Acute myeloid leukemia (AML) is a heterogeneous leukemia characterized by the blockage of myeloid differentiation at different stages, which define distinct AML subtypes. We have recently reported that the ligation of CD44 with 2 activating monoclonal antibodies (mAbs), A3D8 and H90, triggers terminal differentiation of leukemic blasts in AML-M1/2 to AML-M5 subtypes, which are the most frequent ones. However, fresh AML blasts have short in vitro lifespans. Therefore, to find relevant in vitro cellular models for further studying the mechanisms involved in CD44-induced differentiation, we investigated whether CD44 ligation with A3D8 and H90 mAbs can induce terminal differentiation of THP-1, NB4, and HL60 cells, each interesting models of AML-M5 (monoblastic subtype), AML-M3 (promyelocytic subtype), and AML-M2 (myeloblastic subtype), respectively. We also study whether CD44 ligation induces a loss of proliferative capacity, an important feature of late-stage myeloid differentiation. In the second part of our study, we investigated whether A3D8 and H90 anti-CD44 mAbs can induce the differentiation and inhibit the proliferation of KG1a cells, which are very immature AML-M0 blasts. Using functional, antigenic, and cytologic criteria, we presently show that A3D8 and/or H90 induce terminal differentiation of THP-1, HL60, and NB4 cell lines and strongly inhibit their proliferation. Interestingly, cell-specific effects of H90 and A3D8 are observed. We also observe that incubation with A3D8 for 3 to 6 days induces an apoptotic cell death that is moderate in the case of THP-1 and HL60 cells and massive in the case of NB4 cells. Finally, our results demonstrate for the first time that it is possible to reverse the leukemic blockage of KG1a cells by using both an anti-CD44 mAb and retinoic acid. This result may provide a new experimental basis for a differentiative therapy in AML-M0 patients.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous leukemia characterized by the blockage of myeloid differentiation at different stages, which define distinct AML subtypes1 (AML-M0 to AML-M5). Interestingly, the CD44 cell surface antigen is expressed on leukemic blasts in all AML subtypes.2,3 Because CD44 is a signaling receptor4-8 that plays an important role in myelopoiesis (anti-CD44 antibodies profoundly alter the production of myeloid cells in long-term bone marrow cultures9-13), it can be used as a target molecule to induce terminal differentiation of leukemic blasts in all AML subtypes.14 It has been recently reported that the ligation of CD44 with 2 activating monoclonal antibodies (mAbs), A3D815 and H90,16 triggers terminal differentiation of leukemic blasts in AML-M1/2 to AML-M5 subtypes on the basis of antigenic, functional, and cytologic criteria.14 However, because AML blasts have short in vitro lifespans, they could hardly be used for further studying the mechanisms involved in CD44-induced differentiation. Therefore, it seems worthwhile to find in vitro cellular models for further studying these mechanisms.
THP-1 are monoblastic cells originating from an AML-M5 patient (monoblastic subtype).17 NB4 cells are promyelocytic cells that display the PML-RARα–encoding t(15;17) translocation that is specific for AML-M3 (promyelocytic phenotype).18 Moreover, like AML-M3 blasts, NB4 cells can be terminally differentiated into granulocytes by retinoic acid (RA).18 Finally, HL60 are myeloblastic cells isolated from an AML-M2 patient19; they have been used to demonstrate the differentiative activity of RA and to predict its in vivo efficiency. Accordingly, we presently investigate whether CD44 ligation with A3D8 and H90 anti-CD44 mAbs can induce terminal differentiation of THP-1, NB4, and HL60 cell lines. If so, these cell lines would be interesting models for further studying the mechanisms involved in CD44-induced differentiation of AML-M5, AML-M3, and AML-M2 blasts, respectively. In addition, because terminal myeloid differentiation is associated with a loss of proliferative capacity, we also investigate whether A3D8 and H90 anti-CD44 mAbs inhibit the proliferation of these cell lines.
In the second part of our study, we ask whether A3D8 and H90 anti-CD44 mAbs can induce the differentiation and inhibit the proliferation of KG1a cells20 (Table 1). These cells originate from an AML-M0 patient and are considered to be very undifferentiated because, like primitive myeloid progenitors, they display a CD34highCD38−phenotype.21 Interestingly, an important feature of KG1a is its inability to differentiate20 when it is treated by any chemical known to trigger the differentiation of other leukemic cell lines (eg, dimethylsulfoxide, RA, or phorbol esters22).
Main features of KG1a, HL60, NB4, and THP-1 cell lines
| . | KG1a . | HL60 . | NB4 . | THP-1 . |
|---|---|---|---|---|
| Origin | AML-M0 | AML-M2 | AML-M3 | AML-M5 |
| Cytologic features | Myeloblasts | Myeloblasts | Promyelocytes | Monoblasts |
| Immunophenotype | CD34+++CD38− | CD34+++CD38− | CD34+++CD38− | CD34+++CD38− |
| CD11b−CD14−CD15low/− | CD11b−CD14−CD15low | CD11b−CD14−CD15low | CD11b−CD14−CD15low |
| . | KG1a . | HL60 . | NB4 . | THP-1 . |
|---|---|---|---|---|
| Origin | AML-M0 | AML-M2 | AML-M3 | AML-M5 |
| Cytologic features | Myeloblasts | Myeloblasts | Promyelocytes | Monoblasts |
| Immunophenotype | CD34+++CD38− | CD34+++CD38− | CD34+++CD38− | CD34+++CD38− |
| CD11b−CD14−CD15low/− | CD11b−CD14−CD15low | CD11b−CD14−CD15low | CD11b−CD14−CD15low |
KG1a, HL60, NB4, and THP-1 cells are human AML cell lines blocked at distinct stages of granulomonocytic differentiation. They are characterized by specific cytologic and antigenic features closely similar to those displayed by AML blasts from distinct subtypes, as indicated.
We have recently shown that A3D8 anti-CD44 mAb significantly reduces drug-induced apoptosis in NB4 and HL60 cells when it is administered prior to the drug.23 This inhibition takes place until 22 to 40 hours following the addition of A3D8. Here, we found it interesting to investigate the effect of A3D8 on survival of AML cells not treated by chemotherapy drug. This study is all the more relevant because (1) apoptotic death is a feature of normal terminally differentiated granulocytic cells and (2) CD44 can trigger opposite effects on cell survival in different cellular contexts. For example, it promotes survival of B lymphocytes24 and, on the contrary, it triggers apoptosis of mature granulocytes.25
Our present results show that (1) A3D8 and H90 inhibit the proliferation of all AML cell lines; (2) A3D8 and/or H90 induce terminal differentiation of THP-1, HL60, and NB4 cell lines; cell-specific effects of H90 and A3D8 are observed; and (3)A3D8 induces an apoptotic cell death that is moderate in the case of THP-1 and HL60 cells and massive in the case of NB4 cells. Most importantly, our results also show for the first time that it is possible to reverse the leukemic blockage of KG1a cells by using both an anti-CD44 mAb and RA. This result may provide a new experimental basis for a differentiative therapy in AML-M0 patients.
Materials and methods
Cell lines
KG1a cells were kindly provided by P. Mannoni (Marseille, France). NB4 cells are a gift from M. Lanotte (Hôpital St Louis, Paris, France). HL60 and THP- 1 cells were purchased from ATCC (Manassas, VA). All of these cells were cultured in RPMI 1640 containing 10% fetal calf serum, 2 mM/L l-glutamine, 100 μg/mL streptomycin, and 200 U/mL penicillin (GIBCO, Grand Island, NY). The main cytologic, antigenic, and karyotypic features of these cell lines are summarized in Table 1.
Anti-CD44 mAbs, isotypic controls, and RA
CD44 ligation
Cells were seeded in triplicate at 105/mL in RPMI 1640 with 10% fetal calf serum in 96-well tissue culture plates (200 μL/well) (Costar, Cambridge, MA). They were independently treated with specific concentration of anti-CD44 mAbs (H90, A3D8, or J173). As a negative control, cells were incubated with 10 μg/mL immunoglobulin G1 (IgG1) or 20 μg/mL J173 anti-CD44 mAb. RA (10−7 M/L) was used as a positive control for granulocytic differentiation of NB4 and HL60 cells. Plates were incubated at 37°C in a humidified incubator for up to 6 days. They were then processed for proliferation, differentiation, and apoptosis studies as described below.
Cell proliferation
Using trypan blue dye exclusion, the number of viable cells was determined over a 6-day period. Cell proliferation was assessed at day 3 by adding 1 μCi (0.037 MBq) per well of [3H]thymidine (20 Ci/mM [740 GBq/mM]) (NEN Life Science Products, Boston, MA) over 16 hours. Incorporated radioactivity was measured in a β-scintillation counter (Beckman Instruments, Fullerton, CA). The values are reported as the mean ± SD of triplicate wells.
Myeloid differentiation
Myeloid differentiation was evaluated by using the 3 following criteria, as previously described.14
1.
The ability to produce oxidative bursts, evaluated using the nitroblue tetrazolium (NBT) reduction assay. The NBT assay was performed as previously described.14 Briefly, a total of 2 × 105 cells were suspended in 700 μL RPMI 1640 medium and incubated with 50 μg/mL 12-O-tetradecanoyl-phorbol-13-acetate (Sigma) and 0.05% NBT (Sigma) for 30 minutes at 37°C. The reaction was stopped at 4°C, and cells were cytocentrifuged and stained with May-Grünwald Giemsa staining. The percentage of cells containing reduced black deposits was determined in duplicate, under light microscope, by examining 300 cells.
2.
Increased expression of myeloid differentiation antigens CD11b, CD14, and CD15, measured by flow cytometry.CD11b26 characterizes both granulocytic and monocytic differentiation; CD1427 is specific to monocytic differentiation. CD1528 is commonly associated with granulocytic lineage because its expression strongly rises during granulopoiesis. However, CD15 is also moderately increased through monopoiesis.28
For labeling, cells were suspended in RPMI 1640 containing 0.5% bovine serum albumin and at 105 cells per milliliter and then incubated at 4°C for 30 minutes with fluorescein isothiocyanate (FITC)-conjugated mAb to CD14 (IgG2a, 2 μg/mL, Coulter Immunology, Hialeah, FL), CD15 (IgM, 2 μg/mL, Becton Dickinson, San Jose, CA), or CD11b (IgG1, 2 μg/mL, Coulter Immunology). The mAbs were used at saturating concentrations. The mAb binding was measured by flow cytometry relative to isotype-matched control antibodies using a FACS Vantage (Becton Dickinson, Richmond, VA) equipped with an INNOVA70-4 argon ion laser (Coherent Radiation, Palo Alto, CA) tuned at 488 nm and operating at 500 mW. The flow cytometer was calibrated using a panel of fluorescent beads (Becton Dickinson). Each measurement was performed on 3000 cells. Dead cells were labeled by propidium iodide staining and excluded from the analysis.
3.
Cytologic modifications, microscopically observed on May-Grünwald Giemsa–stained cytosmears.
Western blot analysis of PML-RARα oncoprotein
Total cellular protein was extracted from 105control and treated NB4 cells, separated on a 8% sodium dodecyl sulfate acrylamide gel, and electrotransferred to nitrocellulose membranes (BioRad Laboratories, Hercules, CA). Membranes were blocked with 5% skim milk in phosphate-buffered saline for enhanced chemoluminescence (ECL, Amersham Life Science, Arlington Heights, IL) detection. Blots were incubated overnight with a 1:2000 dilution of anti-RARα polyclonal antibody (kindly provided by Dr P. Chambon). After washing 3 times for 20 minutes in phosphate-buffered saline, membranes were secondly hybridized to peroxidase-labeled goat antirabbit antibody for detection with ECL chemoluminescence system.
RT-PCR studies of cytokine transcript expression
Transcript expression of granulocyte (G), granulocyte-macrophage (GM), and macrophage colony-stimulating factor (M-CSF), interleukin (IL)-1β, and IL-3 was investigated by reverse transcriptase–polymerase chain reaction (RT-PCR). Total RNA was extracted from 5 × 105 cells using the Trizol reagent (Life Technologies, Cergy-Pontoise, France) followed by phenol-chloroform extraction and isopropanol precipitation as described. One microgram of total RNA was heated at 70°C for 10 minutes and used as a template for first-strand complementary DNA (cDNA) synthesis using RT and random hexamers (Life Technologies, Cergy-Pontoise, France). The housekeeping gene of glyceraldehyde phosphodehydrogenase was used as a marker transcript to yield a PCR product of 0.24 kilobases. Equilibrated amounts of cDNA were taken for cytokine transcript PCR amplification, within the exponential phase of amplification, using primers and 5′ end-labeled specific oligonucleotidic probes from Clontech (Palo Alto, CA). Primers used for the PCR were as follows: G-CSF: 5′-TTGGACACACTGCAGCTGGACGTCGCCGACTTT-3′ and 5′-ATTGCAGAGCCAGGGCTGGGGAGCAGTCATAGT-3′; IL-1β: 5′-ATGGCAGAAGTACCTAAGCTCGC-3′ and 5′-ACACAAATTGCATGGTGAAGTCAGTT-3′; IL-3: 5′-ATGAGCCGCCTGCCCGTCCTG-3′ and 5′-GCGAGGCTCAAAGTCGTCTGTTG-3′; GM-CSF: 5′-ATGTGGCTGCAGAGCCTGCTGC-3′ and 5′-CTGGCTCCCAGCAGTCAAAGGG-3′; and M-CSF: 5′-ATGACAGACAGGTGGAACTGCCAGTGTAGAGG-3′ and 5′-TCACACAACTTCAGTAGGTTCAGGTGATGGGC-3′.
The PCR protocol consisted of 30 cycles of 94°C for 1 minute, 58°C for 1 minute, and 72°C for 1 minute using a DNA Thermal Cycler (Perkin Elmer Cetus, Norwalk, CT). In all experiments, 2 negative controls were carried through all steps. The PCR amplification products were separated in 1% agarose and visualized by ethidium bromide staining. To demonstrate the specificity of the amplification, the PCR products were transferred onto Immobilon-S membranes (Millipore, France) for hybridization using 5′ end-labeled specific oligonucleotidic probe: G-CSF: 5′-GTGAGGAAGATCCAGGGCGA-3′; IL-1β: 5′-GCCAGAGCTGTGCAGATGAGTA-3′; and IL-3: 5′-CAAGACATTCTGATGGAAAA-3′.
Apoptosis
Cells were enumerated in triplicate using a Malassez hemocytometer, and their viability was determined by trypan blue exclusion test. Apoptosis was measured by 2 methods: (1) DNA strand breaks were labeled with FITC-conjugated deoxyuridine triphosphate (dUTP), using terminal deoxynucleotidyl transferase (TUNEL technique29), according to the manufacturer's recommendations (Boehringer Mannheim, Indianapolis, IN, cat. no. 1684795). The proportion of labeled (apoptotic) cells was measured by flow cytometry analysis relative to negative controls (labeled with dUTP without terminal deoxynucleotidyl transferase). (2)Acridine orange/ethidium bromide staining and enumeration of apoptotic cells (with nuclear chromatin condensation) using fluorescence microscopy was also used.30
Statistical analysis
Means were compared using an adjusted Student t test. Values were considered significantly different whenP < .05.
Results
H90 and A3D8 anti-CD44 mAbs strongly label CD44 expressed on KG1a, HL60, NB4, and THP-1 cell lines
CD44 is expressed on the 4 myeloid leukemia cell lines, and it is strongly labeled by both A3D8 and H90 anti-CD44 mAbs. Its mean fluorescence intensity (MFI) value is approximately 2- to 3-fold higher in KG1a cells than in HL60, THP-1, and NB4 cells (Table2). J173, an additional anti-CD44 mAb that, as shown in the following experiments, does not induce either cell growth inhibition, differentiation, or apoptosis, also labels CD44 as strongly as H90 and A3D8 (Table 2). Therefore, J173 is used as a surface-binding negative control in our experiments.
Binding of anti-CD44 mAbs A3D8, H90, and J173 to human myeloid cell lines
| Anti-CD44 mAb . | Relative MFI of CD44 (relative values, arbitrary units) . | |||
|---|---|---|---|---|
| KG1a . | THP1 . | HL60 . | NB4 . | |
| A3D8 | 501 ± 26 | 190 ± 12 | 246 ± 19 | 295 ± 16 |
| H90 | 553 ± 58 | 178 ± 32 | 274 ± 9 | 311 ± 18 |
| J173 | 495 ± 20 | 195 ± 28 | 277 ± 15 | 282 ± 45 |
| Anti-CD44 mAb . | Relative MFI of CD44 (relative values, arbitrary units) . | |||
|---|---|---|---|---|
| KG1a . | THP1 . | HL60 . | NB4 . | |
| A3D8 | 501 ± 26 | 190 ± 12 | 246 ± 19 | 295 ± 16 |
| H90 | 553 ± 58 | 178 ± 32 | 274 ± 9 | 311 ± 18 |
| J173 | 495 ± 20 | 195 ± 28 | 277 ± 15 | 282 ± 45 |
KG1a, THP-1, HL60, and NB4 cells are incubated with anti-CD44 FITC-conjugated A3D8, H90, or J173 mAbs. The MFI of CD44 is measured by flow cytometry as described in “Material and methods.” Data are the mean of MFI ± 1 SD calculated from 3 independent experiments.
H90 and A3D8 anti-CD44 mAbs inhibit proliferation of HL60, NB4 THP-1, and KG1a cell lines
Measurement of viable cell numbers over a 6-day period shows that addition of 2.5 μg/mL A3D8 anti-CD44 mAb strongly inhibits the growth of the 4 AML cell lines (Figure 1). The strongest inhibition is observed in A3D8-treated NB4 cells: at days 2 and 3, their number is only multiplied 1.5 times versus 3.6 times in controls. Thereafter, the number of A3D8-treated NB4 cells rapidly decreases, namely because of the onset of apoptotic cell death, as we detail in experiments presented below (Figure 3). A3D8 also strikingly slows the growth of HL60, THP-1, and KG1a cells: the maximum number of these cells plateau at day 4 at values 3- to 4-fold lower than the ones of controls (Figure 1A). At day 6, a slight decrease is observed due to limited cell death (17% ± 8%, 22% ± 5%, and 29% ± 4% in HL60, THP-1, and KG1a cell populations, respectively, vs < 10% in controls). In agreement with these results, measurement of [3H]thymidine incorporation performed at day 3 (at the exponential growth phase of control cells) shows that A3D8 strongly inhibits DNA synthesis in all AML cell lines. As shown in Figure 1B, the inhibition is the strongest in NB4 and KG1a cells, in which it reaches about 80%. It is slightly lower but still significant (P < .05) in THP-1 and HL60 cells (about 30% and 60%, respectively).
H90 and A3D8 anti-CD44 mAbs inhibit proliferation of HL60, NB4, THP-1, and KG1a cell lines.
A total of 105 cells/mL were seeded into 96-well plates and incubated in triplicate in the presence of specific anti-CD44 mAbs (2.5 μg/mL A3D8 or 20 μg/mL H90). Controls were cells incubated with isotypic control (20 μg/mL IgG1). At the indicated times, the viable cell number was determined using trypan blue dye exclusion (A). The statistical difference (P < .05) between A3D8-treated cells and controls is reached from day 2 (P < .05). The one between H90-treated cells and controls is reached from day 3 in HL60, NB4, and THP-1 cells and day 2 in KG1a cells. Treatment with the anti-CD44 mAb J173, which is inactive, gives similar results as the isotypic control. ▪: control; ▴: + H90; ■: +A3D8. At day 3 (exponential growth of control cells), [3H]thymidine incorporation into the cellular DNA was measured (B). Data are means ± 1 SD of 3 independent experiments. Data obtained with A3D8- or H90-treated cells are significantly different from those of controls (P < .05). Light grey bar: control; medium grey bar: +A3D8; black bar: + H90.
H90 and A3D8 anti-CD44 mAbs inhibit proliferation of HL60, NB4, THP-1, and KG1a cell lines.
A total of 105 cells/mL were seeded into 96-well plates and incubated in triplicate in the presence of specific anti-CD44 mAbs (2.5 μg/mL A3D8 or 20 μg/mL H90). Controls were cells incubated with isotypic control (20 μg/mL IgG1). At the indicated times, the viable cell number was determined using trypan blue dye exclusion (A). The statistical difference (P < .05) between A3D8-treated cells and controls is reached from day 2 (P < .05). The one between H90-treated cells and controls is reached from day 3 in HL60, NB4, and THP-1 cells and day 2 in KG1a cells. Treatment with the anti-CD44 mAb J173, which is inactive, gives similar results as the isotypic control. ▪: control; ▴: + H90; ■: +A3D8. At day 3 (exponential growth of control cells), [3H]thymidine incorporation into the cellular DNA was measured (B). Data are means ± 1 SD of 3 independent experiments. Data obtained with A3D8- or H90-treated cells are significantly different from those of controls (P < .05). Light grey bar: control; medium grey bar: +A3D8; black bar: + H90.
In the presence of H90 anti-CD44 mAb (20 μg/mL), the total number of viable cells is inhibited from day 3 onward. This inhibition is less pronounced than in A3D8-treated cells, ranging between 30% and 50%. It is, however, highly significant (P < .05 in all cell lines) (Figure 1A). Consistently, H90 also inhibits DNA synthesis, as shown by the significant diminution of [3H]thymidine incorporation measured at day 3 (P < .01, Figure 1B). Contrary to A3D8, H90 does not induce any significant cell death of either cell line.
H90 induces granulocytic differentiation of HL60 and NB4 cells
HL60 cells.
HL60 cells cultured in the presence of H90 (20 μg/mL) for 6 days display increased levels of 2 differentiation antigens: CD11b, which is myeloid-specific, and CD15, which is a granulocytic differentiation antigen. Indeed, as shown in Figure 2A, up to 43% ± 12% of H90-treated cells display CD11b (MFI 12 ± 0.5) versus less than 5% in controls, and the MFI value of CD15 (expressed on all control and treated cells) increases up to 349 ± 77 compared with 166 ± 12 in controls (P < .05). This increase is slightly lower than in RA-treated cells (Figure 2A). Its extent is related to the dose of H90: 6 days after addition of 5 μg/mL and 10 μg/mL H90, 14% ± 7% and 36% ± 5% of HL60 cells display CD11b, and the MFI of CD15 reaches 215 ± 23 and 285 ± 23, respectively. The increase of CD11b and CD15 expression is also time-dependent: It is slight at day 2 after addition of 20 μg/mL H90 (22% ± 9% CD11b+cells; MFI of CD15: 260 ± 12), and it is maximum from day 4 to day 6. Like RA, H90 does not induce any expression of the monocytic-specific antigen CD14. In addition, H90 induces the ability to produce oxidative bursts, as shown by the presence of 62% ± 4% of NBT+ cells. This percentage is as high as the one induced by RA (59% ± 5%, P > .05). Less than 5% of control cells are NBT+. Finally, H90 induces similar cytologic changes as RA, including segmented nuclei, fewer nucleoli, a smaller size, and decreased azurophilic granulations than in controls. These changes are characteristic of differentiated granulocytic cells (metamyelocytes and segmented polymorphs) (Figure 2B). No cytokine transcript expression is detected in HL60 cells 24 hours after the addition of H90. On the contrary, IL-1β and IL-3 transcripts are detected in RA-treated cells.
H90 induces granulocytic differentiation of HL60 and NB4 cells.
(A,C) Increased expression of lineage differentiation antigens. The expression of CD11b, CD14, and CD15 was measured as described in “Materials and methods” and in the legend to Figure 1A. Time and dose-dependency curves are shown. ▪: control; ▴: + H90; ▵: + RA. (B,D) May-Grünwald Giemsa–stained cytosmears of HL60 cells (B) and NB4 cells (D) treated with H90, 10−7 M/L RA, or J173 (controls). Both H90-treated HL60 and NB4 cells showed a segmented nucleus, condensed chromatin, rare nucleoli, and low nucleus-cytoplasmic ratio, all typical for differentiated granulocytic cells (metamyelocytes and band cells) and also observed in RA-treated cells. Magnification: × 630. (E) Degradation of PML-RARα in H90-treated NB4 cells; 24, 48, and 72 hours after addition of H90 (20 μg/mL), PML-RARα and RARα were revealed by successive incubation, first with a 1:2000 dilution of an anti-RAR polyclonal antibody and second with peroxidase-labeled goat antirabbit antibody for detection with ECL chemoluminescence system. The PML-RARα band (approximately 110 kd), present in control NB4 cells, was greatly decreased following addition of H90 (20 μg/mL). This decrease was time-dependent. A similar decrease was observed in RA-treated NB4 cells. The wild-type RARα band (50 kd) was also decreased.
H90 induces granulocytic differentiation of HL60 and NB4 cells.
(A,C) Increased expression of lineage differentiation antigens. The expression of CD11b, CD14, and CD15 was measured as described in “Materials and methods” and in the legend to Figure 1A. Time and dose-dependency curves are shown. ▪: control; ▴: + H90; ▵: + RA. (B,D) May-Grünwald Giemsa–stained cytosmears of HL60 cells (B) and NB4 cells (D) treated with H90, 10−7 M/L RA, or J173 (controls). Both H90-treated HL60 and NB4 cells showed a segmented nucleus, condensed chromatin, rare nucleoli, and low nucleus-cytoplasmic ratio, all typical for differentiated granulocytic cells (metamyelocytes and band cells) and also observed in RA-treated cells. Magnification: × 630. (E) Degradation of PML-RARα in H90-treated NB4 cells; 24, 48, and 72 hours after addition of H90 (20 μg/mL), PML-RARα and RARα were revealed by successive incubation, first with a 1:2000 dilution of an anti-RAR polyclonal antibody and second with peroxidase-labeled goat antirabbit antibody for detection with ECL chemoluminescence system. The PML-RARα band (approximately 110 kd), present in control NB4 cells, was greatly decreased following addition of H90 (20 μg/mL). This decrease was time-dependent. A similar decrease was observed in RA-treated NB4 cells. The wild-type RARα band (50 kd) was also decreased.
NB4 cells.
Like HL60 cells, NB4 cells differentiate into granulocytes when they are treated with H90 anti-CD44 mAb (20 μg/mL). Indeed, from day 4 to day 5 following the addition of H90, up to 77% ± 15% of NB4 cells display CD11b (MFI 33 ± 3) versus 17% ± 3% (MFI 18 ± 13) in controls. In addition, the MFI value of CD15 (that is expressed on all control and treated cells) reaches 374 ± 70 versus 102 ± 12 in controls (P < .05) (Figure 2C). These H90-induced antigenic changes are less pronounced than in the RA-treated group (100% of CD11b+ cells with an MFI of 138 ± 7; MFI value of CD15: 648 ± 53). The expression of both CD11b and CD15 increases proportionally to the dose of H90: the percentage of CD11b+ cells reaches 32% ± 3% and 62% ± 15% 6 days following addition of 5 μg/mL and 10 μg/mL H90. Following addition of 20 μg/mL H90, expression of both CD11b and CD15 is significantly increased at day 2 (24% ± 8% of CD11b+ cells, MFI of CD15: 185 ± 28) and it is maximum from day 4 to 5.
Like RA, H90 does not induce any expression of CD14. In addition, treatment with H90 induces cytologic features characteristic for mature differentiated granulocytic cells and similar to those observed in the RA-treated group (Figure 2D). However, H90 does not induce any significant ability to produce oxidative bursts (percent NBT+ cells < 5%) in NB4 cells, suggesting that the granulocytes are not fully functional.
Western blot analysis performed with a specific anti-RARα mAb22 shows that 2 days after addition of H90, the intensity of the 110 kd band corresponding to PML-RARα is significantly decreased and is no more detectable at day 3 (Figure 2E). This degradation is as rapid as the one induced by RA. Like RA,40 H90 also provokes the degradation of the 50 kd band corresponding to RARα (Figure 2E). No induction of cytokine transcript expression is detected 24 hours after addition of H90-treated NB4 cells.
H90-induced differentiation of HL60 and NB4 cells is dose- and time-dependent: using 20 μg/mL H90, the increase in differentiation antigen expression is detectable from day 2 and has its maximum at day 5.
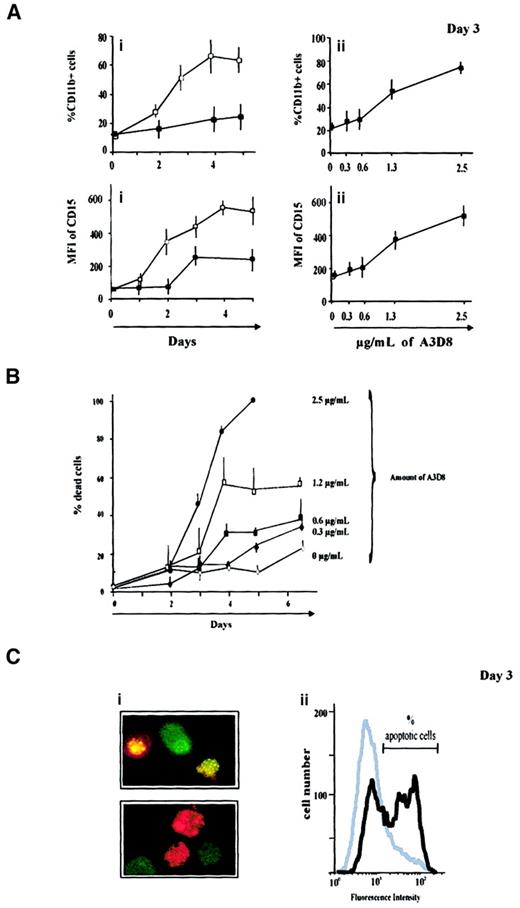
A3D8 induces incomplete cell maturation and apoptosis of NB4 cells
A3D8 induces maturation of NB4 cells (promyelocytic phenotype) toward granulocytic lineage, because it provokes a significant increase of both differentiation antigen expression and oxidative burst production capacity, in a dose- and time-dependent manner. Indeed, as early as 2 days after the addition of 2.5 μg/mL A3D8, NB4 cells display CD15 with an MFI value of 360 ± 44 versus 152 ± 19 in controls, and 53% ± 8% of them express CD11b+ with an MFI of 20 ± 1 (vs 17% ± 1% CD11b+ cells, MFI 18 ± 3, in controls). In addition, the A3D8-treated group displays 42% ± 8% NBT+ cells versus 8% ± 2% in controls. However, no cytologic change is observed at day 2 or after a treatment duration of 7 days (following the addition of lower doses of A3D8, see below). Therefore, these results show that A3D8 induces incomplete maturation of NB4 cells.
In addition, to provoke incomplete differentiation activity, A3D8 induces apoptotic death of NB4 cells in a dose- and time-dependent way (Figure 3B). The trypan blue exclusion test shows that the proportion of dead cells reaches 23% ± 3% at day 6 and 100% at day 4 after addition of 0.3 μg/mL and 2.5 μg/mL of A3D8, respectively.
A3D8 induces incomplete maturation and apoptosis of NB4 cells.
(A) Increased expression of myeloid differentiation antigens. Cells were treated with either 1.25 μg/mL A3D8 (Ai) or with increasing doses of this mAb (Aii). At the indicated days, cells were labeled in triplicate with FITC-conjugated mAbs directed to CD15 and CD11b, and their expression was measured by flow cytometry relative to isotypic controls. Dead cells were labeled by propidium iodide staining and excluded from the analysis. Because CD15 was expressed on all control cells, only its MFI was analyzed. Controls were cells treated with IgG1 or 2.5 μg/mL J173 anti-CD44 mAb, which was inactive. Data are mean values ± 1 SD from 3 independent experiments. ▪: control; ■: + A3D8. (B) Induction of cell death by A3D8. The percentage of dead cells was determined in triplicate, during 6 days, after addition of increasing doses of A3D8 (0.3 μg/mL to 2.5 μg/mL) using trypan blue exclusion assay. It was time- and dose-dependent. Data are mean values ± 1 SD from 3 independent experiments. (C) Apoptotic cells, 3 days following addition of 2.5 μg/mL A3D8. (Ci) Fluorescent staining with acridine orange and ethidium bromide showed nuclei condensation and fragmentation that are apoptosis-specific. Cells at an early stage of apoptosis (without loss of membrane integrity) displayed a bright green–stained nucleus. Apoptic cells that have lost membrane integrity displayed bright orange–stained nuclei as a result of taking up ethidium bromide. Magnification: × 630. (Cii) DNA strand breaks, which are apoptosis-specific, were labeled using TUNEL reaction. Their amount was evaluated by flow cytometry analysis. The proportion of labeled (apoptotic) cells was measured by flow cytometry analysis relative to negative controls labeled with dUTP without terminal deoxynucleotidyl transferase. This technique showed 39% ± 6% of apoptotic cells in the treated group versus less than 5% in controls (treated with J173). One experiment representative of 3 independent experiments is shown.
A3D8 induces incomplete maturation and apoptosis of NB4 cells.
(A) Increased expression of myeloid differentiation antigens. Cells were treated with either 1.25 μg/mL A3D8 (Ai) or with increasing doses of this mAb (Aii). At the indicated days, cells were labeled in triplicate with FITC-conjugated mAbs directed to CD15 and CD11b, and their expression was measured by flow cytometry relative to isotypic controls. Dead cells were labeled by propidium iodide staining and excluded from the analysis. Because CD15 was expressed on all control cells, only its MFI was analyzed. Controls were cells treated with IgG1 or 2.5 μg/mL J173 anti-CD44 mAb, which was inactive. Data are mean values ± 1 SD from 3 independent experiments. ▪: control; ■: + A3D8. (B) Induction of cell death by A3D8. The percentage of dead cells was determined in triplicate, during 6 days, after addition of increasing doses of A3D8 (0.3 μg/mL to 2.5 μg/mL) using trypan blue exclusion assay. It was time- and dose-dependent. Data are mean values ± 1 SD from 3 independent experiments. (C) Apoptotic cells, 3 days following addition of 2.5 μg/mL A3D8. (Ci) Fluorescent staining with acridine orange and ethidium bromide showed nuclei condensation and fragmentation that are apoptosis-specific. Cells at an early stage of apoptosis (without loss of membrane integrity) displayed a bright green–stained nucleus. Apoptic cells that have lost membrane integrity displayed bright orange–stained nuclei as a result of taking up ethidium bromide. Magnification: × 630. (Cii) DNA strand breaks, which are apoptosis-specific, were labeled using TUNEL reaction. Their amount was evaluated by flow cytometry analysis. The proportion of labeled (apoptotic) cells was measured by flow cytometry analysis relative to negative controls labeled with dUTP without terminal deoxynucleotidyl transferase. This technique showed 39% ± 6% of apoptotic cells in the treated group versus less than 5% in controls (treated with J173). One experiment representative of 3 independent experiments is shown.
Two independent and specific assays show that this cell death is apoptotic. The first one uses acridine orange/ethidium bromide staining, which presents specific nuclear condensation and fragmentation (Figure 3C). Using this assay, 18% ± 2% and 53% ± 4% of apoptotic cells are enumerated at days 2 and 3 after addition of 2.5 μg/mL A3D8, respectively (vs 6% ± 2% in controls). The second assay uses flow cytometry analysis after TUNEL reaction that specifically labels DNA strand breaks. It shows that 39% ± 6% of A3D8-treated NB4 cells are apoptotic 3 days after addition of 2.5 μg/mL A3D8 (Figure 3D).
Like H90 (Figure 2E), and as previously reported in the case of fresh AML-M3 blasts, A3D8 (2.5 μg/mL) induces degradation of PML-RARα oncoprotein in NB4 cells. At 24 hours, this degradation is as strong as in RA-treated cells (data not shown).
A3D8 induces monocytic differentiation of THP-1 and HL60 cells
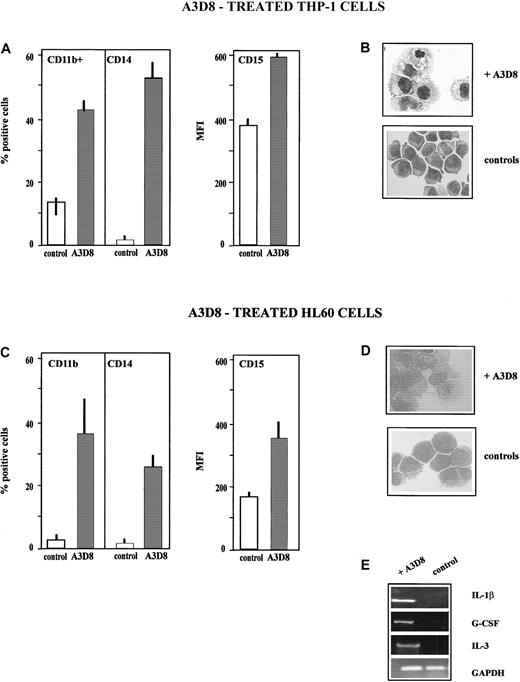
THP-1 cells.
THP-1 cells (monoblastic phenotype) fully differentiate into monocytes after addition of A3D8, as assessed by the following criteria measured at day 5 after addition of 2.5 μg/mL A3D8. First, the treated cells display an increased expression of the monocytic-specific antigen CD14 (52% ± 6% of CD14+ cells, MFI 32 ± 5, vs < 5% in control group) and of the myeloid-specific antigen CD11b (45% ± 6% of CD11b+ cells, MFI 32 ± 3, vs 12% ± 3%, MFI 26 ± 2 in controls); the level of CD15 (expressed on all control and treated cells) is also increased, with an MFI value of 592 ± 12 vs 392 ± 18 in controls (Figure4A). The increase in the expression of these differentiation antigens is time-dependent: it is detectable from day 2 following addition of 2.5 μg/mL A3D8 (17% ± 6% of CD14+ cells, MFI 25 ± 8; 28% ± 7% of CD11b+ cells, MFI 22 ± 6), and it is maximum from day 4 to 6. It is also dose-dependent: using 1.25 μg/mL A3D8, measurement at day 5 evidences 42% ± 5% CD14+ cells (MFI 32 ± 5) and 30% ± 9% of CD11b+ cells (MFI 30 ± 12). A half dose of A3D8 (0.63 μg/mL) does not significantly increase the expression of CD11 and CD14.
A3D8 induces monocytic differentiation of THP-1 and HL60 cells.
The data presented in panels A-E have been determined after addition of 2.5 μg/mL A3D8 at day 5 in the case of THP-1 cells (A,B) and at day 6 in the case of HL60 cells (C,D), except cytokine transcript synthesis, which has been analyzed at 24 hours (E). (A,C) Increased expression of lineage differentiation antigens. The expression of CD11b, CD14, and CD15 was measured as described in “Materials and methods” and in the legend to Figure 1A. (B,D) May-Grünwald Giemsa–stained cytosmears. (B) THP-1 cells: control THP-1 cells showed an immature phenotype: high nucleus-cytoplasmic ratio and numerous nucleoli. In contrast, A3D8-treated THP-1 cells showed a decreased nucleus-cytoplasmic ratio, chromatin condensation, and irregular cytoplasmic contours, all typical of mature monocytes. (D) HL60 cells: control cells showed an immature myeloblastic phenotype: high nucleus-cytoplasmic ratio and numerous nucleoli. A3D8-treated cells show a decrease in nucleus-cytoplasmic ratio, chromatin condensation, and irregular cytoplasmic contours typical of mature monocytes. Magnification: × 630. (E) Induction of synthesis of IL-1β, G-CSF, and IL-3 transcripts in HL60 cells. Total RNA from control and treated HL60 cells was prepared as described in “Materials and methods.” Using primers for glyceraldehyde phosphodehydrogenase, the amounts of cDNA were equilibrated to this internal standard. RT-PCR amplification products were separated by 1% agarose gel electrophoresis with 0.5 μg/mL ethidium bromide and were pictured under UV-light irradiation. Twenty-four hours after addition of A3D8, the expression of IL-1β, G-CSF, and IL-3 transcripts was detected. The specificity of the amplification was assessed by Southern blot hybridization with a specific oligonucleotide probe (data not shown). Pretreatment with genistein, a specific inhibitor of tyrosine kinases, abrogated this expression (data not shown).
A3D8 induces monocytic differentiation of THP-1 and HL60 cells.
The data presented in panels A-E have been determined after addition of 2.5 μg/mL A3D8 at day 5 in the case of THP-1 cells (A,B) and at day 6 in the case of HL60 cells (C,D), except cytokine transcript synthesis, which has been analyzed at 24 hours (E). (A,C) Increased expression of lineage differentiation antigens. The expression of CD11b, CD14, and CD15 was measured as described in “Materials and methods” and in the legend to Figure 1A. (B,D) May-Grünwald Giemsa–stained cytosmears. (B) THP-1 cells: control THP-1 cells showed an immature phenotype: high nucleus-cytoplasmic ratio and numerous nucleoli. In contrast, A3D8-treated THP-1 cells showed a decreased nucleus-cytoplasmic ratio, chromatin condensation, and irregular cytoplasmic contours, all typical of mature monocytes. (D) HL60 cells: control cells showed an immature myeloblastic phenotype: high nucleus-cytoplasmic ratio and numerous nucleoli. A3D8-treated cells show a decrease in nucleus-cytoplasmic ratio, chromatin condensation, and irregular cytoplasmic contours typical of mature monocytes. Magnification: × 630. (E) Induction of synthesis of IL-1β, G-CSF, and IL-3 transcripts in HL60 cells. Total RNA from control and treated HL60 cells was prepared as described in “Materials and methods.” Using primers for glyceraldehyde phosphodehydrogenase, the amounts of cDNA were equilibrated to this internal standard. RT-PCR amplification products were separated by 1% agarose gel electrophoresis with 0.5 μg/mL ethidium bromide and were pictured under UV-light irradiation. Twenty-four hours after addition of A3D8, the expression of IL-1β, G-CSF, and IL-3 transcripts was detected. The specificity of the amplification was assessed by Southern blot hybridization with a specific oligonucleotide probe (data not shown). Pretreatment with genistein, a specific inhibitor of tyrosine kinases, abrogated this expression (data not shown).
Second, treatment with A3D8 induces the capacity to produce oxidative bursts, as shown by 35% ± 5% of NBT+ cells versus less than 5% in control. Third, A3D8-treated THP-1 cells display cytologic changes typical of mature monocytes and not found in control cells: decreased nucleus-cytoplasmic ratio and nucleoli numbers, chromatin condensation, and irregular cytoplasmic contours (Figure 4B). At day 5 after addition of 2.5 μg/mL A3D8, a moderate proportion of apoptotic cells is detected (23% ± 4% vs 12% ± 3% in controls,P < .05). Half amount of A3D8 does not induce significant apoptosis. No induction of cytokine transcript expression is detected in A3D8-treated THP-1 cells. We also notice that RA does not induce the differentiation of THP-1 cells.
HL60 cells.
The addition of A3D8 (2.5 μg/mL) enhances the expression of myeloid differentiation antigens in HL60 cells. Indeed, measurement performed at day 6 shows 37% ± 13% of CD11b+ cells (MFI value of 12 ± 1) versus less than 5% in controls and 25% ± 7% of CD14+ cells (MFI value of 26 ± 5) versus less than 5% in controls. In addition, the MFI value of CD15 (displayed by 100% of the cells in both treated and control groups) increases up to 426 ± 55 versus 166 ± 12 in controls (Figure 4C). The increase in the expression of CD11b and CD14 is detectable since day 2, and it is maximum from day 4 to day 6. This increase is proportional to the amount of A3D8 for concentrations ranging from 0.63 to 2.5 μg/mL (data not shown). Moreover, A3D8-treated HL60 cells display a lower nucleus-cytoplasmic ratio than control cells, chromatin condensation, and irregular cytoplasmic contours typical of differentiated monocytes (Figure 4D). These antigenic and cytologic modifications, which are very similar to those observed in the course of monocytic maturation of THP-1 cells, suggest that A3D8 induces differentiation of HL60 cells toward monocytic lineage. However, A3D8-treated HL60 cells do not display oxidative burst production capacity, indicating that the monocytic differentiation is incomplete. Analysis of cytokine transcript expression using semiquantitative RT-PCR analysis shows an expression of G-CSF, IL-1β, and IL-3 transcripts in HL60 cells 24 hours after the addition of A3D8 (Figure 4E). These transcripts are not detected in control cells. The induction of their expression by A3D8 involves tyrosine kinases because it is fully abrogated by genistein (data not shown).
A moderate proportion of apoptotic cells is detected at day 6 (18% ± 4% vs 8% ± 3% in controls, P < .05). Half amount of A3D8 does not induce significant apoptosis.
Combined administration of H90 (or A3D8) and RA induced the differentiation of KG1a cells
Treatment of KG1a cells with H90 anti-CD44 mAb only increases the expression of CD15, with 58% ± 14% of CD15+ cells (MFI 46 ± 8) vs 31% ± 2% (MFI 22 ± 3) in controls, as measured at day 6. This increase is statistically significant (P < .05). A3D8 has a similar effect as H90 (data not shown). However, other changes currently used to assess myeloid differentiation (eg, increased expression of CD11b, oxidative burst production, cytologic changes) are not detectable. Accordingly, it is concluded that A3D8 and H90 are not able to trigger terminal differentiation of KG1a cells.
In contrast, in the presence of RA, which is inactive when used alone, both A3D8 and H90 anti-CD44 mAbs (used at maximal concentrations: 2.5 μg/mL and 20 μg/mL, respectively) succeed to overcome the differentiation blockage of these very immature leukemia cells. This is shown by the following changes, evaluated at day 5. The level of CD15, CD11b, and CD14 is strikingly increased, with 90% ± 5% of CD15+ cells (MFI value of 46 ± 14), 82% ± 7% of CD11b+ cells (MFI 30 ± 5), versus less than 5% in controls, and 23% ± 6% of CD14+ cells (MFI 15 ± 3), versus less than 5% in controls (Figure5A). In addition, the treated group comprises 30% NBT+ cells versus less than 5% in controls (Figure 5B). Finally, the treated KG1a cells display a decreased nucleus-cytoplasm ratio and pale cytoplasm with irregular contours that are typical of differentiating myeloid cells. However, large nucleoli are still present in the nucleus (Figure 5C). The whole of these antigenic, functional, and cytologic modifications indicates that the treatment combining activating anti-CD44 mAb plus RA triggers KG1a cells to differentiate.
Combined treatment with anti-CD44 mAbs (H90 or A3D8) and RA induces the differentiation of KG1a cells.
(A) Increased expression of lineage differentiation antigens. Cells were labeled in triplicate with FITC-conjugated mAbs directed to CD15 and CD11b. The percentage of CD11b+ and CD15+cells was determined by flow cytometry relative to isotypic controls. (B) Induction of NBT-reducing ability. Data are the percentage of NBT+ cells. They are mean values ± 1 SD of triplicate samples from a representative of 3 experiments. (C) May-Grünwald Giemsa–stained cytosmears of KG1a cells. Cells were treated for 6 days with H90 (20 μg/mL) plus RA (10−7 M/L) or J173 (controls). Control cells showed an immature myeloblastic phenotype: high nucleus-cytoplasmic ratio and numerous nucleoli. Treated cells showed a decrease in nucleus-cytoplasmic ratio, chromatin condensation, and a pale cytoplasm with irregular contours, all typical of maturing monocytes. Cells treated with A3D8 (2.5 μg/mL) and RA displayed similar features. Magnification: × 630.
Combined treatment with anti-CD44 mAbs (H90 or A3D8) and RA induces the differentiation of KG1a cells.
(A) Increased expression of lineage differentiation antigens. Cells were labeled in triplicate with FITC-conjugated mAbs directed to CD15 and CD11b. The percentage of CD11b+ and CD15+cells was determined by flow cytometry relative to isotypic controls. (B) Induction of NBT-reducing ability. Data are the percentage of NBT+ cells. They are mean values ± 1 SD of triplicate samples from a representative of 3 experiments. (C) May-Grünwald Giemsa–stained cytosmears of KG1a cells. Cells were treated for 6 days with H90 (20 μg/mL) plus RA (10−7 M/L) or J173 (controls). Control cells showed an immature myeloblastic phenotype: high nucleus-cytoplasmic ratio and numerous nucleoli. Treated cells showed a decrease in nucleus-cytoplasmic ratio, chromatin condensation, and a pale cytoplasm with irregular contours, all typical of maturing monocytes. Cells treated with A3D8 (2.5 μg/mL) and RA displayed similar features. Magnification: × 630.
No significant apoptosis is detected up to day 6 in either treated or control KG1a cells (< 10% of apoptotic cells).
The whole of the results are summarized in Table 3.
Summary of the effects of anti-CD44 mAbs A3D8 and H90 on differentiation and survival of NB4, THP-1, HL60, and KG1a cell lines
| . | A3D8 . | H90 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Increase in differentiation antigens' expression . | NBT assay . | Cytologic differentiation . | Increase in differentiation antigens' expression . | NBT assay . | Cytologic differentiation . | |||||
| CD11b . | CD14 . | CD15 . | CD11b . | CD14 . | CD15 . | |||||
| NB4 | + | + | + | + | +/− and apoptosis | + | − | + | − | + (granulocytes) |
| THP-1 | + | + | + | + | + (monocytes) | − | − | − | − | − |
| HL60 | + | + | + | − | + (monocytes) | + | − | + | + | + (granulocytes) |
| KG1a | − | − | − | − | − | − | − | − | − | − |
| KG1a + RA | + | + | + | + | Maturing myeloid cells | + | + | + | + | Maturing myeloid cells |
| . | A3D8 . | H90 . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Increase in differentiation antigens' expression . | NBT assay . | Cytologic differentiation . | Increase in differentiation antigens' expression . | NBT assay . | Cytologic differentiation . | |||||
| CD11b . | CD14 . | CD15 . | CD11b . | CD14 . | CD15 . | |||||
| NB4 | + | + | + | + | +/− and apoptosis | + | − | + | − | + (granulocytes) |
| THP-1 | + | + | + | + | + (monocytes) | − | − | − | − | − |
| HL60 | + | + | + | − | + (monocytes) | + | − | + | + | + (granulocytes) |
| KG1a | − | − | − | − | − | − | − | − | − | − |
| KG1a + RA | + | + | + | + | Maturing myeloid cells | + | + | + | + | Maturing myeloid cells |
Discussion
In the present work, we have investigated whether the human myeloid leukemia cell lines THP-1, NB4, and HL60 are interesting models for further studying the mechanisms involved in CD44-induced differentiation of AML-M5, AML-M3, and AML-M2 blasts, respectively. In a second part of our study, we have also investigated whether KG1a cells, which are very immature AML-M0 blasts, can be terminally differentiated via CD44.
Using antigenic, functional, and cytologic criteria, we show that CD44 ligation with activating anti-CD44 mAbs (A3D8 and H90) induces terminal differentiation of THP-1, HL60, and NB4 cell lines and, as expected at late stages of granulomonopoiesis, strongly inhibits their proliferation. Both A3D8 and H90 inhibit proliferation of all 3 cell lines but in contrast show cell-specific effects regarding induction of terminal differentiation. For example, H90 does not trigger differentiation of THP-1, and A3D8 (contrary to H90) only provokes incomplete differentiation of NB4 cells. These results indicate that although inhibition of proliferation might be a necessary condition for CD44-induced differentiation,31 it is not a sufficient one. Second, they strongly suggest that H90 and A3D8 mAbs activate distinct signaling pathways. Interestingly, it has been shown in the context of lymphoid cells that the CD44 receptor can selectively associate with distinct Src family protein tyrosine kinases.32-34 In this regard, it would be worthwhile to determine whether, in a given cell, CD44 engagement with either H90 or A3D8 mAb activates distinct Src tyrosine kinases, some being involved in terminal differentiation and the others not. Notably, H90 triggers differentiation toward granulocytic lineage (HL60 and NB4 cells) and A3D8 toward the monocytic one (HL60 and THP-1 cells). This suggests that H90 and A3D8 are likely to map distinct epitopes of the CD44 molecule (although they cross-block each other from binding, data not shown); these epitopes may be specifically involved in granulopoiesis and monopoiesis, respectively. It is also notable that the most efficient doses of H90 and A3D8 strikingly differ (by a factor 8). This supports the hypothesis that these anti-CD44 mAbs trigger differentiation and/or inhibit cell proliferation through different mechanisms.
In this paper, we show A3D8 induces massive apoptosis of NB4 cells. This is only in apparent contradiction with a previous study reporting an antiapoptotic activity of A3D8 (used at the same concentration and also in NB4 cells).23 Indeed, in the former study, apoptosis was triggered by a chemotherapy drug, whereas in the present one it is induced by A3D8 alone. Also, A3D8 inhibited drug-induced apoptosis 20 to 40 hours following its addition, whereas when it is used alone the apoptosis it triggers is not detected before 3 days. Interestingly, incomplete differentiation precedes this apoptosis. Indeed, although A3D8-treated NB4 cells display 2 features specific to terminally differentiating myeloid cells (increased expression of lineage antigens and oxidative burst production capacity), this differentiation is not achieved, because no cytologic changes are observed. This suggests that although A3D8-induced apoptosis is not a direct consequence of full terminal maturation (like the one occurring in normal granulopoiesis), it may be, however, somehow related to this differentiation process. Notably, the dual effect of A3D8 on NB4 cells (induction of incomplete maturation and apoptosis) shares many similarities with the one exerted by arsenic trioxide on these cells.35 This suggests that A3D8, like arsenic trioxide, may be efficient to improve the eradication of AML-M3 blasts in vivo, on the condition, previously demonstrated, that it will not be administered prior to apoptosis-inducing drugs. Contrary to NB4 cells, HL60, KG1a, and THP-1 cells endow only limited apoptotic death following addition of A3D8 (even when 25 μg/mL A3D8 was used, ie, a 10-times higher concentration than the one commonly used). The greater immaturity of these cells (in the case of HL60 and KG1a) or their monocytic phenotype (in the case of THP-1) is likely to account for their resistance to the apoptotic effect of A3D8. Indeed, it has been shown that survival-maintaining molecules are more abundant in immature versus differentiating myeloid cells and in monocytic versus granulocytic cells.36 This hypothesis remains to be demonstrated.
A3D8 and H90 used alone only inhibit the proliferation of KG1a cells, but they do not induce their differentiation. This is not surprising because, using a great variety of differentiation-inducing molecules that were active in other leukemic cell lines,20 22 it had not been possible so far to reverse the differentiation blockage of KG1a cells. The great immaturity of these cells is thought to partly account for their resistance to differentiation-inducing molecules. For the first time, we present evidence here that by combining an anti-CD44 mAb and RA it is possible to trigger the differentiation of these very immature cells, as evidenced by the increase in differentiation antigen expression and in the proportion of NBT+ cells. Moreover, although the persistence of numerous nucleoli indicates that KG1a cells remain rather immature, their differentiation was cytologically attested by a decrease in both cytoplasmic basophily and in nucleus-cytoplasmic ratio.
In conclusion, we have demonstrated that H90 and A3D8 anti-CD44 mAbs induce terminal differentiation of THP-1 (monoblastic), HL60 (myeloblastic), and NB4 (promyelocytic) cell lines. These results indicate that THP-1, HL60, and NB4 are reliable experimental models to study the mechanisms involved in H90- and A3D8-induced differentiation of fresh AML blasts. Cell-specific effects of H90 and A3D8 have been observed—in particular, a strong apoptotic effect of A3D8 in NB4 cells. This suggests that a combination between the differentiative effect of H90 and the apoptotic one of A3D8 may be of therapeutic interest. Finally, and most importantly, we show for the first time that the differentiation blockage of KG1a cells (that are as immature as AML-M0 blasts) can be overcome by treating these cells with both A3D8 or H90 anti-CD44 mAb and RA. These results may provide an experimental basis for a differentiative therapy combining CD44 ligation and RA in AML-M0.
We thank Dr Eliane Macquarre for the cytologic studies, Bernadette Guerton for her excellent technical assistance, Noémi Smadja for helpful comments and suggestions on the manuscript, and Nicole Vriz for her skillful secretarial assistance.
Supported by Inserm, ANRB-Vaincre le Cancer (Villejuif), Association pour le Recherche contre le Cancer (grant 5121), and La Ligue Contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Florence Smadja-Joffe, Inserm U268, Laboratoire de différenciation hématopoiétique normale et leucémique, Hôpital Paul-Brousse, 14, avenue P.V. Couturier, 94807 Villejuif Cedex, France; e-mail:fjsmadja@infobiogen.fr.

![Fig. 1. H90 and A3D8 anti-CD44 mAbs inhibit proliferation of HL60, NB4, THP-1, and KG1a cell lines. / A total of 105 cells/mL were seeded into 96-well plates and incubated in triplicate in the presence of specific anti-CD44 mAbs (2.5 μg/mL A3D8 or 20 μg/mL H90). Controls were cells incubated with isotypic control (20 μg/mL IgG1). At the indicated times, the viable cell number was determined using trypan blue dye exclusion (A). The statistical difference (P < .05) between A3D8-treated cells and controls is reached from day 2 (P < .05). The one between H90-treated cells and controls is reached from day 3 in HL60, NB4, and THP-1 cells and day 2 in KG1a cells. Treatment with the anti-CD44 mAb J173, which is inactive, gives similar results as the isotypic control. ▪: control; ▴: + H90; ■: +A3D8. At day 3 (exponential growth of control cells), [3H]thymidine incorporation into the cellular DNA was measured (B). Data are means ± 1 SD of 3 independent experiments. Data obtained with A3D8- or H90-treated cells are significantly different from those of controls (P < .05). Light grey bar: control; medium grey bar: +A3D8; black bar: + H90.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.290/6/m_h80121969001.jpeg?Expires=1765922148&Signature=KkraX8zVXHCEDEk2kYo24m7RGXd3-fdlc6TTI3X3rFbltCW7Z4K~dKTF5EPUlOUu5O3ChRPdDDOBofc2TAmmoiRwB51-WgjUVHGTP8RK9sqeP6GQwBIoEbJf-uvY-IkvzTvbuEf9Yofth5IYYii0FuvWbsWez1WtbXIQuvizahko0kbz88rwZrJQ5KuAAwcTVp0jBEGJl~eM6Y9C9u8XiSmGXeJU8wRaYeLp6zFJ7duvz4Dew~dHuv0mjCjFXN9cumG6WCZJVxiYc1jwIzedSsgU1QagxZZC49evmvBDhwSLe3OnjlaIUNkKsdGV7J9nEqnjGu0hQF9Np9wY9Qev7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal