Abstract
Kidney and liver are the major organs of erythropoietin (Epo) synthesis. However, Epo messenger RNA (mRNA) has been detected in several organs, such as brain, lung, and testis. Furthermore, functional Epo receptors have been demonstrated on different cell types, including rat Leydig cells. The aim of the study was to identify testicular cells expressing Epo mRNA and to quantitate its levels by competitive reverse transcriptase–polymerase chain reaction (RT-PCR). Besides whole testis, Epo transcripts were found in Sertoli and peritubular myoid cells, while no signal was detected in Leydig cells. Exposure of Sertoli cells to CoCl2 led to an increase of Epo mRNA level. Semiquantitative competitive RT-PCR presented an increase in the level of Epo mRNA in Sertoli cells stimulated by follicle-stimulating hormone, while exposure of peritubular myoid cells cultures to testosterone reduced Epo mRNA expression. Due to the blood-testis barrier, basal expression of Epo suggests a not yet defined function of this hormone in testis.
Introduction
Besides kidney and liver,1erythropoietin (Epo) messenger RNA (mRNA) has been detected in several other normal organs, such as brain, testis, lung, spleen, placenta,2-6 bone marrow,7 embryonic stem cell–derived embryoid bodies,8 and ovary.9Moreover, functional Epo receptors have been demonstrated on several cell types, such as rodent and human placenta,10 human vascular endothelial cells,11 human and rodent brain,12,13 rat gastric epithelial cells,14rodent and human kidney cells,15 and rat Leydig cells.16 The widespread distribution of Epo mRNA and functional Epo receptors has suggested that Epo may act as a paracrine factor. In testis there is a consistent basal expression of Epo mRNA, and hypoxic exposure leads to increased levels of the transcript in the whole organ,2,6 but the identity of Epo-producing cells in testis is not defined. However, it has been observed that Epo influences rat Leydig cell steroidogenesis in vitro by stimulating testosterone production through a specific receptor-mediated mechanism17; furthermore, in humans, intravenous Epo administration increases testosterone production.18 In the present study, we examined by means of competitive reverse transcriptase–polymerase chain reaction (RT-PCR) whether rat Sertoli, Leydig, and peritubular myoid primary cell cultures express Epo mRNA. Our results revealed that Epo mRNA is expressed in Sertoli and peritubular myoid cells.
Study design
Animals
Twenty-day-old male Sprague Dowley rats (Charles-River, Como, Italy) were killed by decapitation, and testes and kidneys were removed, frozen in liquid nitrogen, and stored at −70°C until RNA extraction.
Cell cultures
Primary Sertoli and peritubular myoid cells were isolated from 20-day-old rat testes by sequential enzymatic digestion with a previously described procedure.19 Sertoli cell clusters were resuspended in Dulbecco modified Eagle medium/F-12 medium (1:1) (Gibco, Paisley, United Kingdom), plated, and maintained at 34°C under humidified atmosphere with 5% CO2. On day 3, plates were exposed to a hypotonic to remove contaminating germ cells. Contamination with peritubular cells, as assessed by staining for alkaline phosphatase, was negligible (1%-2%), and contamination with Leydig cells, evaluated by cytochemical detection of 3β-hydroxysteroid dehydrogenase activity, was virtually absent.
Purified peritubular myoid cells were separated from Sertoli cells by a Percoll discontinuous gradient technique.20 Primary cultures of peritubular myoid cells were maintained at 34°C in 5% CO2 atmosphere in Dulbecco modified Eagle medium/F12 (1:1) containing 10% fetal calf serum (Gibco). Cell purity, never below 97%, was evaluated by alkaline phosphatase activity on adherent cells.
After 4 days, Sertoli cells were incubated with either 100 ng/mL ovine follicle-stimulating hormone (o-FSH-17; NIH, Bethesda, MD), 1 μM testosterone (Sigma, St Louis, MO), or 100 μM CoCl2for 18 hours. Between day 10 and 12, peritubular myoid cells were treated with 1 μM testosterone and 100 μM CoCl2 for 18 hours in 95% air and 5% CO2. After exposure to stimuli, Sertoli and peritubular myoid cells were harvested for total RNA extraction.
Leydig cell–enriched cultures and the rat tumor Leydig cell line LC-540, obtained from American Type Culture Collection, were prepared and cultured using previously described procedures.21 22After 48 hours of cultures, Leydig cells, whose purity was never below 88% as assessed by cytochemical detection of 3β-hydroxysteroid dehydrogenase, and LC-540 cells were harvested for RNA extraction.
Cell viability for cell types was assessed by the trypan blue dye exclusion method.
RT-PCR
The RT-PCR reactions were performed as previously described.23 Aliquots of the reaction mixture were used as template for PCR amplification of Epo complementary DNA (cDNA) with primers 5′-ATTTGCGACAGTCGCGTTCT-3′ (sense) and 5′-GTATCCGCTTGAAGTGTTCG-3′ (antisense), located in exon 2 and exon 5, respectively, of the rat Epo gene. The Epo RT-PCR product was 395 base pairs. Epo mRNA was quantified by competitive RT-PCR. The competitor Epo template was obtained as previously described11 and, after amplification with the same primer set, resulted in a 492–base pair fragment. Fixed or increasing relative amounts of synthetic RNA competitor template were added to the total RNA samples before semiquantitative or quantitative RT-PCR, respectively. The relative amount of amplified target cDNA versus competitor cDNA was quantitated by a densitometric scanner of ethidium bromide–stained agarose gels of the RT-PCR products.
Results and discussion
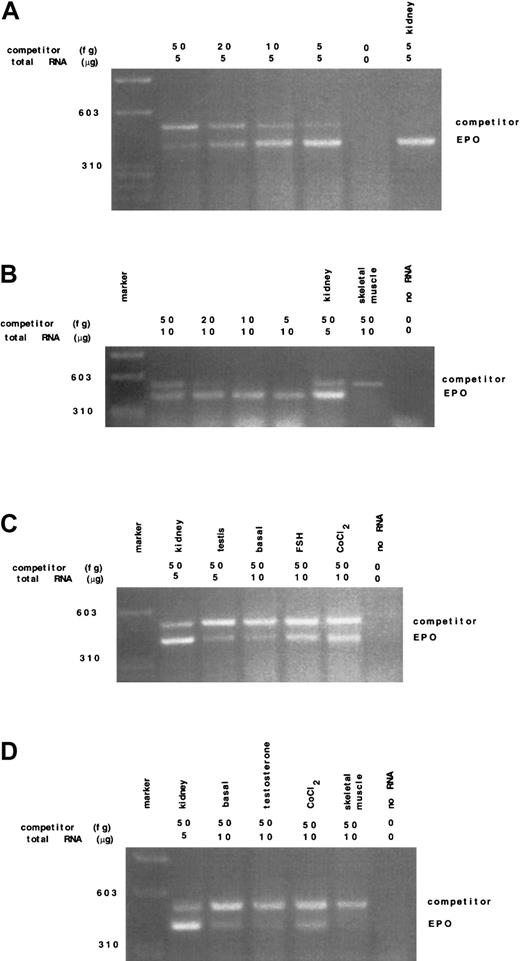
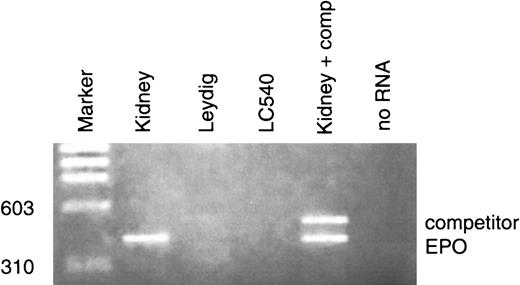
Apart from kidney and liver, several tissues express Epo mRNA in an oxygen-dependent manner. In the testes of healthy adult rodents, the total amount of Epo mRNA has been described to range from 10% to 30% of the level in the kidney2; such expression increased threefold to fourfold after hypoxic stimulation.2 Previous studies have documented that adult rat Leydig cells possess specific Epo binding sites and that recombinant human Epo stimulates testosterone production by these cells17; however, to our knowledge, no data are available concerning which type of cells in testis may express Epo mRNA. In this study we evaluated the main somatic testicular cell types: Sertoli, Leydig, and peritubular myoid cells. Quantitation of Epo mRNA levels in the different experimental conditions was performed by semiquantitative or quantitative competitive RT-PCR assay. The study was performed on 5 different groups of animals; in each group the experiments were carried out at least 3 times. The amount of specific Epo mRNA in whole rat testis was 2.58 ± 0.41 fg/μg total RNA (Figure1A). Primary Sertoli cells cultured at normal oxygen conditions (20% O2) contained 1.31 ± 0.26 fg/μg total Epo mRNA. Exposure of Sertoli cell cultures to 100 μM CoCl2 resulted in an increase of Epo mRNA levels of about three to four times (Figure 1B). Epo mRNA levels in peritubular myoid cells were evaluated by semiquantitative RT-PCR. Using this technique we observed the presence of Epo transcripts also in this cell type. Addition of 100 μM CoCl2 to peritubular myoid cell cultures induced an increase of Epo mRNA levels (Figure 1D). Unlike Sertoli and peritubular myoid cells, neither Leydig cell primary cultures nor the LC-540 cell line presented Epo transcripts (Figure2).
Detection and quantitation by competitive RT-PCR of Epo mRNA in whole rat testis and in Sertoli and peritubular myoid cells in basal conditions and after hormonal stimuli.
(A) Quantitation of Epo mRNA transcripts in rat testis; 5 μg total RNA from rat testis with the indicated amounts of competitor Epo cRNA was reverse transcribed and amplified; no RNA (negative control), kidney total RNA (positive control). (B) Quantitation of Epo mRNA in CoCl2-stimulated rat Sertoli cells; 10 μg total RNA from primary cultures of rat Sertoli cells, 5 μg from kidney, 10 μg from skeletal muscle, and the indicated amounts of Epo cRNA competitor were reverse transcribed and amplified. Sertoli cells were stimulated with 100 CoCl2 for 18 hours. No Epo transcripts were detected from skeletal muscle (negative control). (C) Detection of Epo mRNA transcripts in rat Sertoli cells; 5 μg total RNA from kidney and testis and 10 μg total RNA from rat Sertoli cells with a standard amount of the competitor (50 fg) were reverse transcribed and amplified. Sertoli cells were incubated for 18 hours with 100 ng/mL FSH and 100 μM CoCl2. (D) Detection of EPO mRNA transcripts in rat peritubular myoid cells; 10 μg total RNA from primary cultures of rat peritubular myoid cells in basal condition and stimulated with 100 μM CoCl2 for 18 hours and 5 μg total RNA from rat kidney were reverse transcribed and amplified.
Detection and quantitation by competitive RT-PCR of Epo mRNA in whole rat testis and in Sertoli and peritubular myoid cells in basal conditions and after hormonal stimuli.
(A) Quantitation of Epo mRNA transcripts in rat testis; 5 μg total RNA from rat testis with the indicated amounts of competitor Epo cRNA was reverse transcribed and amplified; no RNA (negative control), kidney total RNA (positive control). (B) Quantitation of Epo mRNA in CoCl2-stimulated rat Sertoli cells; 10 μg total RNA from primary cultures of rat Sertoli cells, 5 μg from kidney, 10 μg from skeletal muscle, and the indicated amounts of Epo cRNA competitor were reverse transcribed and amplified. Sertoli cells were stimulated with 100 CoCl2 for 18 hours. No Epo transcripts were detected from skeletal muscle (negative control). (C) Detection of Epo mRNA transcripts in rat Sertoli cells; 5 μg total RNA from kidney and testis and 10 μg total RNA from rat Sertoli cells with a standard amount of the competitor (50 fg) were reverse transcribed and amplified. Sertoli cells were incubated for 18 hours with 100 ng/mL FSH and 100 μM CoCl2. (D) Detection of EPO mRNA transcripts in rat peritubular myoid cells; 10 μg total RNA from primary cultures of rat peritubular myoid cells in basal condition and stimulated with 100 μM CoCl2 for 18 hours and 5 μg total RNA from rat kidney were reverse transcribed and amplified.
Detection of Epo mRNA in rat Leydig cells and in LC-540 cell line.
The same RT-PCR experimental conditions were used. No Epo transcripts were detected in both cell types; no RNA (negative control), kidney (positive control).
Detection of Epo mRNA in rat Leydig cells and in LC-540 cell line.
The same RT-PCR experimental conditions were used. No Epo transcripts were detected in both cell types; no RNA (negative control), kidney (positive control).
We then tested the effects of the hormonal factors known to specifically maintain Sertoli and peritubular myoid cells function, ie, FSH and testosterone, respectively. Addition of 100 ng/mL FSH to Sertoli cell cultures induced Epo mRNA levels (Figure 1C), whereas we observed a reduction of Epo mRNA expression after treatment of peritubular myoid cell cultures with 1 μM testosterone (Figure 1D). Thus, in the 2 cell types the results have been contrasting, showing FSH-stimulatory and testosterone-inhibiting effects upon Epo mRNA expression. Hormonal regulation has long been known to be involved in the control of Epo expression. Studies performed in vitro with HepG2 cells have shown that thyroid hormones increase hypoxia-induced EPO mRNA levels twofold to threefold, while neither growth hormone, insulinlike growth factor-1, testosterone, nor cortisone have led to an increase in Epo gene expression.24 Thus, FSH would be the first pituitary hormone able to increase Epo mRNA expression in in vitro conditions, although GH has been previously shown to stimulate Epo secretion in anemic patients with chronic renal failure.25
These results, together with the previous description of specific binding sites in Leydig cells, suggest that Epo may play a yet unknown local regulatory role in testis. It is tempting to speculate that testis could respond to specific stimuli with an increased local production of Epo that would stimulate Leydig cell steroidogenesis or could act inside the seminiferous tubule. The elevated intratesticular testosterone concentrations could, in turn, reduce Epo mRNA levels, generating a paracrine-negative feedback.
The authors thank R. H. Wenger for helpful discussions.
Supported in part by the Swiss Nationale Science Foundation (31-16743.99) and by MURST (Ministero Dell' Universitá e Ricerca Scientifica e Technologica).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anna Maria Aglianò, Dipartimento di Medicina Sperimentale e Patologia, Viale Regina Elena, 324-00161 Rome, Italy; e-mail: annamaria.agliano@uniroma1.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal