A reduced-intensity conditioning regimen was investigated in 45 patients with hematologic malignancies who were considered poor candidates for conventional myeloablative regimens. Median patient age was 49 years. Twenty-six patients previously failed autologous transplantation, and 18 patients had a refractory disease at the time of transplantation. In order to decrease nonrelapse mortality, and enhance the graft-versus-tumor effect, a program was designed in which a reduced conditioning with thiotepa, fludarabine, and cyclophosphamide was associated with programmed reinfusions of donor lymphocytes for patients without graft-versus-host disease (GVHD), not achieving clinical and molecular remission after transplantation. GVHD prophylaxis consisted of cyclosporine A and methotrexate. Seventeen patients received marrow cells and 28 received mobilized hematopoietic cells. All patients engrafted. The probability of grades II-IV and III-IV acute GVHD were 47% and 13%, respectively. The probability of nonrelapse mortality, progression-free survival, and overall survival were 13%, 57%, and 53%, respectively. Thirteen patients in complete remission had a polymerase chain reaction marker for minimal disease monitoring; 10 achieved molecular remission after transplantation. Nine patients received donor lymphocytes: one patient with mantle cell lymphoma had a minimal response, one patient with refractory anemia with excess of blasts in transformation achieved complete remission, and 7 patients did not respond. At a median follow-up of 385 days (range, 24 to 820 days), 25 patients (55%) were alive in complete remission. Although longer follow-up is needed to evaluate the long-term outcome, the study shows that this regimen is associated with a durable engraftment, has a low nonrelapse mortality rate, and can induce clinical and molecular remissions.
Introduction
High-dose chemoradiotherapy followed by allogeneic hematopoietic stem cell transplantation (HSCT) is extensively used to treat patients with hematologic malignancies. This procedure is often limited to young patients in good medical condition because of the increased risk of regimen-related toxicity and graft-versus-host disease (GVHD) that occurs with increasing age and poor performance status.1,2 On the other hand, the median age for hematologic malignancies is over 50 years, and then only a minority of patients can benefit from HSCT. In an effort to reduce transplant-related mortality (TRM) in patients over 45 years or heavily pretreated, or affected by medical comorbidities, different nonmyeloablative conditioning regimens have been developed. It has been shown by several groups that donor cell engraftment can be obtained using a fludarabine-containing or low-dose total body irradiation (TBI)–containing regimen.3-10 The rationale for reducing the chemoradiotherapy dosage relies on the concept that the curative potential of HSCT is not solely due to the conditioning regimen but also to the graft-versus-tumor (GVT) effect. A convincing evidence for the GVT effect is that donor lymphocyte infusions (DLIs) can reinduce remissions in patients who have relapsed following allogeneic transplantation.11,12 Patients with chronic myeloid leukemia are most likely to respond, but responses have also been documented in patients with acute leukemia, myelodysplastic syndromes, myeloma, and lymphoma.13 14
Raiola et al have recently shown that the combination of thiotepa and cyclophosphamide provides a successful engraftment in 80% of patients with a rather low nonrelapse mortality (22%).7 Such a moderate-intensity regimen has shown an interesting antileukemia effect in relatively old patients (median age 51 years) with 60% of patients disease-free at 2 years.7 Because of these promising results, we decided to employ thiotepa in the conditioning phase. Thiotepa can promote engraftment through 2 mechanisms: stem cell depletion and immunesuppression.15 Our conditioning regimen has been designed to be particularly active in lymphoid tumors, and includes thiotepa, fludarabine, and cyclophosphamide. The inclusion of fludarabine in the regimen was for 3 main reasons: (1) to improve cytotoxic activity in relapsed lymphomas; (2) to allow a reduction in cyclophosphamide dosage to minimize potential toxicity in heavily pretreated patients; and (3) to augment pretransplantation immunesuppression, in order to improve the engraftment of lymphoid lineage for a better exploitation of GVT effect. To further enhance the GVT effect, we planned the use of DLIs in all patients without GVHD, not achieving clinical and molecular remission after transplantation.
This report describes the results of our pilot trial in 45 patients with hematologic malignancies who were considered poor candidates for conventional conditioning because of age, previous therapies, or medical comorbidities.
Patients, materials, and methods
Eligibility criteria
Patients with hematologic malignancies were enrolled at 5 hospitals in Italy. The study design was approved by the ethics committee and all patients gave written informed consent to participate. Patients with lymphoma, acute leukemia, myelodysplasia, multiple myeloma, and chronic lymphocytic leukemia, between ages 45 and 70 years were considered eligible. Patients were also eligible if they were younger than 45, but were deemed poor candidates for conventional conditioning because of comorbidities (cardiac ejection fraction < 50%; diffusion capacity of carbon monoxide < 50% predicted; abnormal liver function tests) and/or extensive prior therapy (more than 2 lines of conventional chemoradiotherapy or autologous transplantation). Patients were required to have a human leukocyte antigen (HLA)–identical sibling or one-antigen-mismatched family donor as determined by serologic typing for HLA A/B and molecular typing for HLA C, DR/DQ. Demographics and patient characteristics are shown in Table 1. Forty-five patients were enrolled in the study from September 1998 to January 2001.
Patient and disease characteristics
| N | 45 |
| Median age, y (range) | 49 (20-68) |
| Sex, male/female | 28/17 |
| Median no. of prior therapies (range) | 2 (0-5) |
| Median follow-up, d (range) | 372 (24-820) |
| Prior ABMT | 26 |
| CMV status neg/neg | 0 |
| Thiotepa mg/kg, 15/10/5 | 14/24/7 |
| Stem cell source | |
| marrow | 17 |
| PBPC | 28 |
| Median CD 34+ × 10e6/kg, BM/PBPC | 3.8/5.7 |
| Donor type | |
| 6 of 6 matched related | 42 |
| 5 of 6 mismatched related | 3 |
| Diagnosis and status at transplantation | |
| AML/RAEB-t/ALL | 5/6/1 |
| CR1 | 3 |
| ≥CR2 | 2 |
| Untreated | 4 |
| Refractory | 3 |
| Indolent/high-grade lymphoma | 13/11 |
| CR1 | 1 |
| PR | 6 |
| ≥CR2 | 4 |
| Refractory | 9 |
| Hodgkin disease/multiple myeloma | 4/5 |
| CR1 | 0 |
| PR | 2 |
| ≥CR2 | 0 |
| Untreated relapse | 1 |
| Refractory | 6 |
| N | 45 |
| Median age, y (range) | 49 (20-68) |
| Sex, male/female | 28/17 |
| Median no. of prior therapies (range) | 2 (0-5) |
| Median follow-up, d (range) | 372 (24-820) |
| Prior ABMT | 26 |
| CMV status neg/neg | 0 |
| Thiotepa mg/kg, 15/10/5 | 14/24/7 |
| Stem cell source | |
| marrow | 17 |
| PBPC | 28 |
| Median CD 34+ × 10e6/kg, BM/PBPC | 3.8/5.7 |
| Donor type | |
| 6 of 6 matched related | 42 |
| 5 of 6 mismatched related | 3 |
| Diagnosis and status at transplantation | |
| AML/RAEB-t/ALL | 5/6/1 |
| CR1 | 3 |
| ≥CR2 | 2 |
| Untreated | 4 |
| Refractory | 3 |
| Indolent/high-grade lymphoma | 13/11 |
| CR1 | 1 |
| PR | 6 |
| ≥CR2 | 4 |
| Refractory | 9 |
| Hodgkin disease/multiple myeloma | 4/5 |
| CR1 | 0 |
| PR | 2 |
| ≥CR2 | 0 |
| Untreated relapse | 1 |
| Refractory | 6 |
ABMT indicates autologous bone marrow transplantation; CMV, cytomegalovirus; PBPC, mobilized peripheral blood cells; BM, marrow cells; AML, acute myelogenous leukemia; RAEB-t, refractory anemia with excess of blasts in transformation; ALL, acute lymphocytic leukemia; CR, complete remission; and PR, partial remission.
Conditioning regimen and mobilization of donor hematopoietic cells
Patients received 5 mg/kg thiotepa every 12 hours for 2 doses (day −6); 30 mg/kg cyclophosphamide (days −4 and −3); 30 mg/m2 fludarabine (days −4 and −3), 4 hours after cyclophosphamide administration; and transplantation of marrow or mobilized hematopoietic cells on day 0. The dosage of thiotepa was adjusted to 15 mg/kg for patients younger than 45 years and to 5 mg/kg for those older than 60 years. Three patients received transplants of one-antigen-mismatched grafts, and received antithymocyte globulin (10 mg/kg over 4 days) as part of the conditioning regimen.
Patients were required to have a sibling donor (age 18 to 75 years), willing and capable of donating lenogastrim-stimulated peripheral blood hematopoietic cells or bone marrow. Donors received 5 μg/kg lenogastrim subcutaneously every 12 hours; on day 5 and/or 6, large volume leukapheresis was performed and stem cells were reinfused without cryopreservation. Target value of CD34+ cells was 5 × 106/kg of the recipient body weight (range, 3 × 106/kg to 6 × 106/kg). During the initial phase of the trial bone marrow cells were more frequently used in 2 centers, then they were used in case of an unwilling donor or a donor unsuitable for lenogastrim administration. The bone marrow harvest was performed according to standard techniques.
Supportive care
Patients were managed in laminar airflow rooms. All patients received prophylaxis with cotrimoxazole or pentamidine againstPneumocystis carinii infection. Acyclovir and fluconazole or itraconazole prophylaxis were routinely used. Red cell and platelet transfusions were given to maintain hemoglobin levels above 8 g/dL and platelet counts above 10 × 109/L. Blood products were irradiated. Neutropenic patients received broad-spectrum intravenous antibiotics according to each hospital policy for the management of febrile neutropenia. Lenogastrim at 5 μg/kg per day was administered subcutaneously from day +7 until the neutrophil count was at least 1000/μL for 3 consecutive days.
GVHD prophylaxis consisted of 1 mg/kg per day cyclosporine A (CSA) as a continuous infusion, from day −6 to day −2, and then 2 mg/kg per day from days −1 to +15; then CSA was given orally in a twice-daily divided dose (total dose, 4 mg/kg per day). Doses were adjusted to mantain whole-blood steady-rate through levels at 150 ng/mL to 300 ng/mL, and modified as clinically indicated for nephrotoxicity. A dose of 10 mg/m2 methotrexate was given intravenously (iv) on day +1; 8 mg/m2 methotrexate was given on days +3 and +6, followed 24 hours later by a single dose of leucovorin rescue at 25 mg/m2. CSA was administered at full dose through day +100 and, if GVHD did not occur, the dose was tapered by 10% every week thereafter.
Reinfusion of donor lymphocytes
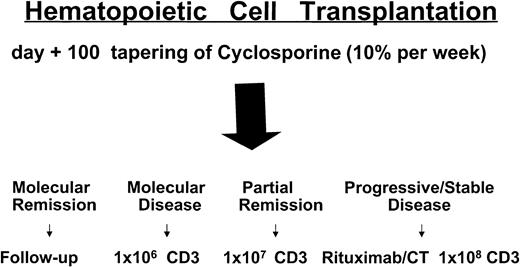
Donors underwent a leukoapheresis to collect lymphocytes prior to mobilization of hematopoietic cells. It has been recently shown that granulocyte colony-stimulating factor (G-CSF) has an immune-modulatory effect on some T-lymphocyte subsets, decreasing their responsiveness to allogeneic stimuli.16 Patients in complete remission (CR) or partial remission (PR) not achieving molecular remission by day +100, without any sign of acute GVHD (aGVHD), tapered CSA (10% every week). If no sign of aGVHD developed 3 weeks after CSA withdrawal, these patients were scheduled to receive donor CD3+lymphocytes. Patients with stable or progressive disease 40 to 60 days after transplantation rapidly tapered CSA (in 7 to 14 days) and then received CD3+ lymphocytes if aGVHD did not occur. For DLI doses see Figure 1.
Treatment plan and timing of donor lymphocyte infusions.
CT indicates chemotherapy; CD3, CD3+ donor lymphocytes.
Treatment plan and timing of donor lymphocyte infusions.
CT indicates chemotherapy; CD3, CD3+ donor lymphocytes.
Chimerism analysis
Myeloid and lymphoid cell populations were sorted using CD13 and CD3 antigens. Chimerism was evaluated using PCR to amplify microsatellites.17 Monitoring started at day +30, and was performed every 30 days for 4 to 6 months, then every 2 months up to 1 year, and then every 4 months. This approach is based on a multiplex PCR amplification of 9 short tandem repeats (STR) loci and the amelogenin locus, which discriminates between X and Y chromosomes. PCR was performed using the AmpFISTR Profiler PCR amplification kit (Perkin Elmer, Monza, Italy). DNA (1 ng) was amplified in a final volume of 25 μL. The tetranucleotide STR loci amplified in this reaction were: D3S1358, vWA, FGA (all labeled with 5-FAM); TH01, TPOX, CSF1PO (all labeled with JOE); D5S818, D132317, D7S820 (all labeled with NED); and in addition, the amelogenin locus (labeled with JOE). The cycle conditions were: 95°C for 11 minutes, followed by 28 cycles at 94°C for 1 minute, at 59°C for 1 minute, and at 72°C for 1 minute. The final elongation step was at 60°C for 45 minutes. Separation and detection of the amplified products was performed on an ABI 377 automated DNA sequencer (PE Biosystems). A denaturing polyacrylamide gel containing 1X TBE, 6 M urea, and 5% Long Ranger (Perkin Elmer) gel solution was used. An internal size standard (Genescan ROX 350, PE Biosystems) and a formamide loading dye solution was added to each sample. After the denaturation, 1.5 μL of this mixture was loaded on the gel and run for 2 hours at 3000 V at 51°C. Results were analyzed using the Genescan 2.1 software (PE Biosystems).
PCR amplification of Bcl-1/IgH and Bcl-2/IgH translocation
Amplification of Bcl-1/JH junction was performed using eminested PCR on diagnostic tissues (marrow or lymph node cells). A consensus primer derived from the 3′ end of JH region JH3: 5′-ACCTGAGGAGACGGTGACC-3′ was used for chromosome 14. The chromosome 11q13-specific oligonucleotides have the following sequences: P2: 5′-GAAGGACTTGTGGGTTGC-3′; P4: 5′-GCTGCTGTACACATCGGT-3′.18For PCR amplification, 1 μg of genomic DNA was amplified with P2 and JH3 primers (10 pmol); 2 μL of the first PCR product was then reamplified with an internal primer P4 and JH3 (10 pmol). Amplified DNAs were analyzed by electrophoresis on 2% agarose gel containing ethidium bromide, and visualized by ultraviolet light.
Amplification of Bcl-2/JH junction was performed using nested PCR on diagnostic tissues (marrow or lymph node cells). In follicular lymphomas, the amplification of both major (MBR) and minor (mcr) breakpoints were performed. Genomic DNA (1 μg) was amplified, using oligonucleotide primers and amplification conditions previously described.19 The reamplification for 30 cycles of a 5 μL aliquot of the first reaction was performed using internal primers. Amplified DNAs were analyzed by electrophoresis on 2% agarose gel containing ethidium bromide, and visualized by ultraviolet light.
PCR amplification and sequencing of immunoglobulin rearranged variable regions
Rearranged variable regions (VDJs) were amplified starting from total cDNA or genomic DNA depending on sample availability. DNAs were derived from diagnostic tissues with at least 20% of tumor cell infiltration. Briefly, 1 μg of genomic DNA or 1 μL of total cDNA, was amplified using 2 sets of consensus sense primers derived from the immunoglobulin heavy-chain (IgH) leader or FR1 region, and an antisense primer derived from the JH 3′ end (JH3 5′-ACCTGAGGAGACGGTGACCAGGGT-3′).20 PCR products were analyzed by electrophoresis on 2% agarose gel. Direct sequencing of amplified DNAs was performed using the Promega fmol sequencing system (Fireuse, Italy) or using automated sequencing. When the sequence quality will not allow a complete reading of CDR regions, DNA was cloned using TA cloning kit (Invitrogen). Restriction enzyme analysis was carried out on plasmid DNAs prepared by the alkaline lysis method, and miniprep plasmid DNAs were then sequenced. The analyses of sequencing data were performed using the PC-GENE software (IntelliGenetics, Mountain View, CA).
PCR detection of residual tumor cells
Bone marrow samples after transplantation were evaluated for the presence of residual tumor cells. DNA (1 μg) was amplified using the Bcl-2, Bcl-1, or IgH assays. The assays for Bcl-2 and Bcl-1 were previously described. Promyelocytic leukemia/retinoic acid receptor-alpha gene (PML/RARα) assay used RNA template and the protocol was the same described by van Dongen et al.21Nested PCR for IgH rearrangement was carried out as follows on DNA samples: a first amplification was performed using a consensus primer derived from the tumor VH family and an antisense derived from the 3′ end of the JH region.22 The first reaction was carried out for 28 cycles (denaturation 94°C for 30 minutes, annealing 64°C for 30 minutes, extension 72°C for 30 minutes) with a final extension of 7 minutes. Amplified DNA (3 μL) were then reamplified using CDRII sense and CDRIII antisense primers using 2.5 U of AmpliTaq Gold (PE Applied Biosystems). The second reaction was carried out for 35 cycles (denaturation 94°C for 30 minutes, annealing depending on specific primers melting temperature for 30 minutes, extension 72°C for 30 minutes) with a final extension of 7 minutes. The first and the second reactions were performed using AmpliTaq Gold (PE Applied Biosystems). Specific primers for the second reaction were selected, when possible, with a slightly higher melting temperature to minimize inappropriate annealing of primers used during the first amplification. PCR products were visualized on a 3% Metaphor agarose gel (FMC Bioproducts, Rockland, ME) stained with ethidium bromide (0.5 μg/mL). Two polyclonal DNAs were always used as negative controls. To avoid false negatives, all the DNA samples failing to produce a PCR product were reamplified, and the DNA quality was tested by amplifying the sequence of p53 exon 5 or N-ras exon 2.
Statistical methods
Actuarial curves were estimated according to the Kaplan-Meier method. Surviving patients were censored on the last day of follow-up. The significance of differences between the curves was estimated by the log-rank test.
Results
Engraftment and chimerism
All 45 patients had a sustained engraftment as defined by neutrophil counts above 0.5 × 109/L and an untransfused platelet count of above 20 × 109/L for at least 3 consecutive days. The median time to recover an absolute neutrophil count of 0.5 × 109/L was 13 days (range, 9 to 22 days) and the median time to achieve platelets above 20 × 109/L was 15 days (range, 8 to 50 days). Patient no. 14 received a one-antigen-mismatched graft, and after being discharged, developed cytomegalovirus (CMV) antigenemia, and became cytopenic on day +50. Chimerism was always full-donor; after treatment with gancyclovir and foscarnet the patient cleared CMV antigen and DNA; on day +80 he received 5 × 106/kg donor CD34+ cells, selected with Clinimacs device (Miltenyi, Germany); he is now alive and well with normal counts.
All patients had chimerism studies performed on peripheral blood, using microsatellite PCR or fluorescent in situ hybridization for X and Y chromosomes. Thirty-eight patients were conditioned using 15 mg/kg (14 patients) or 10 mg/kg thiotepa and in this cohort all the patients achieving marrow remission were full-donor chimeras. Only 12 of these 38 patients had chimerism analyses performed separately on myeloid and lymphoid populations; the results showed that a complete donor chimerism was achieved on both lineages (ie, more than 95% donor cells). Seven patients were conditioned with 5 mg/kg thiotepa and 2 of them were mixed chimeras. Patient no. 31 had 90% donor myeloid cells and 80% donor lymphoid cells at day 30. This changed to 87% and 78%, respectively, on day 60. Patient no. 6 had 98% donor myeloid cells and 70% donor lymphoid cells at day 30. This changed to 100% and 75%, respectively, on day 60. The data seem to suggest that thiotepa dosage is important for engraftment, but the numbers are too small to draw any definitive conclusion.
Acute and chronic graft-versus-host disease
Forty-four patients were evaluable for GVHD (patient no. 45 died at day +24). The estimated probability of grade II-IV aGVHD was 47% at 11 months (95% confidence interval [CI], 39%-56%); the estimated probability of grade III-IV aGVHD was 13% at 11 months (95% CI, 7%-19%). Overall, 20 patients had grade II-IV aGVHD; of these 3 had grade III and 2 had grade IV aGHVD. Changes in the immunesuppressive therapy or lymphocyte infusions were involved in GVHD onset; 15 patients underwent a rapid tapering or withdrawal of CSA for disease recurrence, and 4 of them developed aGVHD; 2 patients had aGVHD after receiving DLIs for disease relapse. Patient no. 40 received 2 infusions, the second one was combined with low-dose interleukin-2 and GVHD occurred. Forty patients were evaluable for chronic GVHD; limited and extensive forms were found in 8 and 11 patients, respectively. Extensive forms were almost always preceded by aGVHD (see Table2). Patient no. 27 developed chronic GVHD after DLIs.
Transplantation outcome and molecular monitoring of residual disease
| Patient . | Diagnosis . | Acute GVHD grade . | Chronic GVHD . | Status d +60 . | MRD d +60 . | Current status/PCR (d) . | Cause of death . |
|---|---|---|---|---|---|---|---|
| 1 | CLL | 1 | Abs | PR | NA | PD (700) | — |
| 2 | CLL | 1 | Lim | CR | Pos | CR/MD (310) | — |
| 3 | CLL | 0 | Abs | CR | Na | CR/MR (130) | — |
| 4 | CLL | 2 | Lim | CR | Neg | CR/MR (380) | — |
| 5 | Waldens | 0 | Abs | SD | NA | SD (220) | — |
| 6 | MZL | 3 | Ext | PR | NA | Dead (234) | Pneumonia/GVHD |
| 7 | FCL | 0 | Abs | CR | Neg | CR/MR (755) | — |
| 8 | FCL | 2 | Abs | CR | Neg | CR/MR (510) | — |
| 9 | FCL | 2* | Ext | CR | Pos | CR/MR (485) | — |
| 10 | MCL | 2 | Abs | PR | Na | Dead (188) | Lymphoma |
| 11 | MCL | 2 | Ext | PR | Na | Dead (698) | Lymphoma |
| 12 | MCL | 3 | Ext | PR | Na | CR (160) | — |
| 13 | MCL | 2 | Ext | CR | Neg | CR/MR (850) | — |
| 14 | DLCL | 1 | Lim | CR | Neg | CR/MR (735) | — |
| 15 | DLCL | 0 | Abs | CR | — | CR (220) | — |
| 16 | DLCL | 1 | Abs | CR | — | PD (490) | — |
| 17 | DLCL | 1 | Abs | CR | — | CR (440) | — |
| 18 | T cell | 2 | Abs | CR | — | CR (340) | — |
| 19 | DLCL | 2 | Na | NA | Na | Dead (69) | TTP |
| 20 | DLCL | 0 | Abs | CR | Na | CR (180) | — |
| 21 | BL | 0 | Abs | CR | Neg | CR/MR (250) | — |
| 22 | DLCL | 1 | Na | PR | Na | Dead (95) | Lymphoma |
| 23 | DLCL | 2 | Lim | CR | — | CR (850) | — |
| 24 | DLCL | 1 | Lim | CR | Neg | CR/MR (850) | — |
| 25 | HD | 1 | Lim | SD | Na | Dead (240) | Hodgkin |
| 26 | HD | 0 | Abs | PD | Na | PD (170) | — |
| 27 | HD | 0 | Ext | SD | Na | PD (600) | Hodgkin |
| 28 | HD | 2* | Lim | CR | — | CR (410) | — |
| 29 | MM | 4 | Ext | PR | Na | Dead (140) | GVHD |
| 30 | MM | 0 | Abs | PR | Na | PR (320) | — |
| 31 | MM | 2* | lim | CR | Na | CR (280) | — |
| 32 | MM | 0 | Abs | PR | Na | CR (260) | — |
| 33 | MM | 2† | Ext | SD | Na | PD (230) | — |
| 34 | AML | 0 | Na | PD | Na | Dead (66) | Leukemia |
| 35 | AML | 0 | Ext | CR | — | CR (680) | — |
| 36 | AML | 0 | Abs | CR | — | CR (230) | — |
| 37 | APL | 1 | Abs | CR | Neg | PD/MD (300) | — |
| 38 | AML | 4* | Ext | PD | Na | CR (160) | — |
| 39 | ALL | 2 | Abs | CR | Neg | CR/MR (750) | — |
| 40 | RAEBt | 3† | Ext | CR | — | Dead (650) | Leukemia |
| 41 | RAEBt | 2 | Na | CR | — | Dead (94) | CNS toxop |
| 42 | RAEBt | 2 | Abs | CR | — | CR (760) | — |
| 43 | RAEBt/CLL | 1 | Abs | CR | CLL neg | RAEBt/CR (445) | |
| CLL/CR/MR | — | ||||||
| 44 | RAEBt/MM | 1 | Abs | CR | — | Dead (500) | Leukemia |
| 45 | RAEBt | na | na | na | na | Dead (24) | Encephalytis |
| Patient . | Diagnosis . | Acute GVHD grade . | Chronic GVHD . | Status d +60 . | MRD d +60 . | Current status/PCR (d) . | Cause of death . |
|---|---|---|---|---|---|---|---|
| 1 | CLL | 1 | Abs | PR | NA | PD (700) | — |
| 2 | CLL | 1 | Lim | CR | Pos | CR/MD (310) | — |
| 3 | CLL | 0 | Abs | CR | Na | CR/MR (130) | — |
| 4 | CLL | 2 | Lim | CR | Neg | CR/MR (380) | — |
| 5 | Waldens | 0 | Abs | SD | NA | SD (220) | — |
| 6 | MZL | 3 | Ext | PR | NA | Dead (234) | Pneumonia/GVHD |
| 7 | FCL | 0 | Abs | CR | Neg | CR/MR (755) | — |
| 8 | FCL | 2 | Abs | CR | Neg | CR/MR (510) | — |
| 9 | FCL | 2* | Ext | CR | Pos | CR/MR (485) | — |
| 10 | MCL | 2 | Abs | PR | Na | Dead (188) | Lymphoma |
| 11 | MCL | 2 | Ext | PR | Na | Dead (698) | Lymphoma |
| 12 | MCL | 3 | Ext | PR | Na | CR (160) | — |
| 13 | MCL | 2 | Ext | CR | Neg | CR/MR (850) | — |
| 14 | DLCL | 1 | Lim | CR | Neg | CR/MR (735) | — |
| 15 | DLCL | 0 | Abs | CR | — | CR (220) | — |
| 16 | DLCL | 1 | Abs | CR | — | PD (490) | — |
| 17 | DLCL | 1 | Abs | CR | — | CR (440) | — |
| 18 | T cell | 2 | Abs | CR | — | CR (340) | — |
| 19 | DLCL | 2 | Na | NA | Na | Dead (69) | TTP |
| 20 | DLCL | 0 | Abs | CR | Na | CR (180) | — |
| 21 | BL | 0 | Abs | CR | Neg | CR/MR (250) | — |
| 22 | DLCL | 1 | Na | PR | Na | Dead (95) | Lymphoma |
| 23 | DLCL | 2 | Lim | CR | — | CR (850) | — |
| 24 | DLCL | 1 | Lim | CR | Neg | CR/MR (850) | — |
| 25 | HD | 1 | Lim | SD | Na | Dead (240) | Hodgkin |
| 26 | HD | 0 | Abs | PD | Na | PD (170) | — |
| 27 | HD | 0 | Ext | SD | Na | PD (600) | Hodgkin |
| 28 | HD | 2* | Lim | CR | — | CR (410) | — |
| 29 | MM | 4 | Ext | PR | Na | Dead (140) | GVHD |
| 30 | MM | 0 | Abs | PR | Na | PR (320) | — |
| 31 | MM | 2* | lim | CR | Na | CR (280) | — |
| 32 | MM | 0 | Abs | PR | Na | CR (260) | — |
| 33 | MM | 2† | Ext | SD | Na | PD (230) | — |
| 34 | AML | 0 | Na | PD | Na | Dead (66) | Leukemia |
| 35 | AML | 0 | Ext | CR | — | CR (680) | — |
| 36 | AML | 0 | Abs | CR | — | CR (230) | — |
| 37 | APL | 1 | Abs | CR | Neg | PD/MD (300) | — |
| 38 | AML | 4* | Ext | PD | Na | CR (160) | — |
| 39 | ALL | 2 | Abs | CR | Neg | CR/MR (750) | — |
| 40 | RAEBt | 3† | Ext | CR | — | Dead (650) | Leukemia |
| 41 | RAEBt | 2 | Na | CR | — | Dead (94) | CNS toxop |
| 42 | RAEBt | 2 | Abs | CR | — | CR (760) | — |
| 43 | RAEBt/CLL | 1 | Abs | CR | CLL neg | RAEBt/CR (445) | |
| CLL/CR/MR | — | ||||||
| 44 | RAEBt/MM | 1 | Abs | CR | — | Dead (500) | Leukemia |
| 45 | RAEBt | na | na | na | na | Dead (24) | Encephalytis |
After cyclosporine withdrawal.
After DLI.
CLL indicates chronic lymphocytic leukemia; MZL, marginal zone lymphoma; FCL, follicular cell lymphoma; MCL, mantle cell lymphoma; DLCL, diffuse large cell lymphoma; BL, Burkitt lymphoma; HD, Hodgkin disease; MM, multiple myeloma; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; ALL, acute lymphocytic leukemia; RAEBt, refractory anemia with excess of blasts in transformation; Abs, absent; Lim, limited; Ext, extensive; PR, partial remission; CR, complete remission; SD, stable disease; PD, progressive disease; MD, molecular disease; MR, molecular remission; CNS, central nervous system; TTP, thrombotic thrombocytopenic purpura; Toxop, toxoplasmosis; Waldens, lymphoplasmacytoid lymphoma; and MRD, minimal residual disease.
Toxicity and nonrelapse mortality
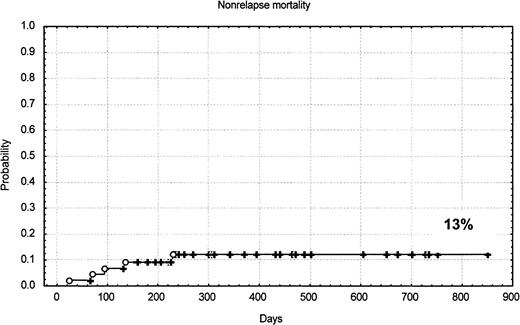
The conditioning regimen was generally well tolerated in terms of mucosal and/or organ toxicity. Five patients died as a consequence of the transplantation: one for GVHD, one for pneumonia and GVHD, one for cerebral toxoplasmosis, one for encephalitis, and one for transplant-associated thrombotic microangiopathy. The estimated probability of nonrelapse mortality was 13% at 10 months (95% CI, 1%-18%). Twenty-eight patients had CMV antigen reactivation and one had CMV gastrointestinal disease. Four of 5 deaths were in patients previously autografted. There were no deaths in patients younger than 54 years. There was no difference in nonrelapse mortality rate among patients receiving marrow or mobilized hematopoietic cells.
Disease response and relapses
Current disease status and transplantation outcome are shown in detail in Table 2. Nine of 11 patients with acute leukemia or RAEB-t achieved CR and 4 of them subsequently relapsed. Patient no. 45 was not evaluable because he died of toxicity at day +24. Eight of 13 patients with indolent and 9 of 10 with high-grade lymphomas achieved CR: one patient with follicular and one with high-grade lymphoma relapsed so far. The patient with follicular lymphoma was treated with 4 rituximab infusion and CSA withdrawal, and he has been in second CR for one year. Disease response in patient no. 19 was not evaluable because he died of toxicity before restaging.
One of 4 patients with Hodgkin disease, and 1 of 5 with myeloma achieved CR. The patient (no. 28) with Hodgkin disease relapsed 74 days after transplantation and went back into remission after CSA withdrawal and GVHD occurrence; she now has chronic GVHD and is still in remission 320 days after the achievement of the second CR. Ten patients with lymphoma or myeloma achieved PR after transplantation, and 4 of them already suffered disease progression. Patient no. 31 had myeloma and was in PR after transplantation; he withdrew CSA because of disease progression 4 months after transplantation. He developed grade II aGVHD and achieved his first CR.
Of 16 patients with chemorefractory disease at the time of transplantation, only 3 achieved CR: one with chronic lymphocytic leukemia, one with mantle cell lymphoma, and one with RAEB-t. The patient with RAEB-t relapsed 110 days after the transplantation. On the contrary, 20 of 27 patients with chemosensitive disease achieved CR.
Donor lymphocyte infusions
Overall, 9 patients received DLIs: one with mantle cell lymphoma (patient no. 10) had a minimal response (25% reduction of adenopathies), and one with RAEB-t (patient no. 40) achieved CR after a second infusion followed by interleukin-2 treatment. The other patients did not show any response. Reasons for not receiving donor lymphocytes were (1) patients had clinical signs of GVHD after transplantation or developed aGVHD after withdrawing CSA (15 cases); (2) patients were in CR without a molecular marker (7 cases); (3) patients were already in clinical and molecular remission (5 cases); (4) early deaths (5 cases); (5) pending or too early for evaluation (4 cases).
Survival analyses
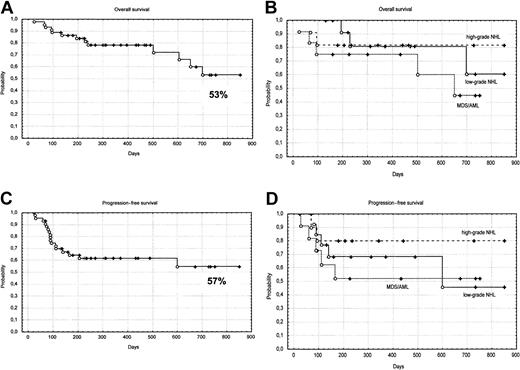
The median follow-up of the patients is only 385 days. The Kaplan-Meier–estimated probabilities of overall survival, progression-free survival, and nonrelapse mortality for all patients are shown in Figure 2 and Figure3. The estimated probability of overall survival at 24 months was 53% (95% CI, 41%-63%). The estimated probability of progression-free survival at 20 months was 57% (95% CI, 36%-72%). The estimated probability of nonrelapse mortality at 10 months was 13% (95% CI, 1%-18%).
Kaplan-Meier survival curves.
(A) overall survival; (B) overall survival according to disease categories; (C) progression-free survival; (D) progression-free survival according to disease categories. MDS indicates myelodysplasia; AML, acute myelogenous leukemia; NHL, non-Hodgkin lymphoma.
Kaplan-Meier survival curves.
(A) overall survival; (B) overall survival according to disease categories; (C) progression-free survival; (D) progression-free survival according to disease categories. MDS indicates myelodysplasia; AML, acute myelogenous leukemia; NHL, non-Hodgkin lymphoma.
PCR-based detection of minimal residual disease
Thirty-five patients had a disease involving the marrow, of those 26 achieved CR. In 13 of them we were able to find a disease-specific marker for PCR analysis, and then they became eligible for the molecular monitoring of minimal residual disease. Molecular markers were generated from diagnostic specimens: 8 were derived from the rearranged variable region of IgH genes, 2 from Bcl-2 gene and one from Bcl-1 gene, one from PML/RARα fusion transcript, and one was based on HLA-A mismatched allele (patient no. 14). The sensitivity of PCR assays has been previously reported.19-21 When HLA allele disparity was used to detect residual host cells in the marrow, the sensitivity was approximately one donor cell in 5000 host cells (data not shown).
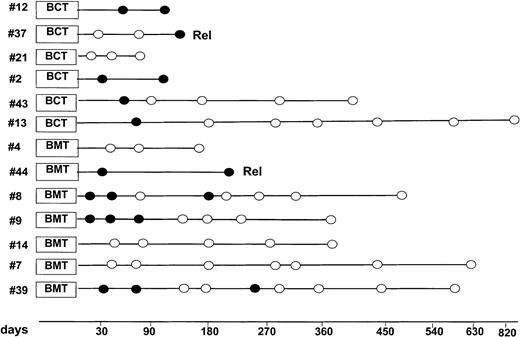
Overall, 10 patients achieved molecular remission: one with acute lymphoblastic leukemia, one with acute promyelocytic leukemia, and 8 with lymphoma. The patient with acute promyelocytic leukemia (patient no. 37) relapsed shortly after HSCT. Patient no. 9 had a peculiar outcome: he had follicular lymphoma and relapsed 80 days after HSCT; he was rescued with 4 rituximab infusions and rapid tapering of CSA (Figure 4). After developing acute and chronic extensive GVHD, he has been in clinical and molecular remission for one year. In this case a critical role for rituximab cannot be ruled out, and a longer follow-up is required. Overall, only 2 of 10 patients who achieved molecular remission after undergoing transplantation did not have any sign of GVHD (Table 2).
Molecular monitoring of minimal residual disease after transplantation.
White and black dots represent PCR negativity and positivity, respectively. Rel indicates relapse; BCT, blood cell transplantation; BMT, bone marrow cell transplantation.
Molecular monitoring of minimal residual disease after transplantation.
White and black dots represent PCR negativity and positivity, respectively. Rel indicates relapse; BCT, blood cell transplantation; BMT, bone marrow cell transplantation.
Discussion
Allogeneic transplantation is a curative treatment modality for several advanced hematologic malignancies. Transplantation efficacy, however, is frequently hampered by its toxicity. Regimen-related toxicity and nonrelapse mortality remain major obstacles to successfully performing allogeneic HSCT. In order to improve the outcome of allografting in high-risk patients, we have developed a program in which reduced intensity conditioning was combined with the use of programmed reinfusions of donor lymphocytes. The intensity of conditioning regimen has been reduced for the following reasons: (1) to decrease mucosal and tissue damage; (2) to minimize the release of inflammatory cytokines; (3) to lower the incidence of infections; and (4) ultimately to reduce the occurrence of severe aGVHD.
The primary endpoints of this study were to explore the incidence of durable engraftment and nonrelapse mortality. The use of a regimen including thiotepa, fludarabine, and cyclophosphamide has been relatively safe in a group of patients that had many high-risk features. High-risk patients were defined by the presence of one or more of the following characteristics: (1) patients treated with more than 2 lines or high-dose chemotherapy before undergoing transplantation; (2) patients older than 50 years; (3) patients affected by other medical comorbidities; and (4) patients with high-risk diagnoses for allogeneic HSCT such as lymphoma, Hodgkin disease, and multiple myeloma. Allogeneic HSCT in such patients gives an overall TRM ranging from 30% to 40% when conventional dose chemoradiotherapy is used.1,2,23-29 In particular, allogeneic transplantation using conventional conditioning following failed autologous transplantation has been associated with a treatment mortality ranging between 50% and 80%.23 29 Undoubtedly, such a high mortality rate may offset a potential for cure, and therefore conventional intensity transplantations have generally been avoided in such patients. With reduced-intensity conditioning, we report a stable engraftment in all patients, and a probability of nonrelapse mortality of 13%, which compares favorably with the results obtained, using more aggressive conditioning regimens (Figure 3). In our study, 26 patients were autografted before allogeneic HSCT, and only 4 patients (15%) died of transplant-related complications, demonstrating that this regimen can be used when a second transplantation has to be considered. Although a conditioning regimen containing thiotepa, fludarabine, and cyclophosphamide appears to have antitumor activity both in leukemia and lymphoma, the follow-up period is still very limited and all patients remain at risk of relapse, and therefore the survival curves shown in Figure 2 should be interpreted with caution. The presence of clinical and molecular remissions, however, represents a promising finding and suggests that the field is worth of investigation.
Another important aim of the study was to evaluate the feasibility of programmed DLIs in the setting of a reduced-intensity program using a T-repleted graft. For a better exploitation of the GVT effect we planned DLIs in all patients without GVHD, not achieving clinical and molecular remission. This strategy proved to be feasible in 9 of 40 (22%) patients surviving more than 100 days. The main reasons for not receiving DLIs were (1) GVHD occurrence; (2) patients did not have a molecular marker; (3) patients were already in molecular remission after transplantation. Nine patients received DLIs, one minimal, one complete, and 7 no response were observed. Therefore, we were not able to clarify the controversial role of DLIs in the setting of reduced-intensity allografting. Our preliminary findings suggest that they may have a limited range of applicability, we were generally not able to show a substantial benefit in the setting of posttransplantation disease persistence or relapse. In summary, most of the patients had a clinical benefit from the transplant or from CSA withdrawal. It is worthy of note that we could see a clear GVT effect in 4 patients (no. 9, no. 28, no. 31, and no. 36) responding to CSA withdrawal. All of these patients responded after the occurrence of GVHD (as previously mentioned one patient received also rituximab infusions).
Several other groups have explored different drug combinations to develop reduced-intensity regimens. Slavin et al pioneered the use of fludarabine in combination with 8 mg/kg busulfan.4 Their initial report consisted of 28 patients with a variety of malignant and nonmalignant disorders. The regimen was well-tolerated, with a disease-free survival rate of 77% at 14 months. Severe aGVHD was a major complication and the cause of death in 4 of 26 patients. However, the median patient age was 32 years (range, 1-56 years), and all appeared to be candidates for conventional allografting. Giralt et al have used a combination of fludarabine and melphalan in advanced leukemias and lymphomas.8 Patients had a median age of 52 years and were considered poor candidates for conventional allografting. Disease-free survival at one year was 57% for patients in first remission or chronic phase, and nonrelapse mortality rate on day 100 was 37%. In a cohort of patients similar to the patients included in our study, fludarabine/melphalan combination seems to show a higher nonrelapse mortality. Kottaridis et al have employed fludarabine/melphalan regimen in combination with Campath-1H antibody.9 Although median age was only 41 years, they have not reported grades III-IV GVHD, and this represents a very interesting finding. The estimated probability of nonrelapse mortality was 11%, which is comparable to our results in a cohort of older patients. The use of T-cell depletion, however, may raise some concern in a setting of heavily pretreated patients. The delayed immune reconstitution may be the cause of serious opportunistic infections for several months after transplantation. In addition, patients failing high-dose chemotherapy can be considered patients affected by a chemorefractory disease and a T-repleted graft can initiate its immunologic activity soon after transplantation.
We have used molecular monitoring as a surrogate marker to evaluate response and GVT activity. The achievement of molecular remissions with reduced conditioning is a novel and somehow promising finding. In chemotherapy and/or autografting programs for leukemia and lymphoma, it has already been shown that molecular remission correlates with a longer disease-free survival.20 30-34 Because of genetic marker availability, molecular analysis was mainly performed in patients with lymphoma. We suppose that the achievement of molecular remission soon after transplantation indicates that our conditoning regimen had a substantial antitumor activity, even in heavily pretreated patients. In patients no. 8, no. 9, no. 13, no. 39, and no. 43, however, a stable PCR-negative status was achieved several months after transplantation suggesting a possible GVT effect. As reported in “Results,” patient no. 9 also received rituximab before developing acute and chronic GVHD. It seems unlikely, however, that a patient relapsing 80 days after transplantation remains for one year in clinical and molecular remission only because of 4 rituximab infusions. Finally, we would like to point out that molecular remissions were found both in patients receiving marrow and mobilized blood grafts.
In conclusion, our results show that thiotepa, fludarabine, and cyclophosphamide regimen is active in promoting the engraftment of allogeneic hematopoietic cells, with a rather low nonrelapse mortality. The regimen retains a substantial antitumor activity providing both clinical and molecular remissions in leukemias and lymphomas. The programmed use of donor lymphocytes was feasible, but their role in this setting is still unclear. GVHD is still the main problem, and the long-term outcome remains unknown and requires further follow-up.
We are deeply indebted to the nursing staffs. We are grateful to Alessandra Pescarollo, Jacopo Peccatori, Andres Ferreri, and Sergio Vai for the helpful discussions during the study design, statistical analyses, and patient care.
This work was supported in part by Associazione Italiana Ricerca sul Cancro, Italy.
Submitted July 6, 2001; accepted August 27, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paolo Corradini, Bone Marrow Transplantation Unit, Istituto Nazionale Tumori, Via Venezian 1, 20133 Milano, Italy; e-mail:paolo.corradini@istitutotumori.mi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal