In lymphoid organs invaded by malignant B-cell lymphomas, the development of reactive CD4+ tumor-infiltrating T cells (TIL-T) at the contact of tumor cells is now firmly established.1,2 It has been reported that lymphoma B cells are capable of proliferating in response to various recombinant signals usually provided by CD4+ T cells, such as interleukin-4 (IL-4), or CD40 ligand.3 However, the exact nature of the functional relationships between CD4+ TIL-T and autologous non-Hodgkin lymphoma (B-NHL) cells remains largely unknown mainly because this question has not yet been extensively investigated in autologous CD4+ T/malignant B-cell coculture systems in vitro. We report here evidence that CD4+ TIL-T have the potential to drive autologous lymphoma B cells toward a terminal differentiated state, in one case of splenic low-grade, marginal zone B-cell lymphoma.
In May 1998, a 67-year-old man presented with a low-grade lymphoma (nonfollicular small-cell lymphoma) with involvement of spleen, blood, periaortic lymph nodes, liver, and bone marrow. The patient underwent splenectomy in July 1998. Histologic, cytologic, and immunophenotypic features were compatible with splenic marginal-zone B-cell lymphoma.4 All malignant B cells were surface IgM+, kappa+, CD19+, CD24+, CD40+, and were negative for IgD, CD23, and CD5. Less than 1% of malignant cells were CD38+ or CD138+. The percentage of CD3+ TIL-T was 9% with 5% CD4+ and 4% CD8+.
A population containing both CD4+ T cells and malignant B cells was negatively selected from total spleen cells by depleting CD8+ T cells, residual NK cells, monocytes, and normal B cells by one round of immunomagnetic bead depletion. Purity was assessed by flow cytometry and CD4+ T/malignant B-cell preparations usually contained 8% to 10% CD4+ T cells and 88% to 90% CD19+ kappa+ B cells. Residual cells not stained by CD3, CD4, CD19, or kappa antibodies were always less than 1%. Cocultures were then performed in the presence of recombinant IL-2 (rIL-2) at 10 UI/mL. Control cultures consisted of purified malignant B cells cultured with rIL-2 but without the presence of CD4+ T cells.
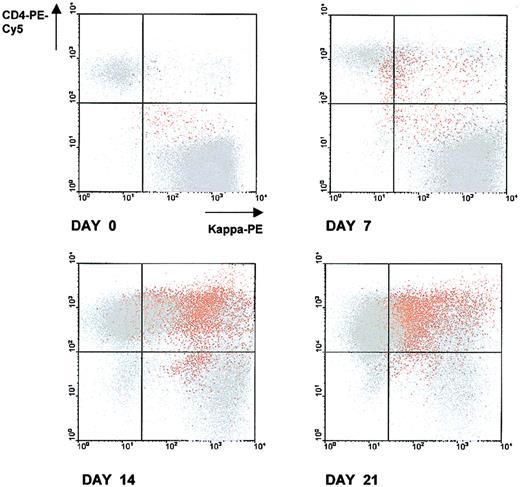
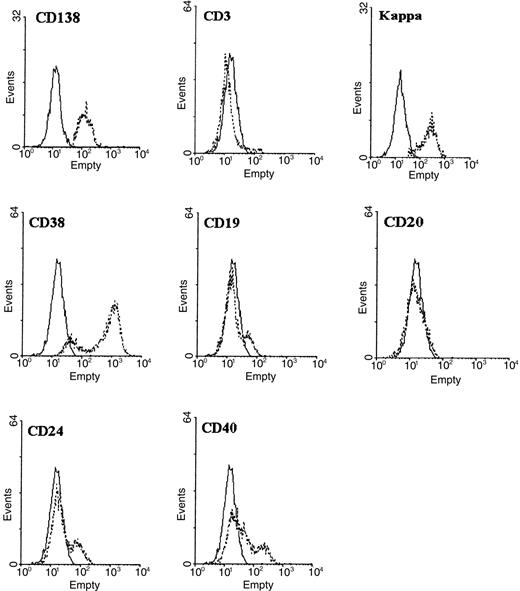
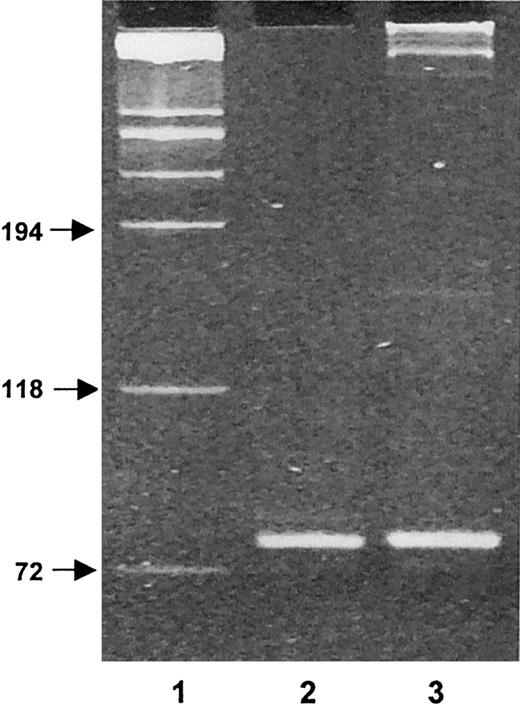
A representative experiment of CD4+ T/malignant B-cell cocultures is given in Figure 1. After 7, 14, and 21 days of coculture, cells were harvested, triple stained with anti-CD4–PE-Cy5, kappa-PE, and CD138-FITC antibodies (Abs) and analyzed by flow cytometry. CD138 (Syndecan-1) is a transmembrane heparan sulfate proteoglycan expressed in Ig-producing, normal and malignant mature plasma cells.5 At analysis, according to a multicolor gating/painting strategy, CD138+cells were gated and colored in orange, and CD138− cells were colored in gray. At the initiation of the coculture (day 0), the spontaneous formation of conjugates between T cells and malignant B cells was negligible. At the contact of malignant B cells, autotumor-reactive CD4+ T cells became activated, expanded, and formed stable conjugates with malignant B cells. Between day 7 and day 21 of the coculture, the percentage of T/B-cell conjugates gradually increased (5% at day 7, 36% at day 14, and 34% at day 21), and this was accompanied by a progressive increase in the number of free T cells (from 6% at day 0 to 51% at day 21). CD138+ cells progressively accumulated in the CD4+ T/B-cell coculture and reached a maximum value of 18% of the total number of cells at day 14 (1%, 4%, 18%, 13%, at days 0, 7, 14, 21, respectively). CD138+ cells were almost exclusively confined within CD4+ T/B-cell conjugates (Figure 1), suggesting that the acquisition of this ag by malignant B cells was dependent on a close association with autotumor-reactive CD4+ T cells. To formally show that the CD138+ cells were truly plasma B cells, these cells were positively purified by an immunomagnetic method at day 14 of the coculture and phenotyped (Figure 2). This method allowed us to obtain a very pure population of CD138+ cells (98%). As expected, CD3, CD19, CD20, and CD24 were low or absent; the expression of CD40 was intermediate, and kappa light chain was expressed at a low level (mean value: 198 units of fluorescence) in comparison to fresh malignant B cells (mean value: 1299). A high proportion (85%) of cells strongly expressed CD38, a relevant marker to plasma cells. These cells exhibited a morphology of plasma cells and contained large amounts of intracytoplasmic kappa chain as estimated on cytospin slides stained with fluorochrome-conjugated anti-kappa Abs. To ensure that these CD138+ plasma cells belonged to the neoplastic clone, IgH gene rearrangements of purified CD138+ cells were investigated by a PCR-based method using Fr3 region primers and compared with IgH gene rearrangements of purified fresh malignant B cells. Both cell populations showed the same rearrangement bands, stressing that the CD138+ cells were B cells and clonally related to the original lymphoma cells (Figure3). Cell-division tracking using PKH26 dye labeling indicated that more than 95% of the malignant B cells remained in a nonproliferative state throughout the coculture, formally excluding that the accumulation of CD138+ cells during the CD4+ T/malignant B-cell coculture might arise from the rapid proliferation of a minor component of CD138+ cells present at the initiation of the coculture. Finally, in control experiments, purified malignant B cells cultured alone in the sole presence of rIL-2 retained a phenotypic profile identical to fresh malignant B cells without any down-regulation of CD19, CD20, CD40, and surface Ig, or up-regulation of CD38 and CD138. Thus, malignant B cells did not show any tendency to spontaneous or rIL-2–induced differentiation in the absence of autologous CD4+ T cells.6-10
Conjugate formation between autotumor reactive CD4+ T cells and malignant B cells in CD4+ T/malignant B-cell cocultures, accumulation of CD138+ cells, and preferential localization of CD138+ cells within conjugates.
After various time intervals, cells were harvested, triple stained with anti-CD4–PE-Cy5, kappa-PE, and CD138-FITC Abs, and analyzed by flow cytometry. At analysis, CD138+ cells were gated and colored in orange; CD138− cells were colored in grey. CD138+ and CD138− cells were then simultaneously visualized on dot plots with CD4-PE-Cy5/kappa-PE parameters. 50 000 events were collected for each dot plot. The quadrant statistics cursors were set using appropriate negative control antibodies. Results are from 1 representative experiment of the 5 performed.
Conjugate formation between autotumor reactive CD4+ T cells and malignant B cells in CD4+ T/malignant B-cell cocultures, accumulation of CD138+ cells, and preferential localization of CD138+ cells within conjugates.
After various time intervals, cells were harvested, triple stained with anti-CD4–PE-Cy5, kappa-PE, and CD138-FITC Abs, and analyzed by flow cytometry. At analysis, CD138+ cells were gated and colored in orange; CD138− cells were colored in grey. CD138+ and CD138− cells were then simultaneously visualized on dot plots with CD4-PE-Cy5/kappa-PE parameters. 50 000 events were collected for each dot plot. The quadrant statistics cursors were set using appropriate negative control antibodies. Results are from 1 representative experiment of the 5 performed.
Purified CD138+ cells exhibit phenotypic features consistent with plasma cells.
At day 14 of the CD4+ T/malignant B-cell coculture, CD138+ cells were positively purified with immunomagnetic beads. The dotted histograms were obtained using the monoclonal antibody (mAb) under study, and the solid histograms were obtained using the respective isotype-matched control Ab. Histograms were obtained from 1 of 2 independent experiments yielding similar results. 2 000 to 5 000 cells were acquired for each histogram.
Purified CD138+ cells exhibit phenotypic features consistent with plasma cells.
At day 14 of the CD4+ T/malignant B-cell coculture, CD138+ cells were positively purified with immunomagnetic beads. The dotted histograms were obtained using the monoclonal antibody (mAb) under study, and the solid histograms were obtained using the respective isotype-matched control Ab. Histograms were obtained from 1 of 2 independent experiments yielding similar results. 2 000 to 5 000 cells were acquired for each histogram.
CD138+ plasma cells belong to the same neoplastic clone than fresh malignant B cells as evidenced by IgH gene rearrangements.
Fr3 PCR products of DNA samples were obtained from fresh malignant B cells and from purified CD138+ cells after 21 days of the CD4+ T/B-cell coculture. Lane 1, molecular weight markers (sizes in base pairs); lane 2, fresh malignant B cells; lane 3, purified CD138+ cells. Results are from 1 representative experiment of the 2 performed.
CD138+ plasma cells belong to the same neoplastic clone than fresh malignant B cells as evidenced by IgH gene rearrangements.
Fr3 PCR products of DNA samples were obtained from fresh malignant B cells and from purified CD138+ cells after 21 days of the CD4+ T/B-cell coculture. Lane 1, molecular weight markers (sizes in base pairs); lane 2, fresh malignant B cells; lane 3, purified CD138+ cells. Results are from 1 representative experiment of the 2 performed.
In the literature, there are now some convincing observations supporting the notion that B-lymphoma cells could be released from their apparent maturation block by external signals. Cerutti et al6 have described that in Burkitt lymphoma, a monoclonal B-cell line was induced to progress throughout a phenotypic differentiation program that approximated the stages of early centroblast, centroblast, centrocyte, and memory B cells, after engagement of CD40 by CD40 ligand and exposure to IL-4 and IL-10. In the presence of IL-6, these cells were driven to terminally differentiated plasma cells. Recently, a second experimental model used a follicular dendritic cell line, HK, and a lymphoma line, L3055, that resembles centroblasts. L3055 cells proliferated continuously in the presence of HK cells, whereas they differentiated into a population with the phenotype of centrocytes after stimulation with CD40 ligand and IL-4.7 In follicular lymphomas, it was formerly reported that allogeneic T cells induced fresh malignant B cells to secrete large amounts of Ig, and this was accompanied by loss of the surface Ig and development of abundant intracytoplasmic Ig.8 In 1995, Kramer et al9 reported a patient with follicular lymphoma who showed in vivo differentiation of malignant cells after autologous bone marrow transplantation and treatment with IL-3. The patient developed a plasmocytosis in blood and bone marrow accompanied by a paraprotein corresponding to the malignant clone and high IL-6–serum concentration. It was proven that the plasma cells were clonally related to the original lymphoma cells. The patient recovered spontaneously and had long-lasting remission, suggesting that terminal differentiation of malignant B cells was beneficial to the patient. More recently, Dogan et al10 described that follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic B-cell population in the interfollicular zone. It was demonstrated that the immunophenotype of the neoplastic interfollicular cells was similar to that of a subpopulation of postfollicular B cells observed outside the follicles in normal lymphoid tissue, with down-regulated expression of follicle-center activation markers such as CD10, CD38, CD80, CD86, and CD95. These cells are thought to be malignant memory B cells that have differentiated from the malignant follicle-center B cells. Thus, differentiation towards a cell with more mature phenotype should be a possible occurrence in low-grade B-cell lymphoma.
Marginal-zone lymphomas tend to have an indolent clinical course.4 In the current case, the patient has remained stable without any treatment after undergoing splenectomy 35 months ago. The results presented here would provide an explanation for this: the tumor cells may have received signals from autotumor-reactive CD4+ T cells leading to a continuous process of differentiation that may be beneficial to the patient. As demonstrated in the current study, in-depth analysis of CD4+ T/malignant B-cell conjugates in CD4+ T/malignant B-cell cocultures should form the basis for valid evaluation of the effects of autotumor-reactive T cells on malignant B cells: induction of differentiation but also growth regulation/apoptosis. These immunologic criteria would provide a strong rationale to engage individual cellular immunotherapy with autologous CD4+ T cells in B-cell NHL.
Supported by grants from the Association pour la Recherche sur le Cancer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal