The development of clinical and histopathologic manifestations of a diffuse elastic tissue defect, resembling inherited pseudoxanthoma elasticum (PXE), has been encountered with a notable frequency in patients with β thalassemia, sickle cell disease, and sickle thalassemia. The PXE-like clinical syndrome, consisting of skin, ocular, and vascular manifestations, has a variable severity in these hemoglobinopathies and it is age-dependent, with a generally late onset, after the second decade of life. The defect is believed to be acquired rather than inherited and related to the consequences of the primary disease. The high prevalence of the findings implicates the elastic tissue injury as one of the main comorbid abnormalities encountered in β thalassemia and the sickling syndromes. In these patients a number of complications, sometimes serious, has been recognized to be related to ocular and vascular elastic tissue defects. Because several organ systems are involved, each medical specialty should be aware of the phenomenon. This coexistence, on the other hand, introduces a novel pathogenetic aspect of PXE and an important research challenge.
Introduction
The clinical spectrum of a chronic disease is often evolving continuously. The closer and more systematic follow-up of patients, the significant improvement of available treatments, and the resulting longer life expectancy lead to a gradual broadening of the clinical picture with new, previously unknown manifestations. This is the case for β thalassemia and the sickling syndromes. Patients with these genetic disorders have manifested signs similar to those found in another hereditary disease, pseudoxanthoma elasticum (PXE). The frequency of this phenomenon, which has attracted the attention of several groups of investigators, and the potential risk of PXE complications prompted this review.
PXE
As conventionally described, PXE is a rare hereditary connective tissue disorder, characterized by generalized degeneration of the elastic fibers with a broad phenotypic expression.1,2First described in 1881 by Rigal,3 its prevalence in the general population ranges between 1/70 000 and 1/160 000.1 Although widely variable, the age of onset averages 13 years.4 The clinical picture consists mainly of cutaneous, ocular, and vascular manifestations; skin histopathology involves swollen, irregularly clumped and multiply fragmented elastic fibers in the middle and deep reticular dermis, with secondary calcium deposition.1,2,5 The term “elastorrhexis” has also been used, on the basis of the pathology findings, to describe such abnormalities.2
The typical cutaneous lesions are small yellowish papules or larger coalescent plaques with an appearance similar to plucked chicken skin.1,2,5 More severely affected skin results in hanging, redundant folds.4,5 In contrast, some patients have a more subtle macular form that requires careful inspection to be recognized.5 Skin lesions develop mainly at areas of flexion, such as the neck, axillae, antecubital and popliteal fossae, inguinal areas, and periumbilical region.1,2,4,5 Mucous membranes, mainly of the inner aspect of the lower lip, may be also affected.4
Angioid streaks are the characteristic ocular manifestations, occurring in 80% of patients with PXE (Figure1)6,7; this combination has been named the Gronblad-Stranderg syndrome.8,9 They are funduscopic findings, caused by breaks of the elastic lamina of the Bruch membrane, with secondary changes of the retinal pigment epithelium and choriocapillaries.6 Not being apparent at birth, angioid streaks are initially seen during the third or fourth decade of life, usually later than the skin manifestations5 and occasionally without the typical cutaneous lesions.10 Although their presentation, color, and distribution may be markedly variable, they typically appear as single or multiple, asymmetrical, bilateral, dark red, brown, or gray bands radiating from the optic disk.5-7
Angioid streaks (arrows) in a patient with β thalassemia (Dr Aessopos' collection).
Magnification, ×8.5.
Angioid streaks (arrows) in a patient with β thalassemia (Dr Aessopos' collection).
Magnification, ×8.5.
PXE-like manifestations in β thalassemia and the sickling syndromes
The first manifestation of a potential elastic tissue defect described in hemoglobinopathies were angioid streaks, the high frequency of which has led to a well-established relationship between the 2 entities. Angioid streaks has been reported in sickle cell disease (SCD) since the late 1950s.13,14 Since then, several papers have been published, presenting a variable frequency of angioid streaks in SCD, ranging from 1% to 22%, depending on patients' age.15-17 Angioid streaks were found to develop in patients with SCD at the age of 25 years on average, although the highest prevalence was reported in a group of 60 Jamaican patients older than 40 years.16 In β thalassemia, angioid streaks were initially described in isolated cases18-20 and subsequently, an occurrence of 20% was reported in a series of 100 patients (Figure 1).21 Being manifested after the age of 20 years, the findings were positively correlated with age also in this group.21 Furthermore, angioid streaks have been encountered in sickle thalassemia22 and a frequency of 10% was reported in a group of 58 cases.23
The etiology of angioid streaks in these hemoglobinopathies has not been clarified. Angioid streaks are generally considered a manifestation of an underlying systemic illness, even if such a disorder cannot be identified at the time that the angioid changes are first noted. PXE, Marfan syndrome, Ehlers-Danlos syndrome, and Paget disease are the most common of these conditions.6,7 In a large series of patients with angioid streaks, 50% had PXE on diagnosis.24 The possibility of a high incidence of this diffuse connective tissue disorder in SCD was raised by the report of the PXE syndrome in at least 7 adults with SCD.25 However, blind skin biopsies, performed in SCD patients with angioid streaks, failed to reveal any evidence of PXE.15,17 Accordingly, angioid streaks were primarily thought to be related to the disease itself and pathogenetic mechanisms, such as vascular obstruction affecting the choriocapillary circulation or chronic hemolysis leading to iron deposition were proposed.13,26 In contrast, another autopsy series of 16 unselected SCD patients included 2 with histopathologic findings identical to PXE in the dermis and the arterial walls of multiple organs.14 A clearer relationship was found by Lippman et al27 in 1985. Based on histopathology and biochemical analysis of skin biopsies taken from 32 consequent SCD patients, these investigators concluded that SCD appears to be associated with a wide spectrum of elastic tissue disorders resembling PXE, although less severe than PXE.27
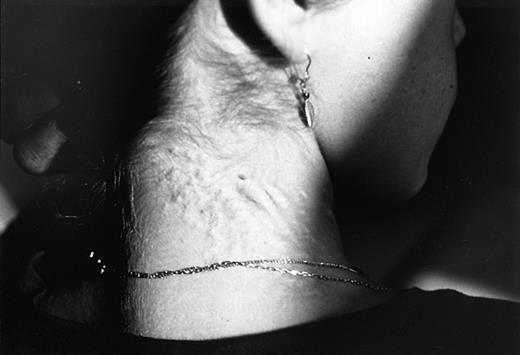
Subsequently, accumulated evidence from the literature has supported the concept of an underlying, generalized elastic tissue defect in the same hemoglobinopathies that were previously associated with angioid streaks. In β thalassemia, as a matter of fact, the presentation of a PXE-like syndrome proved to be more obvious. The coexistence of PXE skin and ocular findings was first reported in 2 cases28and then systematically studied in 100 patients with thalassemia major and thalassemia intermedia.29 In this study, 16% of the patients had PXE cutaneous lesions (Figure2), confirmed by skin histopathology, 20% had angioid streaks, and 26% at least one of the 2 findings. All patients under the age of 19 years as well as 70 family members of those with PXE findings did not show any skin or ocular lesions.29 A more recent report focused on arterial calcifications in 40 older thalassemic patients, mostly with thalassemia intermedia, aged over 30 years (mean, 41.4 years).11 In this study, 55% of patients versus 15% of healthy controls, matched for sex and age (P < .001), had calcification of the posterior tibial artery (Figure3), 20% had typical skin lesions, 52% had angioid streaks, and 85% had one or more of the 3 manifestations. Hence, the complete clinical spectrum of PXE, with skin, ocular, and vascular findings, has been encountered in thalassemia; however, it is also clear that the occurrence of PXE manifestations is more frequent with advancing age. Evidence that thalassemia-associated PXE is structurally identical to inherited PXE was derived by a recent study that compared dermal pathology between the 2 entities applying sophisticated techniques including electron microscopy and immunocytochemistry.30 Of interest is the fact that subclinical disorders of the elastic tissue have been encountered even in the first decade of life. In a surgical series of 45 unselected patients with β thalassemia major, aged between 6 and 25 years (mean, 16 years), spleen, liver, and lymph node biopsies revealed multiple defects of arterial and stromal elastic tissue, characterized as “elastorrhexis,” that were present in an impressively high percentage—96%.31 Only 4 of 45 patients had developed typical PXE skin lesions, although none had angioid streaks at the time of study.31 Similar histopathologic abnormalities were not observed in corresponding specimens of control cases.31 It seems that angioid streaks, cutaneous lesions, and arterial calcifications represent evolving changes of an underlying elastic tissue disorder that starts early in life in patients with thalassemia.
Typical cutaneous manifestations of PXE on the dorsal aspect of the cervical area in a β thalassemic case (Dr Aessopos' collection).
Typical cutaneous manifestations of PXE on the dorsal aspect of the cervical area in a β thalassemic case (Dr Aessopos' collection).
Tibial artery calcification in a 58-year-old patient with β thalassemia (Dr Aessopos' collection).
Tibial artery calcification in a 58-year-old patient with β thalassemia (Dr Aessopos' collection).
Besides SCD and β thalassemia, the spectrum of hemoglobinopathies with clinical PXE findings is broadened by a report of the PXE syndrome in sickle thalassemia.23 Compound heterozygosity for β thalassemia and sickle trait results in a clinical syndrome similar to that of SCD. In this study, 5% of 58 patients had cutaneous lesions typical of PXE, whereas 10% had angioid streaks, all lesions being present after the age of 20 years.23 None of the 25 relatives of patients with angioid streaks, who were also examined, had any similar lesions.23
Pathogenesis and genetics
Classical PXE is characterized by marked genetic heterogeneity; both autosomal dominant and autosomal recessive patterns of inheritance have been reported, but most of the cases appear to be sporadic.1,36,37 Recent studies have mapped the PXE locus to chromosome 16p13.1.38-41 The pathogenetic mutation is believed to affect the MRP6 gene that encodes a cellular transmembrane protein, associated with multidrug resistance.42 43MRP6 is a member of the ABC (adenosine triphosphate–binding cassette) transporters superfamily and its specific biologic function is currently known.
In β thalassemia and the sickling syndromes, a genetic link with PXE is unlikely. Family members who do not have a hemoglobinopathy fail to show any PXE stigmata.23,29 The MRP6 gene and previous PXE candidate genes, encoding elastin, fibrillins 1 and 2, elastin-related glycoproteins, and lysyl-oxidase, are located on different chromosomes than the β-globin gene defects that are responsible for these hemoglobinopathies.42,44-48 In addition, the genotypes of 30 homozygous β-thalassemic patients of Greek origin with PXE-like lesions did not differ from those of the general β-thalassemic population in Greece, indicating that such manifestations develop independently of the β-thalassemia genotype.49 An interesting observation, however, is the fact that the recently defined locus for PXE is located close to the α-globin gene, which is mapped to 16p13.350; nevertheless, there is no evidence for a genetic association between the 2 entities.
Regarding the existence of an acquired form of PXE, no clear data are present in the literature. Posttraumatic, localized, cutaneous lesions, lacking the retinal and vascular stigmata of the inherited type, have been reported in a number of cases.51-53 In contrast, the potentially acquired nature of a PXE syndrome encountered in a couple of cases with chronic renal failure is strongly questioned by some investigators.1,54,55 In β thalassemia and SCD, it is believed that the elastic tissue abnormalities are most likely acquired, despite their clinical, structural, and cytochemical resemblance to inherited PXE. In this context, the terms “PXE-like lesions” and “PXE-like syndrome” were introduced to describe such findings.11,29,31 49 The potential acquired form of the syndrome renders it one of the primary complications of these hemoglobinopathies and may introduce an important novel aspect in the pathogenesis of PXE.
A number of acquired pathogenetic mechanisms for this phenomenon are discussed in the literature. Taking into consideration the occurrence of PXE manifestations in β thalassemia and the sickling syndromes, a rational approach would consider some of the common characteristics of these hemolytic conditions. This concept is strengthened by the report of angioid streaks in other hemolytic states, including inherited spherocytosis and congenital dyserythropoietic anemia type III.56,57 Many studies, however, failed to reveal a statistical relationship between the elastic tissue defects and patients' hematologic parameters. The hemoglobin or serum ferritin levels, the number of blood units received, or the chelation history have not so far been found to have a significant prognostic value.11,20,29,31,49 58
It has been suggested that the elastic tissue injury in these patients may be the result of an oxidative process, induced by the combined and interactive effect of different factors.11 Plasma membrane microparticles, derived from the oxidative damage of red cell membranes by the effect of denatured hemoglobin products and free iron,59-63 are considered to elicit inflammatory and oxidative reactions.64 Moreover, the unbound fractions of hemoglobin and haem, which exceed the binding capacity of haptoglobin and hemopexin in the context of chronic hemolysis, also have powerful oxidative properties.65 Particularly in sickling syndromes, an excessive free radical production follows the postocclusive tissue reperfusion.66 Moreover, iron overload has a central role in multiple organ injury in these hemoglobinopathies. Unbound iron catalyzes the formation of the most toxic hydroxyl radical through the Fenton and Haber-Weiss reactions, causing, in turn, peroxidation of membrane lipids and proteins.67 The accumulated and prolonged effect of the above mechanisms may result in disturbance of elastin metabolism and structural deterioration of elastic fibers.68 Accordingly, oxidative stress constitutes a potential acquired mechanism affecting the same transmembrane proteins, which are implicated in the pathogenesis of hereditary PXE. Indirect evidence of increased and prolonged tissue injury in thalassemic and sickle patients includes activation of polymorphonuclear neutrophils and monocytes and the increased levels of neutrophil elastase and circulating cytokines.49,64 69-72
It should be stated that of all hemoglobinopathies, β thalassemia is the most informative in terms of elastic tissue abnormalities. Extensive clinical and histologic findings have been presented in the literature. However, it is a question whether this is due to the more systematic study of this hemoglobinopathy or due to the greater iron load and the more severe clinical condition of thalassemic patients.
PXE-related complications
The ocular and vascular complications of PXE may become quite serious in the course of the disease. Angioid streaks, although usually asymptomatic, may lead to macular degeneration and choroidal neovascularization that, in turn, can result in deterioration of visual acuity, even blindness.4,6 The vascular complications include gastrointestinal or cerebrovascular bleeding, coronary artery disease, hypertension, and intermittent claudication.1,2,5,10,12 Such clinical manifestations of diffuse arterial involvement often appear quite early, occurring even in childhood. Furthermore, restrictive cardiomyopathy has also been reported,73 and elastic tissue defects of the placenta have been implicated in the development of obstetric complications seen in women with PXE.74-79
The recognition of PXE manifestations and their complications in patients with β thalassemia or sickling syndromes may not be easy. Both PXE and the hemoglobinopathies have a broad clinical spectrum with multiorgan involvement. As a result, PXE-related signs could be overlooked, obscured, or confused with the rest of the clinical manifestations of the primary disease. PXE-related complications may be misinterpreted or recognized only when they become quite severe. In these hemoglobinopathies, such complications have only sporadically been reported. Impaired visual acuity due to subretinal neovascularization and hemorrhage has been described in 2 patients with thalassemia major and another with thalassemia intermedia.18,21,80 Regarding the vascular involvement, it is a question whether it has a contributing role in the development of leg ulcerations, tibial cramps, and easy bruising, features that are frequent in thalassemic and SCD patients. In β thalassemia, fatal intracranial hemorrhages have been described in 2 patients with PXE lesions.81 Concurrently, in SCD, the reported cerebrovascular manifestations include not only vaso-occlusive events but hemorrhages as well; subarachnoid hemorrhage due to ruptured intracranial aneurysms is a recognized cause of morbidity and mortality in adults with SCD.82,83 Interestingly, although investigators did not correlate these events to a generalized elastic tissue defect, pathology showed fragmentation of the internal elastic lamina of the arterial wall of the aneurysms, although aneurysms were multiple and characterized by atypical localization.82 83
Management and guidelines
The surveillance for PXE-like disorders in a hemoglobinopathy population is actually simple. A careful skin inspection, focusing mainly on the neck, axillae, antecubital and popliteal fossae, and abdomen should be performed during the regular follow-up; biopsies of skin lesions are usually required. An annual funduscopic examination by an ophthalmologist beyond the second decade of life and a radiographic examination of the limbs to detect arterial calcifications beyond the third decade are also recommended.
Unfortunately, at present, no specific treatment for PXE exists. The knowledge, however, of the potential complications may lead physicians to take some necessary precautions. A primary concern should be the reduction of the risk of bleeding. Medications such as platelet inhibitors, which are often indicated in both β thalassemia and SCD, should be prescribed cautiously.84 Patients should also be advised to refrain from activities that predispose to hemorrhage, including heavy straining, head trauma, football, wrestling, and weight lifting. In the presence of arterial calcifications, hemoglobin concentration should be kept high to reduce the risk of myocardial and peripheral ischemia. The need for maintaining high hemoglobin levels has also been stressed regarding pregnancy in women with hemoglobinopathies and has an additional importance in view of the potential obstetric complications of PXE.74-79,85 A close follow-up with funduscopy and fluorescein angiography is required in patients with angioid streaks and laser photocoagulation should be considered when choroidal neovascularization develops, to prevent ocular hemorrhages and loss of visual acuity.87,88 The reduction of dietary calcium intake has furthermore been proposed to reduce organ calcium deposition.89-91 However, this recommendation might be inappropriate given the frequent coexistence of osteoporosis in patients with β thalassemia and SCD.
Conclusion
The development of PXE-like elastic tissue disorders has been well documented in SCD, β thalassemia, and sickle thalassemia. The abnormalities are most probably acquired and related to the consequences of the primary disease. In these hemoglobinopathies the clinical syndrome appears to have variable severity and frequency and seems milder, with a later onset, compared to inherited PXE.
The current therapy for hemoglobinopathies has significantly improved survival and if it does not have an equally beneficial effect on elastic tissue, more frequent PXE complications are likely to be seen in the near future. Because these manifestations involve several body systems, each medical specialty should be aware of this entity and its coexistence with hemoglobin disorders. The presence of the entire clinical spectrum of PXE has not so far been described in other diseases. The hemoglobinopathy-associated PXE has, therefore, a particular research interest and may contribute to the better understanding of inherited PXE.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Athanasios Aessopos, First Department of Internal Medicine, University of Athens, Medical School, “Laiko” General Hospital, 17 Aghiou Thoma St, Athens 115 27, Greece; e-mail:aaisopos@cc.uoa.gr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal