The interaction between collagen, von Willebrand factor (VWF), and glycoprotein Ib is the first step in hemostasis and thrombosis especially under high shear conditions. We studied the inhibition of the VWF-collagen interaction by using an antihuman VWF monoclonal antibody 82D6A3 to prevent arterial thrombosis in baboons to develop a new kind of antithrombotic strategy and determine for the first time experimental in vivo data concerning the importance of the collagen-VWF interaction. We used a modified Folts model to study the antithrombotic efficacy of 82D6A3, where cyclic flow reductions (CFRs) were measured in the femoral artery. Administering a dose of 100, 300, and 600 μg/kg resulted in a 58.3%, 100%, and 100% reduction in the CFRs, respectively. When 100 μg/kg 82D6A3 was infused into the baboons, 80% of VWF-A3 domain was occupied, corresponding to 30% to 36% ex vivo inhibition of VWF binding to collagen, with no prolongation of the bleeding time. The bleeding time was also not significantly prolonged when the CFRs were abolished at doses of 300 μg/kg and 600 μg/kg. At these doses 100% of VWF was occupied by the antibody and 100% ex vivo inhibition of the VWF-collagen binding was observed. 82D6A3 has a high affinity for VWF; after 48 hours still 68% VWF (300μg/kg) was occupied with a pharmacologic effect up to 5 hours after administration (80%-100% occupancy). In conclusion, these results clearly indicate that the VWF-collagen interaction is important in vivo in thrombosis under high shear conditions and thus might be a new target for preventing arterial thrombosis.
Introduction
In normal hemostasis and thrombosis, platelets adhere to the subendothelium of damaged blood vessels through an interaction with von Willebrand factor (VWF), which forms a bridge between collagen within the damaged vessel wall and the platelet receptor glycoprotein Ib (GPIb/V/IX), an interaction especially important under high shear conditions.1 This reversible adhesion allows platelets to roll over the damaged area, which is then followed by a firm adhesion through the collagen receptors (GPIa-IIa, GPVI, GPIV, p65, TIIICBP) 2-4 resulting in platelet activation. This leads to the conformational activation of the platelet GPIIb/IIIa receptor, fibrinogen binding, and finally to platelet aggregation.5
The VWF subunit is composed of several homologous domains each covering different functions; VWF interacts through its A1 domain mainly with the GPIb/V/IX complex,6 whereas its A3 domain predominantly interacts with fibrillar collagen fibers.7Under normal conditions platelets and VWF do not interact. However, when VWF is bound to collagen at high shear rate, it is believed to undergo a conformational change allowing its binding with the platelet receptor GPIb/IX/V.8
One line of search for antiplatelet drugs in the prevention of thrombosis is focusing on the inhibition of the VWF-GPIb axis. Compounds that interact with GPIbα, such as the GPIb-binding snake venom proteins echicetin and crotalin,9,10 an antiguinea pig GPIb antibody,11 a recombinant A1 domain fragment (VCL),12 and recently an antihuman GPIb antibody13 or compounds that bind to VWF, such as anti-A1-VWF-monoclonal antibodies (mAbs)14,15 or aurin tricarboxylic acid (ATA),16 indeed, inhibit thrombus formation in vivo.
Specific blockade of the VWF-collagen interaction in vivo has not yet been demonstrated but based on in vitro data could be a novel strategy for the prevention of thrombus formation in stenosed arteries. We here describe for the first time the antithrombotic effect of a murine antihuman VWF mAb 82D6A3, known to bind to the A3 domain and to inhibit VWF binding to fibrillar collagens type I and III but not to collagen VI.17
The antithrombotic efficacy of 82D6A3 was demonstrated in baboons by using a modified Folts model, where cyclic flow reductions (CFRs) due to thrombus formation and its dislodgment are measured in mechanically damaged, severely stenosed femoral arteries.18
Materials and methods
Purification of 82D6A3
82D6A3 was raised and purified from ascites by protein A chromatography.17
Surgical preparation
Baboons (Papio ursinus) used in this study were of either sex and weighed 12 to 18 kg. All procedures were approved by the Ethics Committee for Animal Experimentation of the University of the Free State and Free State Provincial Administration in accordance with the National Code for Animal Use in Research, Education, Diagnosis and Testing of Drug and Related Substances in South Africa. The experimental procedure followed19 was that of the original Folts model18 except that the femoral artery was injured with a spring-loaded forceps. Baboons were anesthetized with ketamine hydrochloride (10 mg/kg, intramuscularly), intubated with a cuffed endotracheal tube, and ventilated by a respirator with oxygen supplemented with 0.5% Fluothane (halothane) to maintain anesthesia. Body temperature was maintained at 37°C with a heating table. A catheter was placed in a femoral vein for drug administration and blood sampling. A segment of a femoral artery was gently dissected free from surrounding tissue and a perivascular ultrasonic flow probe (Transonic Systems, Ithaca, NY) was placed around the distal dissection site. The mean and phasic blood flow were recorded continuously throughout the experiment. Baboons were allowed to stabilize for 30 minutes. The proximal dissection site of the femoral artery was injured by applying 3 occlusions of the artery for 10 seconds with 2-mm interval using a spring-loaded forceps, and a spring-loaded clamp was placed in the middle of the injured site to produce an external stenosis of 65% to 80%. A gradual decline in blood flow due to platelet adhesion and aggregation was observed. When flow was reduced by at least 50%, blood flow was restored by pushing the spring of the clamp to mechanically dislodge the platelet-rich thrombus. This repetitive pattern of decreasing blood flow following mechanical restoration is referred to as cyclic flow reductions (CFRs). Additional endothelial injury and appropriate external stenosis selection was repeated if needed to finally obtain stable CFRs in these baboons. The number of times the thrombus needed to be dislodged determines the number of CFRs.
After a 60-minute control period of reproducible CFRs (t = −60 minutes to 0 minute), test agents (saline or 82D6A3) were given via an intravenous bolus injection (t = 0) and monitoring was continued up to 60 minutes after drug administration (t = +60 minutes). The antithrombotic effect was quantified by comparing the number of CFRs per hour before and after drug administration. If the last flow reduction in the 60-minute recording period was not cyclic, it was considered in the calculations. Blood samples for the different laboratory measurements were drawn at different time points. The doses of 82D6A3 were selected on the basis of preliminary dose-finding studies. Group 1 was a control group where the baboons received saline (n = 2). Group 2 received a high dose of 600 μg/kg 82D6A3 (n = 2). In group 3, the baboons received an initial dose of 100 μg/kg 82D6A3 and after 60 minutes an additional 200 μg/kg 82D6A3 was given (n = 3). In a preliminary study we found that 82D6A3 has a long half-life. This therefore resulted in a final dose of 300 μg/kg. All agents were diluted with saline.
Platelet count, coagulation, and bleeding time
All blood samples were collected on 0.32% (final concentration) trisodium citrate. The platelet count was determined using a Technicon H2 blood cell analyzer (Bayer Diagnostics, Tarrytown, NY). Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured at 37°C using a coagulometer (Clotex II, Hyland, South Africa). The template bleeding time was measured using the Simplate II device (Organon Teknika, Durham, NC). The volar surface of the forearm was shaved and a pressure cuff was applied and inflated at 40 mm Hg. The wound was carefully dabbed every 15 seconds with filter paper. Baseline bleeding time measured in 13 baboons was 2.04 ± 0.59 minutes, with an interanimal coefficient of variation of 29%.
All measurements were performed once at each time point.
Plasma concentration of 82D6A3
Microtiter plates (96-well, Greiner, Frickenhausen, Germany) were coated overnight at 4 °C with 5 μg/mL (in phosphate-buffered saline [PBS], 100 μL/well) goat antimouse IgG whole molecule (Sigma, St Louis, MO). Plates were blocked with 3% milk powder for 2 hours at room temperature (RT). Next dilution series (1:2 in PBS) of the plasma samples (prewarmed for 5 minutes at 37°C) were added for 2 hours at RT and goat antimouse IgG labeled with horseradish peroxidase (HRP) was added for 1 hour at RT. Visualization was obtained with H2O2 and ortho-phenylenediamine (OPD, Sigma) and the coloring reaction was stopped with 4 moL/L H2SO4. The absorbance was determined at 490 nm. After each incubation step, plates were washed with PBS, 0.1% Tween-20, 3 times after coating and blocking steps and 12 times elsewhere. The plasma concentration of 82D6A3 in each sample was calculated from a standard curve where known amounts of 82D6A3 were added to baboon plasma.
VWF-antigen levels
Determination of the VWF-antigen (Ag) levels was performed essentially as described.20 Briefly, microtiter plates were coated with a polyclonal anti-VWF-Ig solution (Dako, Glostrup, Denmark), blocked with 3% milk powder and plasma samples, preincubated for 5 minutes at 37°C, and added to the wells at 1:40 to 1:2560 dilutions. Bound VWF was detected with rabbit antihuman VWF HRP antibodies (Dako). VWF-Ag levels were calculated from a standard curve obtained by adding 1:40 to 1:2560 dilutions to the coated wells of a human plasma pool, known to contain 10 μg/mL human VWF.
VWF occupancy
Microtiter plates (96-well) were coated overnight at 4°C with 125 μL/well of a polyclonal anti-VWF-Ig solution (Dako; 1:1000 in PBS). Plates were blocked with 3% milk powder solution for 2 hours at RT. A dilution series (1:2 in PBS) of the plasma samples (prewarmed for 5 minutes at 37°C) was added for 2 hours at RT. Samples containing 100% occupied VWF were obtained by adding a saturating amount of 82D6A3 (6 μg/mL) to each corresponding baboon plasma. Bound 82D6A3 was detected by addition of goat antimouse IgG-HRP (1 hour at RT). Visualization and wash steps were performed as described above. The VWF occupancy of each sample was calculated as follows: (A490-nm sample/A490-nm sample saturated with 82D6A3) · 100.
Determination of the VWF-collagen binding activity
The enzyme-linked immunosorbent assay (ELISA) was performed essentially as described.20 Briefly, microtiter plates were coated with human collagen type I (Sigma), blocked with 3% milk powder solution and ½ dilution series of baboon plasma (prewarmed for 5 minutes at 37°C) were added. Bound VWF was detected with rabbit antihuman VWF-HRP antibodies. Binding of baboon VWF to collagen in the different plasma samples was compared to the binding of VWF in the respective blood sample taken at time zero (presample), which was set as 100%.
Inhibition of the VWF binding to collagen by 82D6A3
The ELISA was performed as described for the determination of the VWF-collagen binding activity except that serial dilutions of 82D6A3 were either preincubated with constant amounts of VWF in a preblocked plate for 30 minutes or were directly added to the plate containing collagen-bound VWF.
Statistics
Data are expressed as mean ± SD. Student t test (paired) or one-factor ANOVA followed by Fisher test was used for statistical evaluation. P < .05 was considered as statistically significant.
Results
In vitro interaction of 82D6A3 with baboon VWF
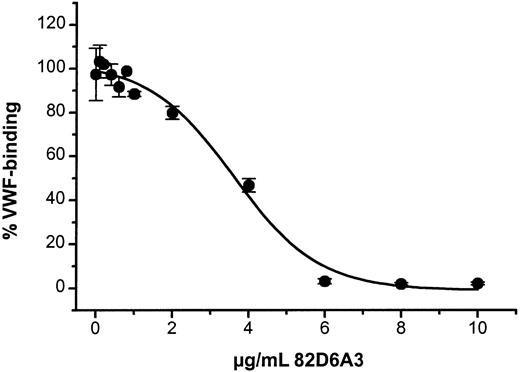
82D6A3, known to inhibit human VWF binding to collagen,17 cross-reacts with baboon VWF. 82D6A3 has a comparable affinity for baboon VWF as for human VWF (median effective concentration [EC50] 200 ng/mL) and inhibited the binding of baboon VWF to collagen with an inhibitory concentration of 50% (IC50) of 3.5 μg/mL (Figure1).
Inhibition of baboon VWF binding to collagen by 82D6A3.
Different concentrations of 82D6A3 (20 μL) were incubated with undiluted baboon plasma (220 μL) for 30 minutes before addition to a collagen-coated plate. Bound VWF was detected with rabbit antihuman VWF antibodies. The data are the mean of 4 measurements obtained with the plasma of 2 different baboons
Inhibition of baboon VWF binding to collagen by 82D6A3.
Different concentrations of 82D6A3 (20 μL) were incubated with undiluted baboon plasma (220 μL) for 30 minutes before addition to a collagen-coated plate. Bound VWF was detected with rabbit antihuman VWF antibodies. The data are the mean of 4 measurements obtained with the plasma of 2 different baboons
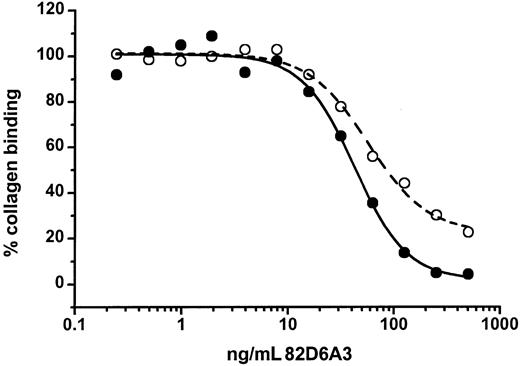
82D6A3 not only prevented binding of VWF to collagen, but also was able to remove the bound VWF from a collagen surface with somewhat lower efficacy (Figure 2).
Inhibition of collagen-bound VWF by 82D6A3.
Different concentrations of 82D6A3 were either preincubated with diluted human plasma (final dilution of 1:46, ie, 217 ng/mL VWF) before addition to a collagen-coated plate (●) or were added to a collagen-coated plate where VWF (final dilution of 1:46, ie, 217 ng/mL VWF) was already bound for 30 minutes (○). Bound VWF was detected with rabbit antihuman VWF antibodies.
Inhibition of collagen-bound VWF by 82D6A3.
Different concentrations of 82D6A3 were either preincubated with diluted human plasma (final dilution of 1:46, ie, 217 ng/mL VWF) before addition to a collagen-coated plate (●) or were added to a collagen-coated plate where VWF (final dilution of 1:46, ie, 217 ng/mL VWF) was already bound for 30 minutes (○). Bound VWF was detected with rabbit antihuman VWF antibodies.
Antithrombotic effect
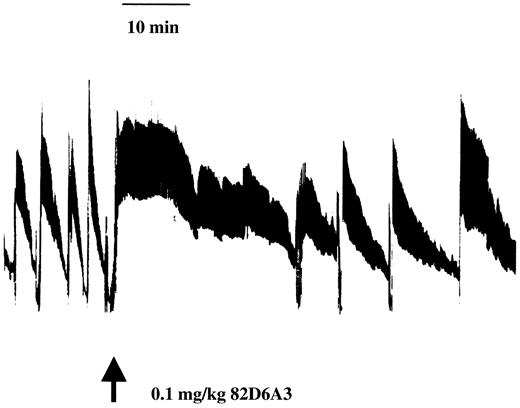
Using a Folts model, the antithrombotic efficacy of 82D6A3 was tested by administering different doses of 82D6A3 to baboons. In Figure3, a representative tracing of CFR data is given.
A representative tracing of CFR data.
After a 60-minute control period, 100 μg/kg 82D6A3 was administered to the baboon and changes in CFRs were monitored for 60 minutes.
A representative tracing of CFR data.
After a 60-minute control period, 100 μg/kg 82D6A3 was administered to the baboon and changes in CFRs were monitored for 60 minutes.
Injection of saline did not affect CFRs (107% ± 7%). In a first study, a dose of 600 μg/kg 82D6A3 resulted in a complete disappearance of the CFRs (Figure 4). This dose was expected to be an overdose because according to theoretical calculations, a dose of 600 μg/kg would result in a plasma concentration of 13 μg/mL (45 mL/kg plasma volume), which in in vitro experiments results in a 100% inhibition of the VWF binding to collagen (Figure 1). As a result, in all subsequent in vivo studies lower doses of 82D6A3 were used. Administration of 100 μg/kg 82D6A3 resulted in a significant reduction of the CFRs by 58.3% ± 4.8% (Figure 4). Administration of a total doses of 300 μg/kg completely abolished the CFRs (Figure 4), which could not be restored by increasing intimal damage or increasing stenosis.
Inhibition of femoral artery CFRs after administration of different doses of 82D6A3 to baboons.
Different doses of 82D6A3 were administered to baboons and the CFRs were measured during 60 minutes. Data represent the mean with n = 2 for 0 and 600 μg/kg and mean ± SD with n = 3 for 100 and 300 μg/kg mAb 82D6A3. * = P < .05; † = P < .01. One-factor ANOVA followed by Fisher test was used for statistical evaluation.
Inhibition of femoral artery CFRs after administration of different doses of 82D6A3 to baboons.
Different doses of 82D6A3 were administered to baboons and the CFRs were measured during 60 minutes. Data represent the mean with n = 2 for 0 and 600 μg/kg and mean ± SD with n = 3 for 100 and 300 μg/kg mAb 82D6A3. * = P < .05; † = P < .01. One-factor ANOVA followed by Fisher test was used for statistical evaluation.
Coagulation, platelet count, and bleeding time
No significant changes in PT and aPTT were observed in any of the animals (data not shown). No major changes in platelet count were observed for the 100 μg/kg (not significant [NS]), 300 μg/kg (NS), and 600 μg/kg 82D6A3 doses (Tables1 and 2). The bleeding time was not significantly prolonged after injection of 100 μg/kg. After injection of 300 μg/kg the bleeding time was increased 2.7 times at 60 minutes and 2.4 times at 150 minutes; however, these changes were not statistically significant. After injection of 600 μg/kg the bleeding time was increased 1.9, 3.0, 2.8, and 1.7 times after 30, 60, 150, and 300 minutes, respectively.
Ex vivo analysis of plasma samples after administration of 100 and 300 μg/kg 82D6A3 to baboons
| Time . | Platelet count (103/mm3) . | Bleeding time (min) . | VWF-Ag levels (μg/mL) . | 82D6A3 levels (μg/mL) . | VWF occupancy (%) . | Collagen binding (%) . |
|---|---|---|---|---|---|---|
| 100 μg/kg (n = 3) | ||||||
| 0 | 286 ± 54 | 2.7 ± 0.4 | 10.2 ± 1.7 | 0 | 2.3 ± 1.3 | 101 ± 7 |
| 30 min | 292 ± 65 | 2.7 ± 0.4 | 10.2 ± 2.5 | 0.4 ± 0.07† | 80 ± 10.8† | 64 ± 7* |
| 60 min | 289 ± 49 | 3.5 ± 2.1 | 8.9 ± 1.4 | 0.4 ± 0.1* | 80 ± 2.4‡ | 69 ± 9 |
| 300 μg/kg (n = 3) | ||||||
| 0 | 286 ± 54 | 2.7 ± 0.4 | 10.2 ± 1.7 | 0 | 2.3 ± 1.3 | 101 ± 7 |
| 30 min | 265 ± 41 | 4.6 ± 0.6 | 8.8 ± 1.4 | 2.9 ± 0.3† | 102 ± 10.4† | 4 ± 1† |
| 60 min | 287 ± 53 | 7.3 ± 2.5 | 9.1 ± 2.4 | 2.8 ± 0.3† | 99 ± 10.6† | 4 ± 1† |
| 150 min | 309 ± 83 | 6.4 ± 3.1 | 9.7 ± 2.7 | 2.6 ± 0.1‡ | 101 ± 7.6† | 4 ± 1† |
| 300 min | 282 ± 7 | 3.15 ± 1.2 | 8.8 ± 0.1 | 2.0 ± 0.5 | 94 ± 0.9† | 4 ± 1† |
| 24 h | 312 ± 46 | 3.25 ± 0.3 | 12.8 ± 1.3 | 0.7 ± 0.2* | 74 ± 31 | 91 ± 18 |
| 48 h | 306 ± 79 | 3 | 13.2 ± 0.8 | 0.2 ± 0.01* | 63 ± 7.8* | 93 ± 0 |
| Time . | Platelet count (103/mm3) . | Bleeding time (min) . | VWF-Ag levels (μg/mL) . | 82D6A3 levels (μg/mL) . | VWF occupancy (%) . | Collagen binding (%) . |
|---|---|---|---|---|---|---|
| 100 μg/kg (n = 3) | ||||||
| 0 | 286 ± 54 | 2.7 ± 0.4 | 10.2 ± 1.7 | 0 | 2.3 ± 1.3 | 101 ± 7 |
| 30 min | 292 ± 65 | 2.7 ± 0.4 | 10.2 ± 2.5 | 0.4 ± 0.07† | 80 ± 10.8† | 64 ± 7* |
| 60 min | 289 ± 49 | 3.5 ± 2.1 | 8.9 ± 1.4 | 0.4 ± 0.1* | 80 ± 2.4‡ | 69 ± 9 |
| 300 μg/kg (n = 3) | ||||||
| 0 | 286 ± 54 | 2.7 ± 0.4 | 10.2 ± 1.7 | 0 | 2.3 ± 1.3 | 101 ± 7 |
| 30 min | 265 ± 41 | 4.6 ± 0.6 | 8.8 ± 1.4 | 2.9 ± 0.3† | 102 ± 10.4† | 4 ± 1† |
| 60 min | 287 ± 53 | 7.3 ± 2.5 | 9.1 ± 2.4 | 2.8 ± 0.3† | 99 ± 10.6† | 4 ± 1† |
| 150 min | 309 ± 83 | 6.4 ± 3.1 | 9.7 ± 2.7 | 2.6 ± 0.1‡ | 101 ± 7.6† | 4 ± 1† |
| 300 min | 282 ± 7 | 3.15 ± 1.2 | 8.8 ± 0.1 | 2.0 ± 0.5 | 94 ± 0.9† | 4 ± 1† |
| 24 h | 312 ± 46 | 3.25 ± 0.3 | 12.8 ± 1.3 | 0.7 ± 0.2* | 74 ± 31 | 91 ± 18 |
| 48 h | 306 ± 79 | 3 | 13.2 ± 0.8 | 0.2 ± 0.01* | 63 ± 7.8* | 93 ± 0 |
Data are mean data ± SD. At each time point, the plasma samples were measured 3 times in 3 different ELISAs for the 3 animal experiments. For statistical analysis, the mean data were used. Studentt test (paired) was used for statistical evaluation.
P < .05.
P < .01.
P < .001.
Ex vivo analysis of plasma samples after administration of 600 μg/kg 82D6A3 to baboons
| . | Platelet count (103/mm3) . | Bleeding time (min) . | VWF-Ag levels (μg/mL) . | 82D6A3 levels (μg/mL) . | VWF occupancy (%) . | Collagen binding (%) . |
|---|---|---|---|---|---|---|
| 0 min | 335 | 1.8 | 14 ± 1.7 | 0 | 6.9 ± 0.1 | 100 ± 0 |
| 30 min | 320 | 3.5 | 11.5 ± 0.9 | 4.5 ± 0.5 | 96 ± 1 | 4 ± 0.2 |
| 60 min | 313 | 5.5 | 10.8 ± 0.1 | 4.8 ± 0.7 | 96 ± 0.2 | 3.5 ± 0.2 |
| 150 min | 356 | 5 | 11.9 ± 1.8 | 3.8 ± 0.5 | 97 ± 4 | 3.5 ± 0.2 |
| 300 min | 334 | 3 | 10.5 | 3.8 ± 0.6 | 97 | 4 |
| 24 h | 347 | ND | 22.9 | 1.4 ± 0.01 | 88 | 45 |
| . | Platelet count (103/mm3) . | Bleeding time (min) . | VWF-Ag levels (μg/mL) . | 82D6A3 levels (μg/mL) . | VWF occupancy (%) . | Collagen binding (%) . |
|---|---|---|---|---|---|---|
| 0 min | 335 | 1.8 | 14 ± 1.7 | 0 | 6.9 ± 0.1 | 100 ± 0 |
| 30 min | 320 | 3.5 | 11.5 ± 0.9 | 4.5 ± 0.5 | 96 ± 1 | 4 ± 0.2 |
| 60 min | 313 | 5.5 | 10.8 ± 0.1 | 4.8 ± 0.7 | 96 ± 0.2 | 3.5 ± 0.2 |
| 150 min | 356 | 5 | 11.9 ± 1.8 | 3.8 ± 0.5 | 97 ± 4 | 3.5 ± 0.2 |
| 300 min | 334 | 3 | 10.5 | 3.8 ± 0.6 | 97 | 4 |
| 24 h | 347 | ND | 22.9 | 1.4 ± 0.01 | 88 | 45 |
Data are mean data ± SD. At each time point, the plasma samples were measured 3 times in 3 different ELISAs for the 2 animal experiments. ND indicates not determined.
82D6A3 plasma concentration, VWF-Ag levels, VWF occupancy, and ex vivo VWF-collagen binding
The 82D6A3 plasma levels remained relatively constant during the first 3 hours of the experiment. Then, they decreased to 69%, 23%, and 7.6% after 300 minutes, 24 hours, and 48 hours, respectively, when 300 μg/kg 82D6A3 was administered (Table 1).
Thirty minutes after injection of the different doses of 82D6A3, plasma VWF-Ag levels decreased slightly and increased above baseline at 24 hours. None of these changes were significant (Tables 1 and 2).
At 60 minutes after administration VWF occupancy was 80% for the 100-μg/kg dose and nearly 100% for the 300-μg/kg and 600-μg/kg doses. VWF remained occupied for an extended period; even 48 hours after the injection of 300 μg/kg 82D6A3, still 63% of the VWF-binding sites were occupied by 82D6A3 (Table 1).
Injection of 100 μg/kg 82D6A3 resulted in an ex vivo inhibition of the VWF-collagen binding of 31% (blood sample taken after 1 hour; Table 1). At doses of 300 μg/kg and 600 μg/kg no interaction between baboon VWF and collagen was observed in samples taken up to 5 hours after the administration of the antibody. Twenty-four hours after the injection of the drug the VWF-collagen interaction recovers (Table1 and 2).
Relation between the ex vivo 82D6A3 plasma levels, VWF occupancy, and collagen binding
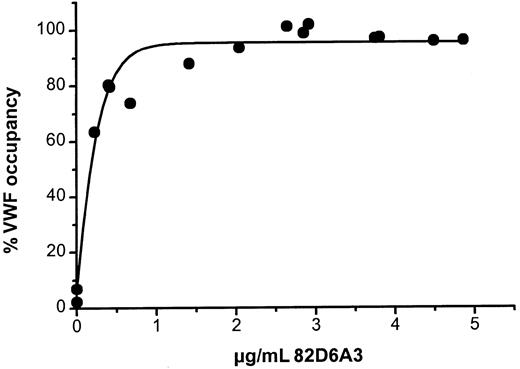
The relationship between 82D6A3 plasma levels and VWF occupancy is illustrated in Figure 5. At plasma concentrations between 1 and 2 μg/mL 82D6A3 nearly 100% of circulating VWF was occupied (Figure 5). There was a good relation between the ex vivo VWF occupancy and the VWF binding to collagen. To obtain inhibition of VWF binding to collagen (20%-40%), a VWF occupancy of at least 80% was required, with complete inhibition at 90% to 100% occupancy (Figure 6). These data were confirmed by in vitro experiments, where different concentrations of 82D6A3 were added to baboon plasma (Figure 6). Occupancy levels of up to 60% resulted in no inhibition of the VWF binding to collagen, whereas up to complete inhibition was observed with 80% to 100% VWF-occupancy levels.
Relationship between the ex vivo VWF occupancy and 82D6A3 plasma levels.
All mean data measured at the different time points in the 3 different dosage studies were used (Tables 1 and 2).
Relationship between the ex vivo and in vitro VWF binding to collagen and the VWF occupancy.
The ex vivo data are the mean data measured at the different time points in the 3 different dosage studies shown in Tables1 and 2 (●) and the in vitro data were obtained by adding known concentrations of mAb 82D6A3 to undiluted baboon plasma and measuring VWF occupancy and collagen binding (○). Data are the mean of 2 determinations in duplicate.
Relationship between the ex vivo and in vitro VWF binding to collagen and the VWF occupancy.
The ex vivo data are the mean data measured at the different time points in the 3 different dosage studies shown in Tables1 and 2 (●) and the in vitro data were obtained by adding known concentrations of mAb 82D6A3 to undiluted baboon plasma and measuring VWF occupancy and collagen binding (○). Data are the mean of 2 determinations in duplicate.
Discussion
Platelet adhesion to a damaged vessel wall is the first step in arterial thrombus formation. The tethering of platelets by VWF to the collagen exposed in the damaged vessel wall is especially important under high shear conditions. Antithrombotics that interfere with the GPIb-VWF axis have been studied in animal models and were shown to be effective.13,14 The present study evaluated for the first time the antithrombotic effects of the inhibition of the VWF-collagen interaction in vivo. For this purpose, we used a monoclonal antihuman VWF antibody 82D6A3, which by binding to the VWF A3-domain, inhibits VWF binding to fibrillar collagens types I and III.17 We here showed that 82D6A3 cross-reacts with baboon VWF and inhibits baboon VWF binding to collagen type I under static conditions. Moreover, in vitro 82D6A3 is able to remove collagen-bound VWF from its surface, which in view of the presence of collagen-bound VWF in the damaged vessel wall, might be a prerequisite for a proper function in vivo. Because the subendothelium, however, not only includes different types of collagen that may interact differently with VWF, and other matrix proteins that may affect the binding of VWF, and because, in addition, it may contain different VWF forms, such as ultralarge forms unprocessed by the VWF-cleaving plasma protease, no direct extrapolation is possible.
A modified Folts model was used to evaluate the antithrombotic efficacy of 82D6A3 under high shear conditions18 in baboons. This model allows study of CFRs due to platelet-dependent thrombi forming at the injured, stenotic site of the artery.21 We demonstrated that inhibition of the VWF-collagen interaction is accompanied by an antithrombotic effect in vivo. Indeed, in all 5 animals receiving doses of 300 μg/kg or higher, a 100% inhibition of the CFRs was obtained, whereas administration of 100 μg/kg resulted in 58% inhibition.
Administration of the lowest 82D6A3 dose (100 μg/kg) to the baboons revealed that when 80% of the VWF-A3 domain sites were occupied (30%-36% inhibition of VWF binding to collagen), 58% reduction in CFRs was observed with no prolongation of the bleeding time. When a 100% decrease of CFRs was observed after administration of 300 μg/kg 82D6A3 (and also of 600 μg/kg) the bleeding time increased 2.7 times (60 minutes, 300 μg/kg), which was not statistically significant. At these doses, 100% VWF was occupied with 82D6A3 and 100% ex vivo inhibition of the VWF binding to collagen was observed. It is interesting to note that when we studied the effect of 300 μg/kg of an anti-GPIIb/IIIa mAb 16N7C2 in exactly the same animal model, a more than 15-fold increase in bleeding time was observed, which was statistically significant.19 Thus the therapeutic window of 82D6A3 seems to be broader when compared to the one of an anti-GPIIb/IIIa blocker in our animal model.19 This suggests that bleeding problems might be less expected when 82D6A3 is used as an antithrombotic agent. The observation that no significant decreases in platelet count and VWF-Ag levels were observed furthermore pointed out that the observed antithrombotic effect is indeed due to the specific inhibition of the VWF-collagen interaction.
82D6A3 has a high affinity for VWF (Kd 0.4 nmol17), which is reflected here by the observation that VWF is still occupied by 63% with the antibody 48 hours after its administration. On the other hand, to be functionally active, at least up to 80% VWF occupancy is needed to have an ex vivo and in vitro inhibition of VWF binding to collagen. This level was maintained for up to 5 hours after the administration of 300 and 600 μg/kg 82D6A3. The observation that up to 80% VWF occupancy is needed to be functionally relevant might be explained by the fact that when VWF is occupied for 74%, the ratio VWF monomer/mAb is 8. So too many VWF subunits are devoid of mAb to have some effect in VWF-collagen binding. It has to be noted, however, that at 100% VWF occupancy, the VWF monomer/mAb ratio (both in vitro and ex vivo) is 2.8, which demonstrates that both in vitro and ex vivo not all VWF-A3 domains are accessible for 82D6A3 binding.
Although many in vitro studies demonstrated that the VWF-collagen interaction is vital for sustaining platelet adhesion under high shear conditions, this study is the first experimental proof of the in vivo importance of this interaction. It is of interest to note that for a very long time no bleeding disorders due to an isolated defect in VWF binding to collagen were known. Only recently 2 patients, mother and daughter, were identified with a moderate bleeding disorder due to Ser968Thr mutation in the A3 domain of VWF resulting in a defective binding of VWF to collagen.22 These recent results together with our data finally unequivocally demonstrate the in vivo relevance of the VWF-collagen interaction.
In conclusion, blockade of VWF-collagen interaction by 82D6A3 reduced platelet thrombus formation in the injured and stenosed baboon femoral arteries in a dose-dependent manner. In view of the lack of effect on the bleeding time, it is worthwhile to develop this approach further to determine its potential to treat acute arterial thrombotic syndromes.
We thank Miss Griet Vandecasteele, Mr Stephan Vauterin, Mr Seb Lamprecht, and Mr Jan P. Roodt for their excellent technical assistance and Dr Jef Arnout for help with the statistical analysis.
Supported by a EU-Biomed grant (PC 96.3517) and a Bilateral Collaboration grant between the Flemish and South African Government (BIL 98/64). D.W. was a Junior Fellow of the KU Leuven. D.W. and K.V. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hans Deckmyn, Laboratory for Thrombosis Research-IRC, K U Leuven Campus Kortrijk, E. Sabbelaan 53, B-8500 Kortrijk, Belgium; e-mail: hans.deckmyn@kulak.ac.be.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal