We have developed a new helper-dependent (HD) adenoviral vector FTC that contains 3 cis-acting sequences as stuffer DNA: a human fragment of alphoid repeat DNA, matrix-attachment regions (MARs), and the hepatocyte control region enhancer. To determine the most robust human coagulation factor IX (hFIX) expression cassette in an adenovirus, we first tested different hFIX expression sequences with or without flanking MARs in first-generation adenoviral vectors. After intravenous infusion of the vector, serum levels of up to 100 000 ng/mL hFIX (normal level, 5000 ng/mL) were obtained at nontoxic doses. In order to make a direct comparison, a first-generation and a gene-deleted vector with identical hFIX expression cassettes were constructed. Both first-generation and HD adenovirus–treated animals demonstrated a threshold effect in a dose-response study. With the administration of 2 × 109transducing particles of either vector, supraphysiological serum levels of hFIX were obtained, with the highest expression (41 000 ng/mL) occurring during the first 2 months after injection. The serum factor IX concentrations, while remaining in the therapeutic range, slowly declined by 95% over a period of 1 year. At this dose, interleukin-6 and tumor necrosis factor–α serum concentrations were elevated in animals that received the first-generation but not the HD vector. This study compares the properties of a gene-deleted and first-generation adenovirus with equivalent expression cassettes and suggests that thecis-DNA elements contained in the vector and expression cassette have important effects on gene expression in vivo.
Introduction
Adenoviral vectors lacking all viral genes are a promising tool for safe and efficient gene transfer in vitro and in vivo.1-3 Adenovirus has several advantages over other viral-based gene therapy approaches, including the ability to produce high titers, efficient infection of a broad range of cell types, and the ability to infect dividing and nondividing cells.4-7However, further development of adenoviral vectors is necessary to make them safer and more efficient. First-generation E1-deficient adenoviruses show toxic effects owing to the production of immunogenic viral proteins. The Cre-loxP helper-dependent (HD) system was developed to generate recombinant adenoviruses in which all viral coding sequences have been deleted.8,9 The helper virus used in this system provides all adenoviral gene products required for replication and packaging of the vector to be supplied in trans. The packaging signal of these helper viruses is flanked by loxP sites to inhibit packaging of the helper-virus genome in stable Cre recombinase–expressing cells. Recombinant adenovirus produced by the Cre-loxP HD system allows for transfer of up to 35 kilobases (kb) DNA with long-term expression in rodents.1,2 Recent studies suggest that the stuffer DNA used for HD vectors may play a major role in production and persistence of transgene expression. HD vectors with stuffer DNA of prokaryotic origin result in transient transgene expression.10Additionally, it was demonstrated that the nature of the stuffer DNA cloned into the HD vector affects its replication during production and the expression levels of the transgene after transduction in vivo.11
The new HD vector presented herein contains a matrix-attachment region (MAR) and a centromeric fragment as “stuffer” DNA. MARs are AT-rich noncoding DNA sequences that often contain topoisomerase II cleavage sites. MARs are believed to be involved in chromatin loop formation by anchoring the DNA to the nuclear matrix, a protein network in the nucleus. Furthermore, MARs may function as insulators for single-copy expression cassettes12 and are capable of increasing expression levels from a transgene.13 The centromeric fragment in our high-capacity vector was a 16.2-kb alphoid repeat DNA fragment from human chromosome 17, which was shown to function as an origin of replication in vitro.14 15
First-generation adenoviruses with different human coagulation factor IX (hFIX) expression cassettes were previously generated, and expression levels from 1 to 5 μg/mL were detected in vivo.16-18 The hFIX expression cassettes selected for this study were based on our recent study using a naked DNA liver delivery approach,19 which demonstrated that expression cassettes containing the hFIX minigene, the hepatocyte control region (HCR), and the apolipoprotein E (ApoE) enhancer as cis-DNA elements show persistent expression at levels of up to 1 μg/mL hFIX in vivo. Therefore, we decided to test these robust expression cassettes in the context of adenoviral vectors. Importantly, while higher levels of gene expression have been demonstrated when an HD vector is used in place of a first-generation vector,1 3 none of these studies directly compare the same expression cassette in both vector systems.
Materials and methods
Construction of plasmid pAdFTC and pAdFTC/hFIX
The pSP72-based (Promega, Madison, WI) plasmid p72BgN5′/3′iso containing the 5′ITR (left inverted terminal repeated of adenovirus type 5, nucleotides [nt] 1-452) and 3′ITR (right inverted terminal repeated of adenovirus type 5, nt 35 796-35 935) was constructed. The HpaI/SacI fragment from p72BgN5′/3′iso containing the 5′ITR was cloned into theHpa/Sac site of the vector pSP72, resulting in p72N5′ITR. The immunoglobulin κ MAR (IgκMAR) was amplified by polymerase chain reaction (PCR) and cloned into the SrfI site of pPCR by using the PCR-Script Amp Cloning Kit (Stratagene, La Jolla, CA). Two copies of the IgκMAR from pPCRMAR were cloned as SacI fragments into the SacI site of p72N5′ITR, resulting in p72N5′ITRIgκMAR. TheBglII/NotI fragment from p72N5′ITRIgκMAR was cloned into the BamHI/NotI site of pBSNheI, in which the KpnI site was changed to an NheI site. The NheI site was changed to a SalI site, resulting in p72N5′ITR IgκMAR/SalI. The 3′ITRSphI fragment from p72BgN5′/3′iso was cloned into theSphI site of pPCR, and a 58-nt multiple cloning site (MCS) was cloned into the AatII site containing the recognition sites for RsrII, BsiwI, MluI,PmeI, PacI, NheI, and AscI. Two copies of the HCR were added into the NheI site of pPCR3′ITRMCS, resulting in pPCR3′ITRMCSHCR. The SphI fragment containing the 3′ITR, MCS, and HCR was cloned into theSphI site of pHM5loxSpeI, and the I-CeuI site changed to an SpeI site, resulting in pHM5 3′ITRMCSHCR.
The plasmid adenoviral vector FTC (pAdFTC) for adenoviral production is based on the plasmid pDYAL containing a 16.2-kb fragment of alphoid repeat DNA from human chromosome 17.15 The alphoid repeat DNA was flanked by a 4.2-kb fragment containing the left terminus of adenovirus type 5 and 2 copies of the IgκMAR. The subclone pDYAL5′ITRIgκMAR was obtained by cloning the SalI fragment from p72N5′ITRIgκMAR/SalI into theSalI site of pDYAL. The 1.2-kb SpeI fragment from pHM5 3′ITRMCSHCR containing the HCR, an MCS with recognition sites for the restriction endonucleases PacI and PmeI, and the right terminus of adenovirus type 5 was cloned into theSpeI site of pDYAL5′ITRIgκMAR, resulting in pAdFTC.
A shuttle plasmid pBS-P/P, based on pBSK/S (Stratagene) was constructed with an MCS between a PacI and a PmeI recognition site by changing the KpnI site of pBSK/S to PacI and the SacI site to PmeI. The SpeI human FIX minigene fragment from pBSh7hF9mgbpA19 was cloned into the SpeI site of pBS-P/P. ThePacI/PmeI hFIX fragment was cloned into thePacI/PmeI site of AdFTC, resulting in pAdFTC/hFIX.
Production of high-capacity adenoviruses using the HD vector AdFTC
The plasmid pAdFTC/hFIX was digested with NotI to release the backbone of pAdFTC/hFIX and transfected with Superfect (Gibco BRL, Rockville, MD) into C7-Cre cells.20 At 18 hours after transfection, cells were infected with a first-generation helper virus containing a packaging signal flanked by lox sites.20 To increase the titer of the HD vector AdFTC, 5 serial passages in C7-Cre cells were performed. To determine the amount of helper-virus particles, aliquots of the crude lysate were used to infect EcR-293 cells (Invitrogen, Carlsbad, CA). The helper virus expresses alkaline phosphatase when induced from the ecdysone promoter and can thus be titered at each passage with alkaline-phosphatase staining. The virions containing the HD-vector genome were purified away from the helper virus by CsCl gradients. After 1 CsCl step gradient and 2 equilibrium gradients, the HD vector can be purified at high titer (5 × 1010transducing particles per milliliter), with helper virus contamination lower than 0.5% as determined by alkaline-phosphatase staining.
Titering of HD vectors
The number of transducing particles of AdFTC/hFIX in HD vector preparations was determined as followed: Hela cells were infected with different volumes of the HD vector preparation. For comparison, Hela cells were infected at different defined multiplicities of infection (MOIs) with the first-generation adenovirus hFIX (fgAdhFIX) with the same expression cassette to generate a standard curve. Cells were incubated for 3 hours, and the genomic DNA was isolated; this was followed by a Southern blot probed with an hFIX complementary DNA (cDNA) probe (HindIII/EcoRI fragment from pAAVCM2). The intensity of the bands for the HD vector was compared with the standard curve.
Generation of first-generation adenoviruses
The BamHI site of pHM5 was changed toSpeI, resulting in pHM5SpeI.21 The PstI IgκMAR fragment from pPCRMAR was cloned into pHM5, and theXbaI chicken lysozyme MAR (ChMAR) fragment from pBS-2x(B-1-X1)13 was ligated into the XbaI site of pHM5. The SpeI fragment from pBSh7hF9mgbpA was cloned into the XbaI site of pHM5, and the I-CeuI/PI-SceI fragment containing the hFIX minigene was cloned into the I-CeuI/PI-SceI site of pAdHM4. First-generation adenoviruses were produced and amplified as previously described.21
Isolation of primary hepatocytes
Primary hepatocytes were isolated as described.22In brief, primary mouse hepatocytes were obtained by collagenase perfusion, and hepatocytes with greater than 90% viability were plated at a density of 6 × 105 on 6-well dishes for the first 6 hours in Dulbecco modified Eagle medium with 10% fetal calf serum and afterward in Williams E medium with 10% fetal calf serum.
Blood analysis
Mouse serum hFIX antigen levels were determined by enzyme-linked immunosorbent assay (ELISA) assay.23 The normal human serum level was 5000 ng/mL. Serum glutamic-pyruvic transaminase (SGPT) (alanine aminotransferase activity) assays were performed by using a diagnostic kit for colorimetric determination of SGPT (Sigma [St Louis, MO] procedure no. 505-OP). To measure the serum levels of interleukin 6 (IL-6) and tumor necrosis factor–α (TNF-α), Pharmingen's BD OptEIA human IL-6 ELISA kit (catalog no. 555 240) and Pharmingen's BD OptEIA human TNF-α ELISA kit (catalog no. 555 268) were used (San Diego, CA).
Animal studies
Mice were injected via tail vein with different amounts of adenoviral vector diluted in phosphate-buffered saline (PBS). To permit comparisons between the present study and those already published, Table 1 converts the number of transducing units per mouse to the total amount of transducing units and viral particles per kilogram body weight.
Summary of vector injections in mice
| MOIs per mouse* . | Total no. transducing units per mouse . | Total viral particle no. per kg body weight . | Total no. transducing units per kg body weight . |
|---|---|---|---|
| 20 | 2 × 109 | 1.8 × 1011 | 9.1 × 1010 |
| 10 | 1 × 109 | 9.1 × 1010 | 4.5 × 1010 |
| 5 | 5 × 108 | 4.5 × 1010 | 2.3 × 1010 |
| 1 | 1 × 108 | 9.1 × 109 | 4.5 × 109 |
| MOIs per mouse* . | Total no. transducing units per mouse . | Total viral particle no. per kg body weight . | Total no. transducing units per kg body weight . |
|---|---|---|---|
| 20 | 2 × 109 | 1.8 × 1011 | 9.1 × 1010 |
| 10 | 1 × 109 | 9.1 × 1010 | 4.5 × 1010 |
| 5 | 5 × 108 | 4.5 × 1010 | 2.3 × 1010 |
| 1 | 1 × 108 | 9.1 × 109 | 4.5 × 109 |
For comparison, the amount of transducing units per mouse, total amount of transducing units, and viral particles per kilogram body weight are shown.
MOIs indicates multiplicities of infection.
For all experiments, mice at the age of 8 weeks were used. It is assumed that a mouse liver at the age of 8 weeks contained approximately 1 × 108 hepatocytes.
Phenotypic correction studies
C57Bl/6 hemophilia B mice were used.24 Three mice per group were injected with 1 × 109, 5 × 108, and 1 × 108 transducing units of AdFTC/hFIX or fgAdhFIX. On day 7, a 0.5-cm section of the tail was clipped from each mouse to measure the bleeding time (time until bleeding stopped).
Southern blotting
For genomic DNA isolation, the liver was removed and homogenized, and a 100-mg portion was used for DNA extraction as previously described.25 26 Then, 20 μg genomic DNA was digested with HindIII, run on a 0.8% agarose gel, and electrotransferred to a Hybond membrane (Amersham, Buckinghamshire, United Kingdom). The blots were hybridized with an α-32]–deoxycytidine triphosphate (dCTP)–labeled cDNA hFIX probe (HindIII/EcoRI fragment from pAAVCM2), by means of a random priming kit (Stratagene).
Results
Different hFIX expression cassettes and cis-DNA elements in first-generation adenoviruses
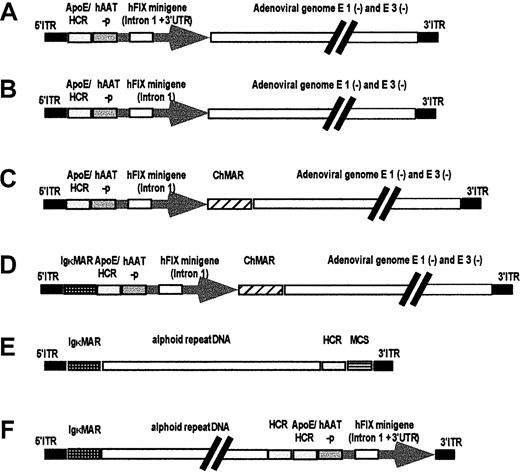
In earlier studies, we compared different hFIX expression cassettes by in vivo hydrodynamic delivery of naked plasmid DNAs into mouse liver.27 The 2 most robust expression cassettes contained the ApoE enhancer and HCR, the human alpha antitrypsin promoter (hAAT-p), and the hFIX minigene (which includes a portion of the first hFIX intron and the bovine growth hormone gene polyadenylation signal) with or without the 3′ untranslated region (Figure 1A-B) and were subsequently used in the current studies.
Constructs for generation of first-generation and HD vectors used in this study.
Sequences used for the production of first-generation adenoviral vectors are based on the plasmid pAdHM4,21 and 2 hFIX expression cassettes, with (A) and without (B) the 3′ untranslated region (3′UTR). The hFIX expression cassette shown in panel B was flanked by 1 or 2 MARs-ChMAR and IgκMAR, (C-D). The structure of the vectors AdFTC and AdFTC/hFIX for gutless adenoviral production are shown in panels E and F. The plasmid pAdFTC is based on the plasmid pDYAL containing a 16.2-kb fragment of alphoid repeat DNA from human chromosome 17. The alphoid repeat DNA is flanked by a 4.2-kb fragment containing the left terminus of adenovirus type 5 (nt 1-452), 2 copies of the Igκ MAR, and a 1.2-kb fragment containing the HCR, an MCS with recognition sites for the restriction endonucleasesPacI and PmeI and the right terminus of adenovirus type 5 (nt 35 796-35 935).
Constructs for generation of first-generation and HD vectors used in this study.
Sequences used for the production of first-generation adenoviral vectors are based on the plasmid pAdHM4,21 and 2 hFIX expression cassettes, with (A) and without (B) the 3′ untranslated region (3′UTR). The hFIX expression cassette shown in panel B was flanked by 1 or 2 MARs-ChMAR and IgκMAR, (C-D). The structure of the vectors AdFTC and AdFTC/hFIX for gutless adenoviral production are shown in panels E and F. The plasmid pAdFTC is based on the plasmid pDYAL containing a 16.2-kb fragment of alphoid repeat DNA from human chromosome 17. The alphoid repeat DNA is flanked by a 4.2-kb fragment containing the left terminus of adenovirus type 5 (nt 1-452), 2 copies of the Igκ MAR, and a 1.2-kb fragment containing the HCR, an MCS with recognition sites for the restriction endonucleasesPacI and PmeI and the right terminus of adenovirus type 5 (nt 35 796-35 935).
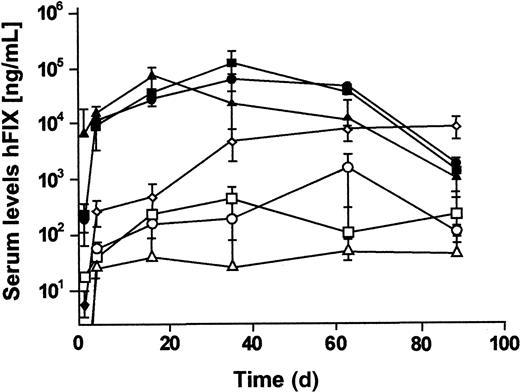
To determine if a MAR would affect gene expression, we constructed different vectors containing the hFIX expression cassette with or without the 3′ untranslated region, and variations of 2 well-characterized MARs: the chicken lysozyme 5′MAR13 and the IgκMAR28 (Figure 1C-D). The first-generation adenoviruses were injected into the tail vein of C57Bl/6 mice. At an MOI of 20 (2 × 109 transducing particles), serum hFIX levels of 20 000 to 100 000 ng/mL were detected during the first 9 weeks after injection for all vectors (Figure2). This level of gene expression was 4 to 20 times higher than that previously achieved in studies using first-generation hFIX expressing adenoviral vectors.16-18 29 Interestingly, the vector with the hFIX minigene containing the 3′ untranslated region resulted in persistent hFIX expression for more than 9 weeks, whereas all other vectors showed a 40-fold decline in hFIX expression 11 weeks after injection. However, therapeutic levels of serum factor IX (200 to 1000 ng/mL) were maintained with all the vectors during the same period. At the lowest MOI of 0.2 (2 × 107 transducing units), no expression was detected for any of the vectors (not shown). No significant differences in hFIX serum levels were observed when adenoviruses with and without MARs were used. No acute liver toxicity was observed by serum alanine aminotransferase activity (SGPT) performed 1 day after injection (Table 2).
Human FIX expression in C57Bl/6 mice after injection of the recombinant first-generation adenoviral vectors.
Mice were injected with first-generation adenoviral vectors shown in Figure 1A-D. At different time points, blood samples were collected and analyzed by ELISA for hFIX expression. For the first-generation adenovirus shown in Figure 1A, an MOI of 20 was injected (♦). For the first-generation adenoviruses shown in Figure 1B (○ and ●), Figure1C (■ and ▪), and Figure 1D (▵ and ▴), an MOI of 2 (■/ ○/ ▵) and an MOI of 20 (●/▪/▴) was injected. Mean ± SD is shown (n = 3 per group).
Human FIX expression in C57Bl/6 mice after injection of the recombinant first-generation adenoviral vectors.
Mice were injected with first-generation adenoviral vectors shown in Figure 1A-D. At different time points, blood samples were collected and analyzed by ELISA for hFIX expression. For the first-generation adenovirus shown in Figure 1A, an MOI of 20 was injected (♦). For the first-generation adenoviruses shown in Figure 1B (○ and ●), Figure1C (■ and ▪), and Figure 1D (▵ and ▴), an MOI of 2 (■/ ○/ ▵) and an MOI of 20 (●/▪/▴) was injected. Mean ± SD is shown (n = 3 per group).
Serum glutamic-pyruvic transaminase levels in mice receiving first-generation adenovirus with different human factor IX expression cassettes 1 day after injection
| Injected transducing particles . | SGPT levels by virus, day 1 . | |||
|---|---|---|---|---|
| FgAdhFIXs, U/mL . | FgAdhFIXs ChMAR, U/mL . | FgAdhFIXs ChMAR IgκMAR, U/mL . | Negative control, U/mL . | |
| 1 × 109 | 7.6 ± 4.2 | 11.5 ± 2.7 | 35.8 ± 5 | 11.7 ± 4.8 |
| 1 × 108 | 6.8 ± 2.8 | 8.4 ± 3 | 17.0 ± 4.1 | – |
| 1 × 107 | 6.5 ± 2.4 | 16.6 ± 8.5 | 18.5 ± 5.1 | – |
| Injected transducing particles . | SGPT levels by virus, day 1 . | |||
|---|---|---|---|---|
| FgAdhFIXs, U/mL . | FgAdhFIXs ChMAR, U/mL . | FgAdhFIXs ChMAR IgκMAR, U/mL . | Negative control, U/mL . | |
| 1 × 109 | 7.6 ± 4.2 | 11.5 ± 2.7 | 35.8 ± 5 | 11.7 ± 4.8 |
| 1 × 108 | 6.8 ± 2.8 | 8.4 ± 3 | 17.0 ± 4.1 | – |
| 1 × 107 | 6.5 ± 2.4 | 16.6 ± 8.5 | 18.5 ± 5.1 | – |
Serum glutamic-pyruvic transaminase levels measure serum alanine aminotransferase activity; data are presented in international units.
SGPT indicates serum glutamic-pyruvic transaminase; fgAd, first-generation adenoviral vector; hFIX, human factor IX; ChMAR, chicken lysozyme matrix-attachment region.
Development of an HD vector expressing hFIX
We generated a new high-capacity adenoviral vector (AdFTC) that contained 3 cis-acting sequences as stuffer DNA: a human centromeric fragment containing alphoid repeat DNA, IgκMAR, and the HCR for hepatocyte-restricted transgene expression. AdFTC also contained an MCS that allowed for the production of deleted adenoviruses with any gene of interest, ranging in size between 4 kb and 14 kb (to keep the total size of the HD vector between 26 and 36 kb) (Figure 1E). The HD vector, AdFTC, contained the hFIX expression cassette (minigene and 3′ untranslated region) that was shown to be the most robust in the first-generation vector. The resulting HD vector AdFTC/hFIX (Figure 1F) was produced as described in “Materials and methods” (the helper-virus contamination after CsCl gradients was below 0.5%), and the total amount of transducing units was determined.
Comparison of the expression levels of the HD and first-generation adenovirus containing the identical expression cassette for hFIX
For all comparisons, the same number of adenoviral transducing particles (determined by Southern blot; see “Materials and methods”) were used. The HD and first-generation adenoviruses (fgAdhFIX), which contained identical expression cassettes, were compared in primary hepatocytes in vitro. The data in Figure3A show the result of transduction experiments performed with AdFTC/FIX at different MOIs while the studies in Figure 3B directly compare AdFTC/hFIX and fgAdhFIX. For example, at an MOI of 100, AdFTC/hFIX–transduced cells had hFIX levels of up to 5400 ng per 6 × 105 cells per day, 5 times more than that obtained with the first-generation vector.
Transgene expression in vitro in primary mouse hepatocytes for the HD vector AdFTC/hFIX and the first-generation adenovirus fgAdhFIX.
Primary mouse hepatocytes (6 × 105 viable hepatocytes) were plated on collagen I–coated 6-well dishes. The media was changed daily for hFIX determinations by ELISA. (A) Human FIX expression levels from transduction of AdFTC/hFIX in primary hepatocytes at an MOI of 1 (○/●), an MOI of 10 (▵/▴), and an MOI of 100 (■/▪). (B) Comparison of the transgene (hFIX) expression levels for AdFTC/hFIX (▵/▴) and fgAdhFIX (○/●). Two independent experiments are shown.
Transgene expression in vitro in primary mouse hepatocytes for the HD vector AdFTC/hFIX and the first-generation adenovirus fgAdhFIX.
Primary mouse hepatocytes (6 × 105 viable hepatocytes) were plated on collagen I–coated 6-well dishes. The media was changed daily for hFIX determinations by ELISA. (A) Human FIX expression levels from transduction of AdFTC/hFIX in primary hepatocytes at an MOI of 1 (○/●), an MOI of 10 (▵/▴), and an MOI of 100 (■/▪). (B) Comparison of the transgene (hFIX) expression levels for AdFTC/hFIX (▵/▴) and fgAdhFIX (○/●). Two independent experiments are shown.
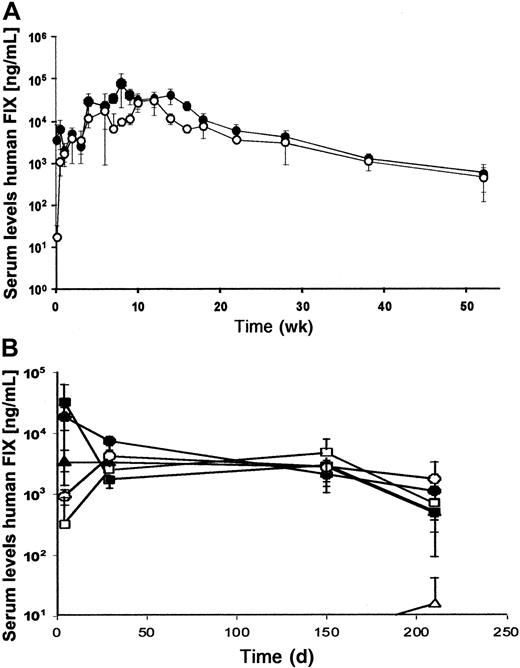
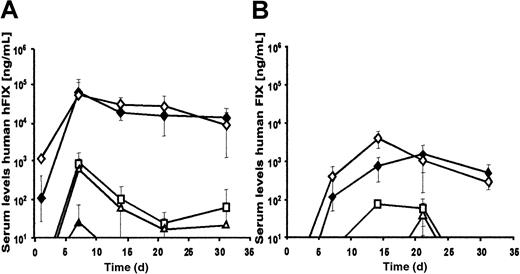
To study adenoviral-mediated expression from these vectors in vivo, 2 × 109 transducing units of AdFTC/hFIX or fgAdhFIX vector were infused into C57BL/6 mice (Figure4A). Sustained levels of serum hFIX were achieved in both groups of animals. Interestingly, serum hFIX was observed more quickly in animals that received the HD adenovirus. The highest hFIX expression levels were obtained during the first 2 months (approximately 41 000 ng/mL for AdFTC/hFIX and approximately 11 000 ng/mL for fgAdhFIX). The expression levels stabilized for the following 4 months at approximately 15 000 ng/mL followed by a slow decline. At 1 year after injection, 5% of the original serum levels of hFIX serum were detected. No significant increase in liver enzyme (SGPT) levels were detected in mice treated with AdFTC/hFIX and fgAdhFIX at 1 day or at later time points after injection (Table3).
Long-term comparison of the hFIX expression levels of the HD adenovirus AdFTC/hFIX and the first-generation adenovirus fgAdhFIX in vivo in C57Bl/6 mice and C57Bl/6 hemophilia B mice.
(A) First, 2 × 109 transducing particles of AdFTC/hFIX (●) or fgAdhFIX (○) (n = 3 per group) were injected into mice. (B) C57Bl/6 hemophilia mice were injected with 1 × 109transducing units AdFTC/hFIX (▪) or fgAdhFIX (■); 5 × 108 transducing units AdFTC/hFIX (●) or fgAdhFIX (○); or 1 × 108 transducing units AdFTC/hFIX (▴) or fgAdhFIX (▵) (n = 3 per group). Serum hFIX levels were periodically determined.
Long-term comparison of the hFIX expression levels of the HD adenovirus AdFTC/hFIX and the first-generation adenovirus fgAdhFIX in vivo in C57Bl/6 mice and C57Bl/6 hemophilia B mice.
(A) First, 2 × 109 transducing particles of AdFTC/hFIX (●) or fgAdhFIX (○) (n = 3 per group) were injected into mice. (B) C57Bl/6 hemophilia mice were injected with 1 × 109transducing units AdFTC/hFIX (▪) or fgAdhFIX (■); 5 × 108 transducing units AdFTC/hFIX (●) or fgAdhFIX (○); or 1 × 108 transducing units AdFTC/hFIX (▴) or fgAdhFIX (▵) (n = 3 per group). Serum hFIX levels were periodically determined.
Serum glutamic-pyruvic transaminase levels in mice receiving either the fgAdhFIX or the helper-dependent vector AdFTC/hFIX
| Day after injection . | SGPT levels by virus . | ||
|---|---|---|---|
| AdFTC/hFIX . | FgAdhFIX . | Negative control, U/mL . | |
| 1 | 47.0 ± 10.1 | 25.7 ± 2.7 | 18.3 ± 6.2 |
| 3 | 31.7 ± 2.3 | 22.0 ± 3.5 | – |
| 7 | 32.7 ± 2.2 | 13.7 ± 7.5 | – |
| 14 | 31.7 ± 0.9 | 15.3 ± 8.0 | – |
| 21 | 26.3 ± 8.6 | 14.7 ± 2.3 | – |
| 42 | 5.7 ± 4.7 | 18.0 ± 9.3 | – |
| Day after injection . | SGPT levels by virus . | ||
|---|---|---|---|
| AdFTC/hFIX . | FgAdhFIX . | Negative control, U/mL . | |
| 1 | 47.0 ± 10.1 | 25.7 ± 2.7 | 18.3 ± 6.2 |
| 3 | 31.7 ± 2.3 | 22.0 ± 3.5 | – |
| 7 | 32.7 ± 2.2 | 13.7 ± 7.5 | – |
| 14 | 31.7 ± 0.9 | 15.3 ± 8.0 | – |
| 21 | 26.3 ± 8.6 | 14.7 ± 2.3 | – |
| 42 | 5.7 ± 4.7 | 18.0 ± 9.3 | – |
Serum glutamic-pyruvic transaminase levels measure serum alanine aminotransferase activity; data are presented in international units.
See Table 2 for other abbreviations.
One potential explanation for the 95% decline in hFIX expression levels was the development of antibodies against the transgene hFIX. However, we performed a reverse ELISA27 and found that no antibodies against hFIX were present (data not shown). To further investigate the source of the 95% decline in hFIX expression levels over the course of 1 year, we analyzed the vector DNA from genomic liver DNA of recipients. Three C57Bl/6 mice per group were injected with 2 × 109 transducing particles of either AdFTC/hFIX or fgAdhFIX, and at both 5 days and 1 year after injection, liver genomic DNA was isolated and a Southern blot was performed. We found that the copy number of the transduced adenoviral vector DNA per cell significantly dropped from about 4.5 copies per cell at 5 days after injection to fewer than 0.51 copies per cell at 1 year after injection (Figure 5).
DNA analysis of transduced adenoviral vector DNA in genomic liver DNA.
C57Bl/6 mice were injected with 2 × 109 transducing particles of either AdFTC/hFIX or fgAdhFIX. At both 5 days and 1 year after injection, genomic liver DNA was isolated and digested with HindIII, and a Southern blot was performed (n = 2 per group). Blots were hybridized with an α-32]dCTP–labeled cDNA hFIX probe, and the copy was determined by comparing the signal intensity of the resulting band with a standard curve that was obtained by digesting known amounts of the plasmid pBSh7hF9mgbpA containing the identical expression cassette withHindIII.19
DNA analysis of transduced adenoviral vector DNA in genomic liver DNA.
C57Bl/6 mice were injected with 2 × 109 transducing particles of either AdFTC/hFIX or fgAdhFIX. At both 5 days and 1 year after injection, genomic liver DNA was isolated and digested with HindIII, and a Southern blot was performed (n = 2 per group). Blots were hybridized with an α-32]dCTP–labeled cDNA hFIX probe, and the copy was determined by comparing the signal intensity of the resulting band with a standard curve that was obtained by digesting known amounts of the plasmid pBSh7hF9mgbpA containing the identical expression cassette withHindIII.19
Cytokine responses
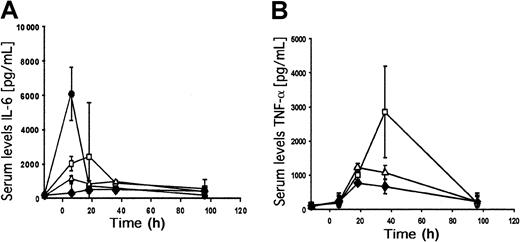
To compare the early innate immune responses, we measured serum concentrations of IL-6 and TNF-α at 6, 18, 36, and 96 hours after adenoviral delivery. Mice were injected with 2 × 109transducing units of the first-generation vector fgAdhFIX or the HD adenoviral vector AdFTC/hFIX. As a negative control, PBS was injected. As a positive control for a robust IL-6 response, we injected 1 × 1010 transducing units of the first-generation vector Ad.hAAT, which expresses alpha 1–antitrypsin cDNA under the transcriptional control of the Rous sarcoma virus promoter.26 At a dose of 2 × 109transducing units, serum IL-6 concentrations were higher for fgAdhFIX compared with the HD vector AdFTC/hFIX (Figure6A). The first-generation vector had a peak IL-6 level at 18 hours after injection (up to 2200 pg/mL IL-6). AdFTC/hFIX injection resulted in a slight increase in IL-6 levels compared with the vehicle control. In mice that received 1 × 1010 transducing units of Ad.hAAT, serum IL-6 levels reached 7500 pg/mL (Figure 6A). After 96 hours, IL-6 serum concentrations returned to normal levels in all groups.
Time course of serum cytokine concentrations after injection of 2 × 109 transducing units of either the first-generation vector fgAdhFIX or the HD vector AdFTC/hFIX.
Serum IL-6 (A) and TNF-α (B) concentrations. Serum samples were collected 12 hours before and 6 hours, 18 hours, 36 hours, and 96 hours after viral injection. ■ = 2 × 109 transducing units fgAdhFIX; ▵ = 2 × 109 transducing units AdFTC/hFIX; ♦ = vehicle (PBS); ● = 1 × 1010 transducing units of the first-generation adenoviral vector Ad.hAAT expressing alpha 1–antitrypsin under the control of the Rous sarcoma virus promoter. Mean ± SD is shown.
Time course of serum cytokine concentrations after injection of 2 × 109 transducing units of either the first-generation vector fgAdhFIX or the HD vector AdFTC/hFIX.
Serum IL-6 (A) and TNF-α (B) concentrations. Serum samples were collected 12 hours before and 6 hours, 18 hours, 36 hours, and 96 hours after viral injection. ■ = 2 × 109 transducing units fgAdhFIX; ▵ = 2 × 109 transducing units AdFTC/hFIX; ♦ = vehicle (PBS); ● = 1 × 1010 transducing units of the first-generation adenoviral vector Ad.hAAT expressing alpha 1–antitrypsin under the control of the Rous sarcoma virus promoter. Mean ± SD is shown.
The peak serum TNF-α levels were 36 hours after injection for the first-generation (fgAd/FIX) adenoviral vector–injected mice. These elevated TNF-α levels were about 3-fold higher than the levels obtained with the HD vector (Figure 6B). After 96 hours, the TNF-α serum concentration dropped to normal levels compared with the vehicle-only control group.
Dose-response curve for AdFTC/hFIX and fgAdhFIX
To determine the lowest amount of the high-capacity AdFTC/hFIX and first-generation fgAdhFIX vectors required to generate therapeutic serum levels of hFIX (approximately 50 ng/mL), a dose-response analysis was performed. Mice were injected with AdFTC/hFIX or fgAdhFIX at 5-fold dose increments ranging from an MOI of 10 (1 × 109 transducing particles) to 0.01 (1 × 106 transducing particles).
Although for both vectors there was a threshold dose response, gene expression became detectable at a lower dose in the HD adenovirus–treated animals (Figure 7). On day 7 after injection, up to 29 000 ng/mL serum hFIX was measured in animals receiving an MOI of 5 and 10 or 5 × 108 and 1 × 109 transducing particles of AdFTC/hFIX. At the lower MOIs (MOI 1 and 0.5), therapeutic levels with serum hFIX concentrations of up to 1200 ng/mL were still detected. In contrast, the first-generation adenoviral vector resulted in serum hFIX concentrations of 500 ng/mL and 150 ng/mL hFIX at MOIs of 10 and 5, respectively, which is about 58 times less than that obtained with the HD vector at the same MOIs. At the lower MOIs (MOI 1 and 0.5), no therapeutic serum levels of hFIX (less than 50 ng/mL) were detected with the use of the first-generation fgAdhFIX vector at 7 days after injection.
Dose response curves.
Dose-response curves for the HD adenovirus AdFTC/hFIX (A) and the first-generation adenovirus fgAdhFIX (B). Mice were injected with varying doses of adenovirus ranging from 1 × 109transducing particles to 1 × 106 transducing particles. ♦ = 1 × 109 transducing particles; ⋄ = 5 × 108 transducing particles; ■ = 1 × 108 transducing particles; ▵ = 5 × 107 transducing particles; ▴ = 1 × 107 transducing particles; ○ = 5 × 106 transducing particles; ● = 1 × 106 transducing particles. Mean ± SD is shown (n = 3 per group).
Dose response curves.
Dose-response curves for the HD adenovirus AdFTC/hFIX (A) and the first-generation adenovirus fgAdhFIX (B). Mice were injected with varying doses of adenovirus ranging from 1 × 109transducing particles to 1 × 106 transducing particles. ♦ = 1 × 109 transducing particles; ⋄ = 5 × 108 transducing particles; ■ = 1 × 108 transducing particles; ▵ = 5 × 107 transducing particles; ▴ = 1 × 107 transducing particles; ○ = 5 × 106 transducing particles; ● = 1 × 106 transducing particles. Mean ± SD is shown (n = 3 per group).
Phenotypic correction in hemophilic mice after injection of AdFTC/hFIX and fgAdhFIX
To test for phenotypic correction of the bleeding diathesis, C57Bl/6 hemophilia-B mice were injected with 1 × 108, 5 × 108, or 1 × 109 transducing particles of AdFTC/hFIX, or 1 × 109 transducing particles of fgAdhFIX. At 3 days after injection of the HD adenovirus, hFIX serum concentrations ranged from 34 000 ng/mL at the highest dose to 3300 ng/mL at the lowest dose (Table 4). On day 6 after injection, a tail-clipping assay was performed. Bleeding times were substantially reduced from no clotting (longer than 30 minutes) in vehicle-treated mice to 3.1 ± 0.5 minutes for AdFTC/hFIX–treated mice, values similar to those found in normal animals. Therefore, these results demonstrate that low doses of HD adenovirus AdFTC/hFIX were sufficient to correct the bleeding diathesis in hemophilic mice. Human factor IX expression levels in C57Bl/6 hemophilic mice (demonstrated in Figure 4B) showed an expression profile similar to that of normal C57Bl/6 mice (Figure 4A). As observed in normal C57Bl/mice (Figure 7B), there was a dose-threshold effect for the first-generation adenoviral vector, but the threshold level was lower than in normal C57Bl/6 mice (5 × 108transducing units).
Phenotypic correction of C57BI/6 hemophilia-B mice treated with different amounts of the high-capacity AdFTC/hFIX or fgAdh/FIX
| Group . | Treatment: virus (transducing particles) . | Serum levels hFIX, ng/mL . | Tail clip bleeding time, min . |
|---|---|---|---|
| 1 | AdFTC/hFIX (1 × 109) | 34 227 ± 14 457 | 3.1 ± 0.5 |
| 2 | AdFTC/hFIX (5 × 108) | 12 143 ± 5 447 | 3.7 ± 0.3 |
| 3 | AdFTC/hFIX (1 × 108) | 3 341 ± 1 227 | 3.8 ± 0.4 |
| 4 | FgAdhFIX (1 × 109) | 910 ± 137 | 5.2 ± 0.2 |
| 4 | Vehicle (PBS) | 0 | No clotting (> 30 min) |
| Group . | Treatment: virus (transducing particles) . | Serum levels hFIX, ng/mL . | Tail clip bleeding time, min . |
|---|---|---|---|
| 1 | AdFTC/hFIX (1 × 109) | 34 227 ± 14 457 | 3.1 ± 0.5 |
| 2 | AdFTC/hFIX (5 × 108) | 12 143 ± 5 447 | 3.7 ± 0.3 |
| 3 | AdFTC/hFIX (1 × 108) | 3 341 ± 1 227 | 3.8 ± 0.4 |
| 4 | FgAdhFIX (1 × 109) | 910 ± 137 | 5.2 ± 0.2 |
| 4 | Vehicle (PBS) | 0 | No clotting (> 30 min) |
On day 3 after injection, an enzyme-linked immunosorbent assay for human factor IX expression levels was performed, and on day 6, a tail clip assay was performed. All groups are of hemophilic mice.
PBS indicates phosphate-buffered saline. For other abbreviations, see Table 2.
Discussion
For gene expression studies in vivo, remarkably similar levels of transgene expression were found in the expression cassette that encodes hFIX with the use of both a first-generation and a new HD vector deleted for all viral coding sequences. This was in contrast to an HD adenoviral vector that contained the complete human alpha 1–antitrypsin gene and a first-generation vector that contained the corresponding cDNA, where large differences in the absolute amount and persistence of gene expression were observed.1 In that case, the same expression cassette was not used, and the differences in the promoter and gene/cDNA sequences probably played a major role in the difference in expression patterns.
The hFIX expression cassette used in this study resulted in supraphysiological hFIX expression levels of up to 100 000 ng/mL at a low MOI from either a first-generation or a gutless adenovirus; this level was about 10 times higher than reported in other studies using adenovirus as a vehicle for hemophilia-B gene therapy.16-18 The slow decline in gene expression over a period of 8 months is not unlike that observed in nonhuman primates infused with an HD vector that contained the human alpha 1–antitrypsin gene.30
The IgκMAR and the ChMAR flanking the hFIX expression cassette in our first-generation recombinant adenoviruses had no significant effect on expression levels of the transgene. One reason might be that the enhancers ApoE and HCR, and the hAAT promoter driving the hFIX transgene, play a similar role to the MARs. Further investigation using cDNAs without enhancers and with and without MARs may resolve this issue.
In this study, we demonstrate an in vivo dose-threshold effect for an HD adenoviral vector. More importantly, the dose threshold for the HD adenovirus was approximately 5 × 108 transducing particles or 4 times lower than a first-generation adenovirus (Figure 7). Bristol et al31 recently published an in vivo dose-threshold effect for a third-generation adenovirus (E1, E2A, and E3 deleted). The threshold level of about 1 × 1010 to 2 × 1010 particles equals our observed threshold level for the first-generation adenovirus of about 2 × 1010 particles. Using a low titer of adenovirus for gene therapy is important to decrease the potential toxicity from helper virus contaminants and/or vector capsids. Most of the current HD adenoviral vectors contain 0.1% to 0.5% contaminating adenoviral helper-virus particles, which might contribute to cell-mediated immune response and/or cytotoxicity. One potential explanation for differences in the threshold level for the first-generation and the gutless adenoviral vector is related to the de novo expression of adenoviral genes from a first-generation adenoviral vector.32-35Lozier et al29 showed that in rhesus macaques, liver toxicity from a first-generation adenovirus expressing hFIX is dose dependent. Because of the low dose required for efficacy, our new high-capacity adenovirus with an optimized expression cassette holds great promise for gene therapy, since it should significantly reduce toxicity from helper-virus genomes and possible toxicity from capsids.
The threshold effect is probably due at least in part to uptake and degradation of viral particles by nonparenchymal Kupffer cells in the liver26,36-38 that then directly and indirectly result in cytokine responses. We found higher serum levels of IL-6 and TNF-α after injection of the first-generation compared with the HD vector during the first phase of the immune response, which occurs during the first 4 days after injection (Figure 6).26 39-41 Whether or not this was somehow responsible for the observed differences in the threshold-dose response will require additional investigation.
Antibodies directed against the transgene product can cause a slow decline in transgene expression levels as shown for factors VIII and IX.2,27,42-44 However, we eliminated anti-hFIX antibodies as the explanation for the 95% decline in hFIX expression levels. Because the vector DNA copy number dropped significantly over the course of 1 year with each vector (Figure 5), we speculate that the natural turnover of hepatocytes and/or development of a cell-specific immune response directed against viral antigens contained within the vector particle are most likely responsible. A cell-mediated immune response was previously demonstrated in another HD adenoviral vector.2 Presumably, the viral antigens derived from the particles are processed by antigen-presenting cells prior to their recognition by the antigen-dependent immune cells.
We demonstrated long-term expression of the transgene hFIX from an HD and first-generation adenovirus in C57Bl/6 mice. The hFIX transgene in the recombinant adenoviral vectors was driven by the liver-specific hAAT promoter and liver-specific enhancers. Pastore et al45 showed that the tissue specificity of a promoter can influence long-term expression of the transgene. For example, a humoral immune response directed against the transgene product occurred when a ubiquitous but not a liver-specific promoter was used in a first-generation vector. Additionally, it was found that variations in recombinant adenoviral genome persistence and the length of transgene expression from mouse hepatocytes are dependent on the mouse strain.46 47 Hepatocyte-restricted gene expression might explain the observed high and long-term hFIX expression levels not only from a high-capacity vector but also from a first-generation adenoviral vector.
Recent studies suggest that the stuffer DNA in the HD vector can have an effect on transgene expression.10 11 Our HD vector AdFTC/hFIX contains a 16.2-kb human centromeric fragment, an IgκMAR, and cis-acting elements in the transgene expression cassette (ApoE, HCR, and a portion of the first hFIX intron). It remains to be determined which cis-acting element in either the transgene expression cassette or the HD vector AdFTC has a predominant effect on the level and persistence of transgene expression. Because our new HD vector contains an MCS that will allow us to insert any transgene expression cassette, we can use this system to attempt to unravel thecis-acting DNA elements required to generate a high-capacity vector that might have a wide variety of different gene therapy applications.
We would like to thank Leonard Meuse and Hui Xu for technical assistance. We gratefully thank Jeffrey Chamberlain (University of Washington, Seattle) for providing C7-Cre cells and the helper virus for adenoviral production. We further thank Michele Calos (Stanford University, Stanford) for providing the subclone pDYAL with the alphoid repeat DNA fragment.
Supported by National Institutes of Health grant R01 DK49022. A.E. is a recipient of a postdoctoral fellowship by the Deutscher Akademischer Austauschdienst in cooperation with the Dr Mildred Scheel Cancer Foundation and is a current recipient of the Judith Graham Pool Fellowship of the National Hemophilia Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark A. Kay, Department of Pediatrics and Genetics, School of Medicine, Stanford University, Stanford, CA 94305; e-mail: markay@stanford.edu.


![Fig. 5. DNA analysis of transduced adenoviral vector DNA in genomic liver DNA. / C57Bl/6 mice were injected with 2 × 109 transducing particles of either AdFTC/hFIX or fgAdhFIX. At both 5 days and 1 year after injection, genomic liver DNA was isolated and digested with HindIII, and a Southern blot was performed (n = 2 per group). Blots were hybridized with an α-32]dCTP–labeled cDNA hFIX probe, and the copy was determined by comparing the signal intensity of the resulting band with a standard curve that was obtained by digesting known amounts of the plasmid pBSh7hF9mgbpA containing the identical expression cassette withHindIII.19](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/11/10.1182_blood.v99.11.3923/6/m_h81122627005.jpeg?Expires=1769155852&Signature=O4EUDDhnNapd4GtuNL-47HnFGjaMwY7vkHGjtsEMVQGy~Cnu1MCSFM06yfOAYQqJGvOqPAT2caB16C0KO6f5At6f-gkwrLbrJvllvsnmpZgNkEGFw~Tkg0iT9pIKqSB-X1Z7blApr6SH-IAnXFfAgQHHiC0rvQVtBOCjViEbSOSwKX73EU7cmrFkTHCOYdEmb6iXMgqmXPCpXV11TOEbNY60lVs2YPfF7nzl6a6I19Da4MD~6rI62TJnXIUwZihyPbMDkyRWphaWvp7v0oKThlxgYc-7SA6Kwz2P4m6SvDuRe8onDe08f3n8vj0amxPNxrvkL0LgsO5T-CzwRMOF2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal