih7HEnyHvIDCPLAZLpwEe1Dj6ocXVCNlap6lhMPuqYQidTVcHFrjgT-OXn6lAqZW4GmirNYRTBFH1Wy4HZcIDzBJ6SkhV75A~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal