We have previously demonstrated that 5-aza-2′-deoxycytidine (decitabine) augments fetal hemoglobin (HbF) levels in patients with sickle cell anemia (SS) who did not respond to hydroxyurea (HU). The present study was designed to determine the effect of repeated decitabine dosing on HbF levels and hematologic toxicity over a 9-month treatment period. Seven patients (5 HU nonresponders) were entered. One patient had α-thalassemia sickle cell anemia. Decitabine was administered by intravenous infusion at a starting dose of 0.3 mg/kg per day, 5 days a week for 2 weeks, followed by a 4-week observation period. If the absolute neutrophil count dropped below 1000, the dose was reduced by 0.05 mg/kg per day in the next cycle. A drug dose was obtained for each patient, and it resulted in an elevated HbF without neutropenia (absolute neutrophil count nadir greater than 1500) or evidence of cumulative toxicity. Average HbF and average maximal HbF levels attained during the last 20 weeks of treatment for the 6 SS patients increased to 13.93% ± 2.75% and 18.35% ± 4.46%, respectively, from a pretreatment mean of 3.12% ± 2.75%. Mean and mean maximal hemoglobin (Hb) levels increased from 7.23 ± 2.35 g/dL to 8.81 ± 0.42 g/dL and 9.73 ± 0.53 g/dL, respectively. Individual maximal F-cell number observed during the trial was 69% ± 10.12%. The absence of cumulative toxicity may allow shorter intervals between drug treatments, which may lead to higher hemoglobin and HbF levels after several treatment cycles and, therefore, to greater clinical improvement.
Introduction
Patients with sickle cell anemia (SS) who have elevated fetal hemoglobin (HbF) levels have fewer painful crises and improved survival.1 The therapeutic benefits of elevated HbF are greater when the cellular distribution of HbF is more uniform.2 Recent studies using hydroxyurea (HU) to increase HbF in the treatment of sickle cell anemia have clearly demonstrated the clinical importance of augmenting HbF levels.3,4 HU was shown to reduce the frequency of painful crises, hospitalizations, and acute chest events and the need for blood transfusions in adults with SS. Individual HbF responses to HU varied; some patients attained HbF levels in excess of 30% of total hemoglobin (Hb), whereas others had little or no response.4 Although HbF augmentation is important in reducing disease severity, decreased neutrophils associated with HU cytotoxicity may also have additional benefit.5
We had originally shown that the DNA demethylating agents, 5-azacytidine and its analog 5-aza-2′-deoxycytidine (decitabine), were powerful inducers of HbF synthesis.6,7 The mechanism of action of these agents in inducing HbF synthesis is likely to be related to DNA demethylation rather than cytotoxicity alone.8 With these considerations in mind, we recently demonstrated that decitabine increased mean HbF levels from 2.28% ± 1.61% to 12.70% ± 1.81% in a group of HU nonresponder patients, suggesting that decitabine be considered an option in this group of patients.9 Therefore, a repeated dose trial was initiated to determine whether prolonged decitabine treatment could maintain HbF levels up to a 36-week period without toxicity. Data presented here indicate that low-dose (0.15-0.3 mg/kg per day) intravenous or subcutaneous decitabine administration is an effective therapy for patients who do not respond to HU.
Patients, materials, and methods
Patient selection
This study was a single-arm, repeated-dose trial of decitabine in patients with sickle cell anemia to determine whether HbF levels could be maintained for an extended period (36 weeks) without toxicity. Six patients with sickle cell anemia and one patient with α-thalassemia sickle cell anemia (α-thal SS), all older than 18 years of age, were enrolled after they agreed and provided written informed consent. The trial was approved by the Institutional Review Board of the University of Illinois at Chicago. All patients were previously enrolled in the dose-escalation trial,9 and 5 of the 7 patients were HU nonresponders. As described in the previous paper,9 the 5 HU nonresponders (Table1, patients 1-5) had been taking HU for more than 1 year in the double-blind, randomized multicenter study of hydroxyurea trial and failed to demonstrate a response. Compliance was demonstrated at that time by measuring drug levels in urine and conducting pill counts.
Hemoglobin and HbF levels before and during the last 20 weeks of treatment with decitabine
| Patient . | HbF % . | Hb g/dL . | ||||
|---|---|---|---|---|---|---|
| Before . | Average . | Maximum . | Before . | Average . | Maximum . | |
| 1 | 0.8 | 12.40 ± 1.25 | 14.4 | 6.2 | 9.05 ± 0.48 | 9.6 |
| 2 | 6.8 | 14.55 ± 1.32 | 16.3 | 8.2 | 9.37 ± 0.60 | 10.3 |
| 3 | 1.4 | 12.75 ± 2.28 | 17.2 | 6.0 | 8.34 ± 0.55 | 9.5 |
| 4 | 0.6 | 10.80 ± 2.05 | 14.4 | 7.2 | 8.28 ± 0.52 | 9.0 |
| 5 | 2.9 | 16.42 ± 2.81 | 24.6 | 8.0 | 8.91 ± 0.57 | 9.6 |
| 7 | 6.2 | 16.70 ± 2.55 | 23.2 | 7.8 | 8.92 ± 0.79 | 10.4 |
| Mean ± SD | 3.12 ± 2.75 | 13.93 ± 2.35 | 18.35 ± 4.46 | 7.23 ± 0.94 | 8.81 ± 0.42 | 9.73 ± 0.53 |
| Patient . | HbF % . | Hb g/dL . | ||||
|---|---|---|---|---|---|---|
| Before . | Average . | Maximum . | Before . | Average . | Maximum . | |
| 1 | 0.8 | 12.40 ± 1.25 | 14.4 | 6.2 | 9.05 ± 0.48 | 9.6 |
| 2 | 6.8 | 14.55 ± 1.32 | 16.3 | 8.2 | 9.37 ± 0.60 | 10.3 |
| 3 | 1.4 | 12.75 ± 2.28 | 17.2 | 6.0 | 8.34 ± 0.55 | 9.5 |
| 4 | 0.6 | 10.80 ± 2.05 | 14.4 | 7.2 | 8.28 ± 0.52 | 9.0 |
| 5 | 2.9 | 16.42 ± 2.81 | 24.6 | 8.0 | 8.91 ± 0.57 | 9.6 |
| 7 | 6.2 | 16.70 ± 2.55 | 23.2 | 7.8 | 8.92 ± 0.79 | 10.4 |
| Mean ± SD | 3.12 ± 2.75 | 13.93 ± 2.35 | 18.35 ± 4.46 | 7.23 ± 0.94 | 8.81 ± 0.42 | 9.73 ± 0.53 |
Drug administration
Decitabine was supplied, as a lyophilized powder for injection in 50-mg vials (SuperGen, Dublin, CA). Each vial was reconstituted with 5 mL sterile water for injection, and the calculated dose was administered by direct intravenous injection in a total volume of 5 mL over a period of 2 minutes. In one patient who had poor venous access, decitabine was injected subcutaneously in the upper arm at a concentration of 10 mg/mL (1.3-1.9 mL). Patients were allowed to leave the clinic as soon as the postinjection assessment—consisting of a physical examination, determination of vital signs, and inspection of the local injection site—was completed.
Treatment plan
The starting dose of decitabine was based on our previous experience.6 Patients received a starting dose of 0.3 mg/kg per day for 5 days a week for 2 weeks, followed by 4 weeks of observation. The 4-week observation period was based on our previous observation that the nadir in absolute neutrophil count (ANC) occurred 5 weeks after the start of therapy and resolved before the sixth week. Thus the recovery of neutropenia would be expected prior to the next treatment cycle. The patient with α-thal SS was started at a dose of 0.15 mg/kg per day because neutropenia resulted at a higher dose in the previous trial. Complete blood counts were conducted weekly and as necessary. Toxicity was evaluated by the National Cancer Institute Common Toxicity Criteria. Dose modifications for neutropenia were as follows: for an ANC nadir less than 1500 but more than 1000 in 2 consecutive treatment cycles, the dose was reduced by 0.05 mg/kg per day in the next treatment cycle. For an ANC nadir less than 1000 occurring in any one cycle, the dose of decitabine was reduced by 0.05 mg/kg per day in the next treatment cycle. Baseline HbF and F cells were determined on all patients. HbF was repeated weekly, and F cells were measured at peak HbF.
Laboratory methods
Results
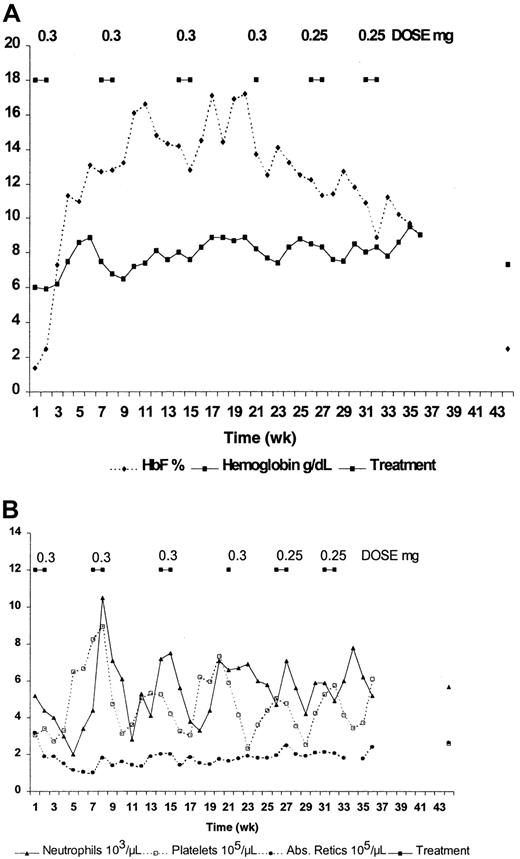
Seven patients with sickle cell anemia (6 SS and one α-thal SS) were treated for up to 36 weeks with decitabine. These patients were assigned the same numbers they were assigned in the previously published dose-escalation study.9 Except for mild neutropenia, there were no other hematologic toxicities, nor were there any cumulative hematologic toxicities. No nonhematologic toxicities were noted. Five patients (patients 3, 4, 5, 7, 8) completed 6 cycles (36 weeks), and 2 completed 5 cycles (patients 1, 2). Four patients (patients 1, 2, 5, 7) required dose reduction because of neutropenia (ANC less than 1500). Two patients (patients 3, 4) had dose reductions in the absence of toxicity to determine the effect on HbF and total Hb levels in patients who had not reached their maximal tolerated dose. Results from 2 patients are shown in detail in Figures1 (patient 3) and 2 (patient 2), and they underscore the variability in hematologic response to decitabine.
Effect of intravenous administration of decitabine on blood components of patient 3.
(A) HbF and hemoglobin. (B) Absolute reticulocyte count, absolute neutrophil count, and platelet count.
Effect of intravenous administration of decitabine on blood components of patient 3.
(A) HbF and hemoglobin. (B) Absolute reticulocyte count, absolute neutrophil count, and platelet count.
Patient 3 (Figure 1) was treated at the starting dose of 0.3 mg/kg per day for 4 cycles. A dose of 0.25 mg/kg per day was used for the last 2 cycles. The fourth cycle consisted of only a 1-week treatment period, because the drug delivery was delayed. This reduction in treatment duration amounted to a dose reduction for this patient, and it occurred before the planned reduction during cycles 5 and 6. HbF increased from 1.4% to approximately 17%, and total hemoglobin increased from 6.0 to 8.9g/dL during 2 successive cycles at this dose (Figure 1A). Maximum F-cell number measured in this patient was 70%. Except for an ANC nadir of 2000, which occurred during the first cycle, the ANC nadirs remained above 3000. These nadirs occurred at approximately weeks 5 to 6 of each treatment cycle, corresponding to the time of peak platelet count (Figure 1B). Even though the ANC for this patient remained above 2000, patient 3 received a dose of 0.25 mg/kg per day for the last 2 cycles. This allowed us to determine the effect of a reduced dose on total hemoglobin level, HbF percentage, absolute reticulocyte count, and ANC. It is clear that the decrease in HbF during the last 3 cycles can be attributed to dose reduction. Interestingly, the total hemoglobin level remained elevated, reaching a peak of 9.5 g/dL following the last treatment cycle. Even with the dose reduction, average HbF and total hemoglobin during the last 20 weeks of treatment were 12.75% ± 2.28% and 8.34% ± 0.55 g/dL, respectively (Table1). In addition, there was no indication of cumulative hematologic toxicity. The average absolute reticulocyte count during the last 20 weeks was 192 ± 26 × 103/μL after dropping from 320 before treatment to 102 after the first cycle, and the average ANC was 5710 ± 120 after dropping from 5200 before treatment to 2000 during the first cycle (Figure 1B, Table 1). The decrease in absolute reticulocyte count might have resulted in part from the 2.3 g/dL increase in total hemoglobin level.
Treatment for patient 2 (Figure 2) began at the starting dose of 0.3 mg/kg per day and was administered subcutaneously, at 10 mg/mL in a volume of 1.9 mL, rather than intravenously because of poor venous access. The patient felt pain at the site of injection that lasted for 1 to 2 minutes, but there was no evidence of a change in skin appearance, and there was no skin sloughing over the injection site. HbF increased from 6.8% to 13.3% (Figure 2A), whereas the ANC decreased from 5600 to 900 during the fifth week after the beginning of treatment (Figure 2B). For the second cycle the dose was reduced to 0.25 mg/kg per day. The ANC again decreased to 900, but the HbF increased to 16.3%, suggesting that the dose reduction did not impact HbF level. Because the ANC nadir was less than 1000, the dose in cycle 3 was reduced to 0.2 mg/kg per day, resulting in an ANC nadir of 2600. HbF level peaked at 16.5%, again comparable to the level attained with the starting dose. This patient was treated for 2 additional cycles at 0.2 mg/kg per day. The ANC remained greater than 1500, the average total Hb was 9.37 ± 0.6 g/dL, and average HbF was 14.55% ± 1.32% during the last 20 weeks of treatment (Figure 2A, Table 1). The maximum F-cell number measured in this patient was 58%. Dose reduction not only lessened neutropenia, it also appeared to result in higher average total Hb levels. It should be noted that this patient missed 3 injections during the last treatment cycle because of out-of-state travel. This 30% reduction in injection number may be responsible for the slight reduction in maximum HbF level observed during the last treatment cycle.
Effect of subcutaneous administration of decitabine on blood components of patient 2.
(A) HbF and hemoglobin. (B) Absolute reticulocyte count, absolute neutrophil count, and platelet count.
Effect of subcutaneous administration of decitabine on blood components of patient 2.
(A) HbF and hemoglobin. (B) Absolute reticulocyte count, absolute neutrophil count, and platelet count.
The values for ANC, platelet count, and absolute reticulocyte count for patient 2 are displayed in Figure 2B. This figure clearly depicts the reduction in the ranges of ANC and platelet counts that occur with the decrease in decitabine dose and length of treatment. Again, there was no indication of cumulative hematologic toxicity in this patient.
Patient 8, diagnosed as having α-thal SS, had a maximum tolerated dose of only 0.15 mg/kg per day based on our previous study. This patient was the only patient who did not have a dose reduction during the 36 weeks of study. The ANC remained greater than 1900, and there was no evidence of cumulative toxicity. Average HbF during the last 20 weeks of treatment increased from a pretreatment value of 11.9% to 20.8% ± 1.43%. Maximal HbF levels were 22.5%, 23.1%, and 22.8% during cycles 4, and 5, and 6, respectively, compared with a maximum of 21.6% during cycle 2 of the first 3 cycles. Therefore, in the absence of dose reduction, there was no temporal decrease in HbF level.
Although there was a marked and consistent increase in HbF in patient 8, surprisingly there was no increase in total Hb. Similar to other patients with α-thal SS, patient 8 had elevated HbF and F cells at baseline, suggesting that increased HbF expression had little effect on cell survival. The results obtained from this patient were excluded from Table 1 and from joint analysis with the other patients because of the difference in genotype.
HbF and total hemoglobin levels measured during the last 20 weeks of treatment for the 6 SS patients are summarized in Table 1. The average HbF percentage increased from a pretreatment level of 3.12% ± 2.75% to 13.93% ± 2.35%, and the average maximum HbF increased to 18.35% ± 4.46%. The average Hb increased from 7.23 ± 0.94 to 8.81 ± 0.42 g/dL, and the average maximum Hb increased to 9.73 ± 0.53 g/dL. Although periodic depressions in ANC occurred 5 to 6 weeks after the beginning each treatment cycle, the average ANC during the last 20 weeks of the study (4200 ± 1350) was not different from the pretreatment average (4670 ± 1560). In addition, Hb and HbF levels measured 8 to 10 weeks after the administration of the last dose of decitabine (7.82 ± 0.42 and 5.53 ± 3.76, respectively) approached pretreatment values. The maximum F-cell number measured among all 7 patients averaged 69% ± 10.12%.
Discussion
Seven patients with sickle cell anemia were treated with decitabine. Our results clearly demonstrate that the dose of decitabine can be individualized for each patient, which results in marked elevations in HbF, F cell, and total Hb levels without marked neutropenia (ANC greater than 1500). It also appears that in the absence of dose reduction, there is no decrease in the maximal HbF levels attained during the later treatment cycles. Although it is likely that therapeutic benefits are associated with these elevations, the present study was not designed to measure them. Future long-term studies are required for a more precise evaluation of clinical benefits.
All patients except patient 8 (α-thal SS) had an increase in total hemoglobin. Interestingly, total hemoglobin levels remained high after dose reduction, even though HbF levels declined. One explanation for this finding is that the reduction in HbF might have resulted from a decrease in F/F cell (%HbF/F cell) in the absence of a significant reduction in F cells. F-cell analysis was performed only at the time of peak HbF level; therefore, sufficient data are unavailable to support the above interpretation. Another possibility is that the maintenance of total hemoglobin level resulted from increased red cell production at the lower dose. However, absolute reticulocyte counts did not increase at lower doses of decitabine.
The lack of an increase in total Hb displayed by patient 8 may reflect the effect of the α-thal gene. Patients with α-thalassemia generally have less severe disease because limited α chain production leads to decreased mean corpuscular HbS concentration,12 13 reduced sickling, and increased cell survival. Given that the level of HbF at the beginning of decitabine therapy was almost 12% in this patient, there might not have been an incremental increase in red cell survival resulting from the increased HbF. Alternatively, increased red cell survival might have been masked by reduced red cell production.
The absence of cumulative hematologic toxicity over a 36-week period suggests that the interval between treatment cycles can be reduced without an increase in toxicity. By shortening the interval, newly synthesized F cells can be added to the circulation more frequently than with the longer interval. The number of F cells remaining in the circulation that can be attributed to the preceding treatment cycle would depend on the length of the interval between treatment cycles and the F-cell half-life. The shorter the interval, the more frequently F reticulocytes are added to the circulation. Therefore, a shorter interval should lead to a greater number of F cells, and this would be expected to reduce symptoms. However, long-term patient compliance might decrease with such a schedule because of the requirement for more frequent clinic visits. An oral dosage form or a self-administered subcutaneous formulation of decitabine might overcome this limitation and should be considered. Our previous experience in baboons9 and other studies of patients with sickle cell disease14 indicate that an oral form of decitabine, compounded with the cytidine deaminase inhibitor tetrahydrouridine, could be developed. Our successful experience with repeated subcutaneous dosing of decitabine in one patient in this trial indicates that this method should be pursued in an upcoming trial, while approval of an oral formulation is awaited.
Supported by VA Merit Review (J.D., D.L.) and Supergen Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joseph DeSimone, VA Chicago West Side Division, 820 S Damen Ave (151C), Chicago, IL 60612; e-mail: jdesimon@uic.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal