Abstract
The murine bone marrow retroviral transduction and transplantation model of chronic myelogenous leukemia (CML) imperfectly mimics human CML because the murine CML-like disease causes death of all animals from an overwhelming granulocytosis within 3 to 4 weeks. In this report, mice reconstituted with P210BCR/ABL-transduced bone marrow cells received posttransplantation therapy with either the tyrosine kinase inhibitor STI571 or placebo. Compared with the rapidly fatal leukemia of placebo-treated animals, 80% of the STI571-treated mice were alive on day 74, with marked improvement in peripheral white blood counts and splenomegaly. There was decreased tyrosine phosphorylation of STAT5, Shc, and Crk-L in leukemic cells from STI571-treated animals, consistent with STI571-mediated inhibition of the Bcr/Abl tyrosine kinase in vivo. In some STI571-treated animals Bcr/Abl messenger RNA and protein expression were markedly increased. In contrast to the polyclonal leukemia of placebo-treated mice, STI571-treated murine CML was generally oligoclonal, suggesting that STI571 eliminated or severely suppressed certain leukemic clones. None of the STI571-treated mice were cured of the CML-like myeloproliferative disorder, however, and STI571-treated murine CML was transplanted to secondary recipients with high efficiency. These results demonstrate the utility of this murine model of CML in the evaluation of novel therapeutic agents against Bcr/Abl-induced leukemias. This improved murine chronic-phase CML model may be a useful tool for the study of STI571 resistance, CML progression, and the anti-CML immune response.
Introduction
The chimeric protein Bcr/Abl, the product of the oncogene BCR/ABL, is a dysregulated nonreceptor tyrosine kinase that plays a central role in the pathophysiology of Philadelphia chromosome–positive (Ph+) hematologic malignancies.1 Although the mechanism remains to be precisely defined, recent studies have suggested that Bcr/Abl leads to hematopoietic transformation by disturbing the complex balance of signaling pathways governing proliferation, differentiation, adhesion, and apoptosis.2 Not surprisingly, integration of these diverse effects of Bcr/Abl into a cohesive model has proved difficult with the use of currently available cell culture systems; however, the retroviral-based mouse model of Bcr/Abl–induced leukemia has provided some important insights. In addition to bolstering the hypothesis thatBCR/ABL is the causative agent of chronic myelogenous leukemia (CML), this model has been an important tool in the analysis of Bcr/Abl hematopoietic target cells3-7 and in identifying signaling pathways important in the induction of leukemia.8-12 This murine model of CML also has the potential to be a useful tool in the evaluation of therapeutic agents against Bcr/Abl-induced leukemias, particularly in experiments not feasible in human subjects.
In the murine bone marrow (BM) retroviral transduction and transplantation model of CML, lethally irradiated mice are reconstituted with syngeneic BM transduced with a replication-defective Bcr/Abl retrovirus. Previous studies have demonstrated that mice treated in this fashion develop a fulminant myeloproliferative disorder, characterized by massive splenomegaly and striking peripheral blood and BM granulocytosis.3-5 However, in contrast to the human disease, the murine CML-like illness is uniformly fatal because mice in this model have never received any antileukemic therapy. To determine whether the established murine BM retroviral transduction and transplantation model could be extended to provide an animal model for CML in chronic phase, we randomized mice to oral treatment with the tyrosine kinase inhibitor STI571 or placebo beginning on day 10 after BM transplantation. Mice were monitored clinically and by serial peripheral blood count for evidence of leukemia. STI571 significantly prolonged the survival of animals in this murine model of CML, with the majority of animals demonstrating a marked reduction in splenomegaly and, in some cases, almost complete normalization of their peripheral white blood counts (WBCs), reminiscent of human CML in chronic phase. Although all animals exhibited a prolonged survival with STI571 treatment, approximately 20% of animals died of leukemia despite treatment with the drug. Interestingly, even among animals that responded to STI571, serial blood counts revealed a heterogeneous pattern of hematologic response to the drug. To gain insight into possible explanations for differing clinical responses to STI571 treatment, peripheral blood, BM, and spleen cells from responding and nonresponding animals were subjected to Western, Northern, and Southern blot analysis.
Materials and methods
STI571
The tyrosine kinase inhibitor STI571 was kindly provided by Elisabeth Buchdunger (Novartis, Basel, Switzerland). For the animal studies, stock solutions of 5 mg/mL and 10 mg/mL were prepared fresh in water, sterile filtered, and administered to mice in a volume of 250 μL by gavage twice a day.
Animal studies
All experiments using animals described in this study were performed according to a research protocol approved by the Animal Care and Use Committee at the University of Texas Southwestern Medical Center. For the murine BM transplantation experiments, Balb/c mice 4 to 12 weeks of age (Harlan, Indianapolis, IN) were primed with 5-fluorouracil and used as BM donors to reconstitute lethally irradiated syngeneic recipients, essentially as described.13 BM cells were transduced with a helper-free replication-defective P210BCR/ABL retrovirus, obtained by transient transfection of 293T cells by means of an ecotrophic retroviral packaging construct.14 Mice were started on STI571 or placebo (the same volume of diluent water) beginning on day 10 after BM reconstitution (day 0) by means of an STI571 regimen of 50 mg/kg every morning and 100 mg/kg every evening by gavage. STI571 was administered in a volume of 250 μL sterile water by means of straight or curved animal feeding needles (Harvard Apparatus, Holliston, MA). Mice tolerated the therapy well, and no interruption of therapy was necessary. Mice were followed clinically 3 times a week, and periodic peripheral blood counts were obtained by tail vein blood draw as indicated. For the survival analysis portion of this study, the death endpoint was determined either by spontaneous death of the animal or by elective killing of the animal because of signs of pain or suffering according to established criteria. Some of the STI571-treated animals were electively killed well after the survival studies to perform the required molecular studies. For the secondary BM transplantation experiments, mice received a single sublethal dose of radiation (4.5 Gy) just prior to reconstitution with 1 × 106syngeneic BM cells from leukemic animals. Secondary transplant recipients were clinically monitored and analyzed as described above. In some cases, secondary recipients were given STI571 treatment according to the same dose and schedule as the primary animals.
Antibodies and immunoblot
RIPA protein lysates containing 2% vol/vol aprotinin, 2 mM sodium orthovanadate, 100 mM sodium fluoride, 10 μg/mL leupeptin, 2 μg/mL pepstatin, 4 mM Pefabloc SC (Roche Diagnostics, Mannheim, Germany), and 2 mM phenylmethyl sulfonyl fluoride were prepared from whole spleen, BM, or peripheral granulocytes, normalized by OD595 (Bio-Rad Protein assay, Bio-Rad Laboratories, Hercules, CA), and subjected to sodium dodecyl sulfate (SDS)/polyacrylamide gel electrophoresis. Gels were then electrophorectically transferred to a nitrocellulose membrane overnight at 4°C. Membranes were probed with either anti-STAT5, anti-Shc, anti-Crk-L (all from Santa Cruz Biotechnology, Santa Cruz, CA), antiphosphotyrosine (Upstate Biotechnology, Lake Placid, NY), anti-Abl (Oncogene Research Products, Boston, MA), or anti-pSTAT5 (Cell Signaling Technology, Beverly, MA) antibodies and detected by enhanced chemiluminescence (Amersham, Arlington Heights, IL).
Southern and Northern blot
To prepare genomic DNA from hematopoietic tissues isolated from mice treated or not treated with STI571, Tris/SDS/proteinase K lysis was used followed by standard phenol-chloroform-isoamyl alcohol extraction. Then, 10 μg genomic DNA was digested either with the restriction enzyme BglII to determine the number of unique proviral integration sites or with XbaI (digests on either side of the retroviral long terminal repeat) to verify the full-length integrity of the provirus. Samples were resolved on a 0.8% Tris/borate/EDTA gel, and complete DNA digestion was confirmed by ethidium bromide staining. Nucleic acids were then bound to a nylon membrane (Zetaprobe) (Bio-Rad) by capillary transfer overnight. Membranes were hybridized with a 32P-labeled ClaI fragment (Radprime DNA labeling system, Gibco BRL, Rockville, MD) derived from the pGD vector neomycin-resistance gene and analyzed by autoradiography.
Total RNA was isolated from hematopoietic tissues by Trizol-based (Gibco BRL) extraction. We resolved 10 μg total RNA by formaldehyde denaturing gel electrophoresis, followed by capillary transfer onto a nitrocellulose membrane overnight. To detect P210BCR/ABL messenger RNA (mRNA), membranes were hybridized with a 32P-labeled DNA probe spanning the Bcr and Abl junction. Cis gene expression was evaluated by means of a 32P-labeled fragment from a cis expression plasmid.15
Results
Mice with the CML-like illness demonstrate marked clinical improvement with the tyrosine kinase inhibitor STI571
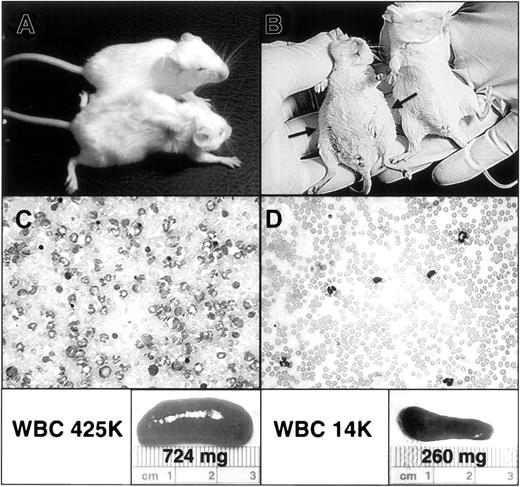
Animals treated with placebo (water by gavage twice a day) generally began to show signs of illness by late in the second week. Clinically, mice exhibited decreased spontaneous activity, such as grooming and ambulation, and an increase in resting respiratory rate. The latter, due to progressive pulmonary hemorrhage and hemothorax from massive granulocytic infiltration of pulmonary arterioles and capillaries, was the likely immediate cause of death in all animals that died from from the CML-like illness (data not shown).7 13 Examination also revealed progressive splenomegaly due to granulocytic infiltration and some degree of extramedullary erythropoiesis (data not shown). The splenomegaly and increased respiratory rate probably contributed to the decreased oral intake manifested by these animals late in the course of their illness. This clinical picture contrasted sharply with that observed in mice receiving STI571 treatment. STI571-treated animals demonstrated fewer signs of systemic illness, including a healthier-appearing coat, a more normal level of physical activity, and evidence of adequate hydration (Figure 1A). Physical examination also revealed a lesser degree of splenomegaly, with a decrease in abdominal distention, and a more normal respiratory rate. At autopsy, there was a striking difference in the magnitude of splenomegaly between placebo- and STI571-treated animals (Figure 1B) and in the degree of granulocytosis on peripheral blood smears (Figure 1C-D). Interestingly, STI571-treated animals also showed decreased pulmonary hemorrhage, despite significant pulmonary parenchymal infiltration by maturing granulocytes (data not shown).
Mice demonstrate clinical and hematopoietic response to STI571 treatment.
(A) An STI571-treated animal (top) is compared with placebo-treated animal (bottom) on day 21 after reconstitution with BM cells transduced with P210BCR/ABL retrovirus. Note the overall weakened appearance of the placebo-treated animal, including decreased skin turgor, reflecting volume depletion due to decreased oral intake in the setting of massive hepatosplenomegaly. In contrast, the STI571-treated animal demonstrates no clinical evidence of illness. (B) A ventral view of the same mice demonstrating the pronounced abdominal distention of the placebo-treated animal (left) secondary to marked hepatosplenomegaly (arrows), which is lacking in the STI571-treated mouse. (C-D) A representative blood smear and spleen from a placebo-treated (C) and STI571-treated (D) mouse, demonstrating the significant reduction in granulocytosis and splenomegaly obtained in STI571-responding animals. The respective white blood cell count (WBC) and spleen size and weight are also shown (lower panels). For the sake of comparison, the average WBC and spleen weight of control healthy mice (no. = 3) of the same strain and age are 8400/μL and 95.5 mg, respectively.
Mice demonstrate clinical and hematopoietic response to STI571 treatment.
(A) An STI571-treated animal (top) is compared with placebo-treated animal (bottom) on day 21 after reconstitution with BM cells transduced with P210BCR/ABL retrovirus. Note the overall weakened appearance of the placebo-treated animal, including decreased skin turgor, reflecting volume depletion due to decreased oral intake in the setting of massive hepatosplenomegaly. In contrast, the STI571-treated animal demonstrates no clinical evidence of illness. (B) A ventral view of the same mice demonstrating the pronounced abdominal distention of the placebo-treated animal (left) secondary to marked hepatosplenomegaly (arrows), which is lacking in the STI571-treated mouse. (C-D) A representative blood smear and spleen from a placebo-treated (C) and STI571-treated (D) mouse, demonstrating the significant reduction in granulocytosis and splenomegaly obtained in STI571-responding animals. The respective white blood cell count (WBC) and spleen size and weight are also shown (lower panels). For the sake of comparison, the average WBC and spleen weight of control healthy mice (no. = 3) of the same strain and age are 8400/μL and 95.5 mg, respectively.
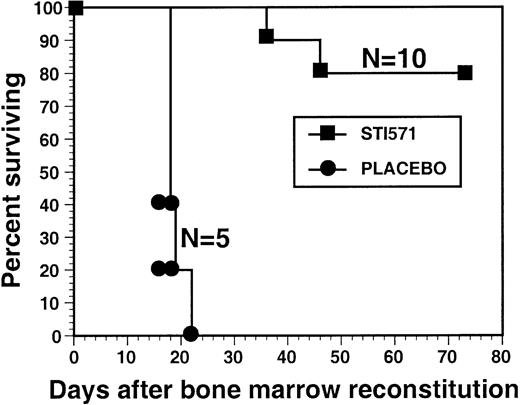
STI571 treatment significantly prolonged the survival of animals in a murine model of CML
To study the effect of STI571 on hematologic response and overall survival in the murine BM retroviral transduction and transplantation model of CML, mice of identical age were randomized to receive either STI571 or the same volume of placebo (water) twice a day beginning on day 10 after BM transplantation. Some animals began STI571 or placebo treatment 48 hours after BM transplantation with similar results (Figure 2 and data not shown). Our STI571 treatment regimen consisted of 50 mg/kg every am and 100 mg/kg every pm by gavage and was based, with some modification, on previous pharmacokinetic studies of STI571 in Bcr/Abl tumor-bearing mice.16,17 Mice tolerated administration of the drug well, without evidence of overt drug toxicity. Animals were continued on the assigned STI571 or placebo treatment regimen until (1) sufficient clinical evidence of symptomatic disease progression was present to warrant euthanasia; (2) death; or (3) elective killing of the animal for purposes of analysis. By day 21, 100% of placebo-treated animals had died from the CML-like illness, characterized by an overwhelming granulocytosis (average WBC, 409 000/μL), marked splenomegaly (Figure 1), BM and spleen infiltration with maturing myeloid cells, and pulmonary hemorrhage, consistent with previous results.3,5,6,13 18 In contrast, 80% of animals treated with STI571 were alive at day 74. Among the mice showing evidence of hematologic response to STI571 treatment (termed responding), the average WBC was only 23 000/μL, compared with an average WBC of 409 000/μL in the placebo-treated animals (Table 1). There was also a marked decrease in splenomegaly in STI571-treated mice, with an average spleen weight of 290 mg in the responding animals, compared with 864 mg in the placebo-treated mice. In this cohort, only 2 STI571-treated animals died of the CML-like illness, 1 animal at day 36 with a WBC of 373 000/μL, and the other on day 46 with a WBC of 457 000/μL. Interestingly, even these animals demonstrated evidence of a decreased leukemia burden, with an average spleen weight at autopsy of 409 mg, more than 50% less than the placebo-treated mice (Table 1).
STI571 prolongs the survival of mice in a murine model of CML.
Lethally irradiated mice were reconstituted with syngeneic BM cells transduced with a replication-defective P210BCR/ABL retrovirus and were then randomized to posttransplantation treatment with either STI571 or placebo. The survival curve depicts the percentage of animals alive at the indicated time point after BM reconstitution (day 0). The number of mice in each arm (N) is also shown.
STI571 prolongs the survival of mice in a murine model of CML.
Lethally irradiated mice were reconstituted with syngeneic BM cells transduced with a replication-defective P210BCR/ABL retrovirus and were then randomized to posttransplantation treatment with either STI571 or placebo. The survival curve depicts the percentage of animals alive at the indicated time point after BM reconstitution (day 0). The number of mice in each arm (N) is also shown.
Mice with the chronic myelogenous leukemia–like myeloproliferative disorder treated with STI571 demonstrate a marked reduction in white blood count and spleen weight
| . | STI571-treated* . | Placebo . |
|---|---|---|
| WBC, average, ×103/μL | ||
| Responding† | 23 (± 12) | 409 (± 12) |
| All | 153 (± 84) | |
| Spleen weight, mg | ||
| Responding† | 290 (± 40) | 864 (± 92) |
| All | 320 (± 33) |
| . | STI571-treated* . | Placebo . |
|---|---|---|
| WBC, average, ×103/μL | ||
| Responding† | 23 (± 12) | 409 (± 12) |
| All | 153 (± 84) | |
| Spleen weight, mg | ||
| Responding† | 290 (± 40) | 864 (± 92) |
| All | 320 (± 33) |
Standard errors are depicted in parentheses.
The data depicted are from the mice in Figure 2 and are representative of at least 3 independent bone marrow transplantation experiments.
Mice experiencing a hematologic response to STI571, recorded at time of analysis.
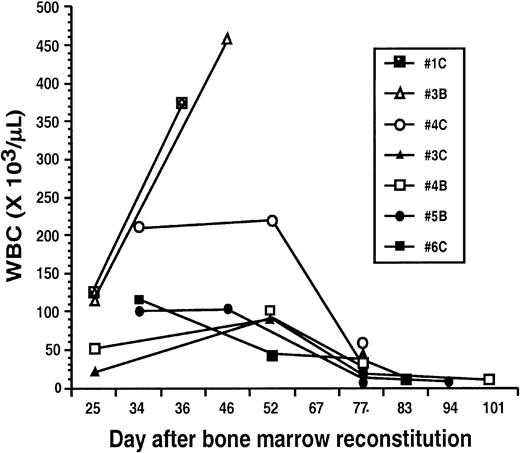
Mice treated with STI571 demonstrated considerable heterogeneity in their pattern of hematologic response
The prolonged survival experienced by the STI571-treated animals provided the opportunity to monitor periodic WBCs. There were considerable differences in the pattern of hematologic response exhibited by mice receiving STI571 treatment (Figure3). For example, 2 animals (nos. 3C, 4B) had WBCs ranging between 20 000 and 50 000/μL on day 25 after BM reconstitution, and by day 52 had demonstrated some elevation of their WBCs to the 71 000 to 92 000/μL range. However, both animals subsequently experienced a decrease in their WBC to 30 000/μL and 10 000/μL at days 77 and 101, respectively. Two animals had WBCs in the 100 000/μL range as late as day 34 (nos. 5B, 6C), but ultimately their WBC declined to 8000/μL and 14 000/μL by days 83 and 94, respectively. One animal (no. 4C) appeared to have disease quite resistant to STI571, with a WBC of 209 000/μL at day 34, splenomegaly, and moderate signs of systemic illness. Its clinical status remained largely unchanged at day 52 when the WBC was 220 000/μL; over the ensuing 3 weeks, however, splenomegaly decreased and improvement in the mouse's activity level was noted, in the setting of a decrease in WBC to 30 000/μL by day 77. Overall, 42% of evaluable mice achieved a WBC lower than 15 000/μL; 25% a WBC between 15 000 and 60 000/μL; and 8% a WBC between 60 000 and 100 000/μL; 25% of evaluable mice had primary refractory disease, with an average WBC of 386 000/μL at the time of death. Interestingly, even the mice that never exhibited evidence of hematologic response to STI571 (nos. 1C, 3B) showed prolonged survival compared with placebo-treated mice, with an average survival of 39 days.
Mice afflicted with the CML-like illness demonstrate a heterogeneous hematologic response to STI571.
Animals reconstituted with BM cells transduced with a replication-defective P210BCR/ABL retrovirus were started on treatment with STI571 beginning on day 10 after BM reconstitution. Serial peripheral blood counts were obtained at the indicated days. For comparison, blood counts from mice no. 1C and no. 3B, which failed to respond to STI571 and ultimately died from the CML-like illness, are shown at left.
Mice afflicted with the CML-like illness demonstrate a heterogeneous hematologic response to STI571.
Animals reconstituted with BM cells transduced with a replication-defective P210BCR/ABL retrovirus were started on treatment with STI571 beginning on day 10 after BM reconstitution. Serial peripheral blood counts were obtained at the indicated days. For comparison, blood counts from mice no. 1C and no. 3B, which failed to respond to STI571 and ultimately died from the CML-like illness, are shown at left.
None of the differences in rate of hematologic response was due to difficulty in drug administration to particular animals, or to any changes in the STI571 regimen. This heterogeneous pattern of hematologic response was observed in other STI571-treated mice in 3 independent retroviral transduction and BM transplantation experiments (data not shown). There was no obvious difference in outcome whether mice received the identical STI571 regimen beginning on either day 10 or 48 hours after BM transplantation (data not shown). In no case was the CML-like illness completely prevented by STI571 treatment, even when treatment was begun 48 hours after transplantation. None of the STI571-responding animals progressed while on STI571 during this study, so after completion of the survival studies, STI571-treated animals were electively killed for the purpose of further analysis. Therefore, despite these animals' receiving identical STI571 treatments, there were distinct patterns of clinical and hematologic response to STI571 in this mouse model of CML.
Hematopoietic cells from STI571-treated animals with murine CML demonstrate decreased tyrosine phosphorylation of Bcr/Abl and other proteins by immunoblot
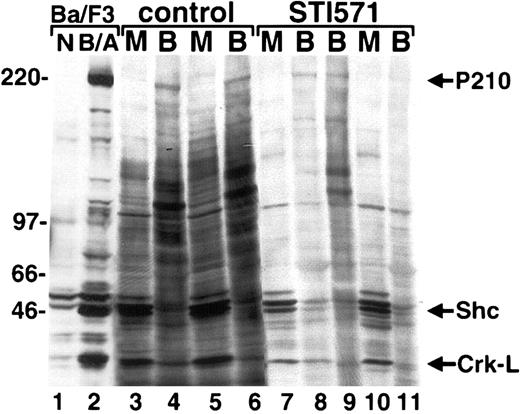
STI571, the product of rational drug design, is a 2-phenylaminopyrimidine–derivative compound that profoundly inhibits Abl and Bcr/Abl kinase activity16,19 and has been demonstrated to interfere with the tyrosine phosphorylation of Bcr/Abl and other proteins in Bcr/Abl cell lines20,21 and in mouse xenografts.17 Peripheral blood and BM protein lysates from STI571- and placebo-treated mice were analyzed by antiphosphotyrosine immunoblot to determine the effect of STI571 on the intensity and number of antiphosphotyrosine immunoreactive bands. Since neutrophils generally composed at least 95% of the peripheral blood in this mouse model of CML (Figure 1C-D, and data not shown), the peripheral blood of these animals was a quite pure source of Bcr/Abl-expressing malignant cells for analysis. Consistent with inhibition of the Bcr/Abl tyrosine kinase, cells from mice receiving treatment with STI571 (Figure4, lanes 7-11) generally demonstrated fewer tyrosine-phosphorylated bands than placebo-treated animals (lanes 3-6). There was also a tendency for a decrease in tyrosine phosphorylation of proteins that have been previously demonstrated to be targets of Bcr/Abl. Tyrosine phosphorylation of Shc22-24 was often decreased in STI571-treated animals, although in many cases it was relatively preserved compared with control animals (Figure 4, and data not shown). The adaptor molecule Crk-L is another prominent target of Bcr/Abl, and its tyrosine phosphorylation has been a useful marker of Bcr/Abl tyrosine kinase activity.25-28 As expected, a decrease in tyrosine phosphorylation of Crk-L was generally observed in STI571-treated animals, suggesting this molecule may provide a convenient biologic marker of STI571 response in Bcr/Abl–induced leukemias in vivo.29
STI571 alters the intensity of protein tyrosine phosphorylation in primary hematopoietic cells from mice with the CML-like illness.
Protein lysates from peripheral blood granulocytes (B) or BM (M) from the indicated placebo (control, lanes 3-6) and STI571-treated (lanes 7-11) mice were analyzed by antiphosphotyrosine immunoblot. A Ba/F3 cell line transduced with P210 (B/A) or retroviral vector alone (N) is shown at left as a control. The positions of P210BCR/ABL, Shc, and Crk-L are shown at far left.
STI571 alters the intensity of protein tyrosine phosphorylation in primary hematopoietic cells from mice with the CML-like illness.
Protein lysates from peripheral blood granulocytes (B) or BM (M) from the indicated placebo (control, lanes 3-6) and STI571-treated (lanes 7-11) mice were analyzed by antiphosphotyrosine immunoblot. A Ba/F3 cell line transduced with P210 (B/A) or retroviral vector alone (N) is shown at left as a control. The positions of P210BCR/ABL, Shc, and Crk-L are shown at far left.
Interestingly, a general decrease in protein tyrosine phosphorylation was not observed in all STI571-treated animals. Mice that never demonstrated significant evidence of a hematologic response to STI571 (Figure 4, lane 9; WBC, 337 000/μL; spleen, 790 mg) generally, but not invariably, exhibited a pattern and intensity of protein tyrosine phosphorylation similar to placebo-treated animals (Figure 4, lanes 3-6). Because of transautophosphorylation, tyrosine phosphorylation of Bcr/Abl may also serve as an approximate indicator of its kinase activity in vivo.30 Generally, STI571-treated mice demonstrated decreased Bcr/Abl tyrosine phosphorylation by immunoblot compared with control mice, but some animals demonstrated relatively preserved Bcr/Abl tyrosine phosphorylation (Figure 4, lane 8), suggesting the possibility of a compensatory response to STI571 in some animals, perhaps due to an alteration in Bcr/Abl expression.
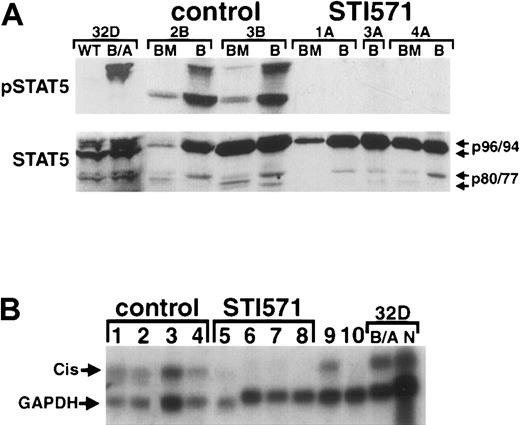
STI571-treated animals demonstrate a lack of constitutive STAT5 tyrosine phosphorylation and decreased expression of the STAT5-inducible gene cis
STAT5, constitutively activated in cells expressing Bcr/Abl,31-35 has recently been demonstrated to play an important role in Bcr/Abl-induced transformation36-38 and the CML-like myeloproliferative disorder.12 Previously, we have demonstrated that Bcr/Abl activates STATs in a Janus kinase–independent manner,32 suggesting that interference with the Bcr/Abl tyrosine kinase may have profound effects on constitutive STAT5 activation in vivo. To investigate this further, BM and peripheral blood protein lysates from STI571-treated and STI571-untreated mice with the CML-like illness were examined by phospho-specific immunoblot for evidence of constitutive STAT5 activation. As expected, placebo-treated animals demonstrated consistent evidence of constitutive STAT5 activation: both full-length (p96 and p94, respectively) and carboxy-terminal isoforms (p80 and p77, respectively) of STAT5A and STAT5B in peripheral blood cells, and predominantly p80 and p77, respectively, in BM cells (Figure5A). As a control, 32D cells transduced with P210BCR/ABL demonstrated constitutive activation of full-length STAT5A and STAT5B, but only faint p80/p77 activation (Figure 5A, left), consistent with previous results.32 In contrast to the untreated animals, there was no evidence of constitutive activation of any STAT5 isoform in mice treated with STI571, despite preserved STAT5 expression (Figure5A, right).
STI571 inhibits constitutive STAT5 activation andcis gene expression in primary hematopoietic cells from animals with the CML-like illness.
(A) Protein lysates from either peripheral blood granulocytes (B) or whole BM cells obtained from placebo-treated (2B, 3B) or STI571-treated (1A, 3A, 4A) mice were analyzed by immunoblot with an antibody recognizing activated STAT5 (pSTAT5, upper panel), or an antibody against the amino-terminal region of STAT5, which recognizes both full-length STAT5A/B and carboxy-terminal isoforms of STAT5 (lower panel). As a control, lysates from wild-type (WT) or P210BCR/ABL–expressing (B/A) 32D cells are shown at left. The positions of full-length STAT5A and STAT5B (p96 and p94, respectively) and the truncated forms of each (p80 and p77, respectively) are shown at the arrows. (B) Total RNA from spleen (lanes 2,4,7), blood (lanes 1,6), and BM (lanes 3,5,8) from either placebo-treated (control) or STI571-treated mice with the CML-like illness was analyzed by Northern blot by means of the indicated radiolabeled DNA probe. Location of the STAT5-responsive genecis15 and control glyceraldehyde-3-phosphate dehydrogenase are indicated at the arrows. Total BM RNA from secondary BM transplant recipients with the CML-like illness, treated (lane 10) and untreated (lane 9) with STI571, is shown at right. As a control, RNA from the myeloid cell line 32D expressing P210BCR/ABL (B/A) or retroviral vector alone (N) is shown at far right. Lanes 3, 4, and 6-8 represent samples from different tissues from the same animals.
STI571 inhibits constitutive STAT5 activation andcis gene expression in primary hematopoietic cells from animals with the CML-like illness.
(A) Protein lysates from either peripheral blood granulocytes (B) or whole BM cells obtained from placebo-treated (2B, 3B) or STI571-treated (1A, 3A, 4A) mice were analyzed by immunoblot with an antibody recognizing activated STAT5 (pSTAT5, upper panel), or an antibody against the amino-terminal region of STAT5, which recognizes both full-length STAT5A/B and carboxy-terminal isoforms of STAT5 (lower panel). As a control, lysates from wild-type (WT) or P210BCR/ABL–expressing (B/A) 32D cells are shown at left. The positions of full-length STAT5A and STAT5B (p96 and p94, respectively) and the truncated forms of each (p80 and p77, respectively) are shown at the arrows. (B) Total RNA from spleen (lanes 2,4,7), blood (lanes 1,6), and BM (lanes 3,5,8) from either placebo-treated (control) or STI571-treated mice with the CML-like illness was analyzed by Northern blot by means of the indicated radiolabeled DNA probe. Location of the STAT5-responsive genecis15 and control glyceraldehyde-3-phosphate dehydrogenase are indicated at the arrows. Total BM RNA from secondary BM transplant recipients with the CML-like illness, treated (lane 10) and untreated (lane 9) with STI571, is shown at right. As a control, RNA from the myeloid cell line 32D expressing P210BCR/ABL (B/A) or retroviral vector alone (N) is shown at far right. Lanes 3, 4, and 6-8 represent samples from different tissues from the same animals.
To determine whether the STI571-dependent decrease in constitutive STAT5 activation might also affect the induction of STAT5-inducible genes, total RNA was prepared from hematopoietic cells of STI571-treated and STI571-untreated animals with the CML-like myeloproliferative disorder. RNA from spleen, BM, and peripheral blood granulocytes was then analyzed by Northern blot by means of a radiolabeled fragment from cis, a STAT5-inducible gene.15 Consistent with the results of the pSTAT5 immunoblot analysis (Figure 5A), constitutive cis gene activation was detected in spleen (Figure 5B, lanes 2 and 4), peripheral blood granulocytes (lane 1), and BM (lane 3) from untreated mice with the CML-like disease. In contrast, hematopoietic cells from STI571-treated mice showed a marked reduction in constitutivecis gene expression (lanes 5-8). Secondary recipients of the CML-like myeloproliferative disorder from previously STI571-treated CML mice also showed constitutive cis gene expression (lane 9). Interestingly, BM cells from secondary CML animals “re-treated” with STI571 (lane 10) still showed a decrease in constitutivecis expression, to levels similar to those observed in drug-treated primary animals (lanes 5-8). Together, these results demonstrate that STAT5 is constitutively activated in hematopoietic cells from mice with the CML-like myeloproliferative disorder and that STI571 treatment is associated with a profound decrease in constitutive STAT5 tyrosine phosphorylation and cis gene expression.
The CML-like illness of STI571-treated animals is readily transplantable to secondary recipients, regardless of the response of the primary animal to STI571
Mice with the CML-like myeloproliferative disorder demonstrated a high rate of hematologic response to STI571, with approximately 75% of animals experiencing a reduction in WBC and 42% achieving a WBC below 15 000/μL. Given the excellent hematologic response of many mice to STI571, we were interested in whether this drug might cause sufficient reduction in Bcr/Abl–expressing cells to impair transplantation of the disease to syngeneic secondary recipients. To address this question, equal numbers of BM cells from STI571-treated animals experiencing either an excellent hematologic response (STI571 no. 4, responsive) or failing therapy (STI571 no. 6, nonresponsive) were used to reconstitute sublethally irradiated recipients (Table2). Primary animal STI571 no. 4 (Table 2, upper section) had experienced an excellent hematologic response to STI571, with a WBC of 10 000/μL and no signs of clinical illness at the time of BM harvest (day 101; 91 days of STI571 treatment). All 4 animals reconstituted with BM cells from animal STI571 no. 4 developed the CML-like myeloproliferative disorder, with an average latency of only 24 days. These mice had characteristic CML-like peripheral blood smears, marked splenomegaly, and striking granulocytosis, with 2 animals dying from leukemia with WBCs of 624 000/μL and 760 000/μL. One of the secondary animals with the CML-like illness was started on STI571 treatment at the onset of clinical leukemia symptoms, but died 5 days later from CML and hind-limb paralysis, the latter characteristic of the ALL-like illness sometimes observed in this animal model of Ph+ leukemia (data not shown).13
The CML-like illness is readily transplantable to secondary recipients, irrespective of the hematopoietic response of the bone marrow donor to STI571 at the time of transplantation
| Secondary recipient . | Treatment . | Outcome . | Spleen weight, mg . | WBC, per μL . |
|---|---|---|---|---|
| Secondary BMT donor: STI571 no. 4, | ||||
| STI571-responsive*,† | ||||
| No. 1 | None | Died d23 CML | ND | ND |
| No. 2 | None | Died d23 CML | 700 | 760 000 |
| No. 3 | None | Died d23 CML | 746 | 624 000 |
| No. 4 | STI571 begun d23 | Died d28 CML, HLP | ND | ND |
| (WBC = 176 000/μL) | ||||
| Secondary BMT donor: STI571 no. 6, STI571 nonresponsive*,‡ | ||||
| No. 1 | None | Died d22 CML | 1140 | ND |
| No. 2 | STI571 begun d22 | Died d31 CML, HLP | 577 | 112 000 |
| (WBC = 33 000/μL) | ||||
| No. 3 | None | Died d27 CML | ND | ND |
| No. 4 | None | NED d117 | ND | 6 000 (d29) |
| Secondary recipient . | Treatment . | Outcome . | Spleen weight, mg . | WBC, per μL . |
|---|---|---|---|---|
| Secondary BMT donor: STI571 no. 4, | ||||
| STI571-responsive*,† | ||||
| No. 1 | None | Died d23 CML | ND | ND |
| No. 2 | None | Died d23 CML | 700 | 760 000 |
| No. 3 | None | Died d23 CML | 746 | 624 000 |
| No. 4 | STI571 begun d23 | Died d28 CML, HLP | ND | ND |
| (WBC = 176 000/μL) | ||||
| Secondary BMT donor: STI571 no. 6, STI571 nonresponsive*,‡ | ||||
| No. 1 | None | Died d22 CML | 1140 | ND |
| No. 2 | STI571 begun d22 | Died d31 CML, HLP | 577 | 112 000 |
| (WBC = 33 000/μL) | ||||
| No. 3 | None | Died d27 CML | ND | ND |
| No. 4 | None | NED d117 | ND | 6 000 (d29) |
BMT indicates bone marrow transplant; ND, not done; HLP, hind-limb paralysis; NED, no clinical evidence of disease.
CML animals treated with STI571 were used as BMT donors for sublethally irradiated syngeneic recipients.
For this donor, WBC = 10 000/μL at time of BM harvest, which was 101 days after BM reconstitution and after 91 days of STI571 treatment.
For this donor, WBC = 329 000/μL at time of BM harvest, which was 34 days after BM reconstitution and after 24 days of STI571 treatment.
The primary animal STI571 no. 6 (Table 2, lower section) had failed STI571 treatment, with a WBC of 329 000/μL at day 34 (day 24 of therapy) when BM cells were harvested. Three of the 4 secondary BM transplant recipients from this animal developed the CML-like myeloproliferative disorder at an average of 27 days after reconstitution, with 1 of these animals also showing evidence of hind-limb paralysis. This last-mentioned animal was started on STI571 on day 22, with a WBC of 33 000/μL and no other clinical evidence of disease except mild splenomegaly, but still died of leukemia 9 days later. Interestingly, one of the secondary recipients from this animal has shown no evidence of leukemia, with a WBC of only 6000/μL at day 29 and no evidence of clinical illness at day 117. These results demonstrate that the CML-like illness of mice treated with STI571 is transplantable to secondary recipients with high efficiency (88%), regardless of whether the primary animal had failed STI571 or had an excellent response to the drug.
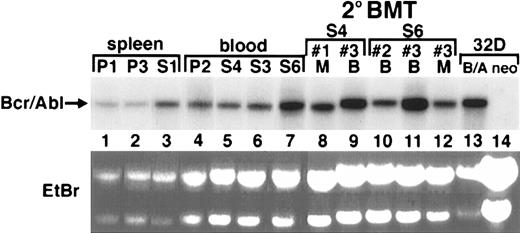
Some STI571-treated animals demonstrate increased Bcr/Abl mRNA and protein expression in peripheral blood, BM, and spleen
Recent studies have observed increased Bcr/Abl expression in Bcr/Abl-expressing cell lines subjected to STI571 for prolonged periods, suggesting the possibility that STI571 could be associated with a biologic selection for increased Bcr/Abl expression in some circumstances.21 39 To determine whether this phenomenon could also occur in vivo, the levels of Bcr/Abl mRNA and protein expression were examined in a group of STI571-treated and control mice. Total RNA was obtained from the peripheral blood, BM, and spleen of placebo- and STI571-treated animals and analyzed by Northern blot. In some cases, the amount of Bcr/Abl mRNA was similar in STI571-treated (S) and placebo-treated (P) animals (Figure6, P2 compared with S4 and S3), while in other cases there was a marked increase in Bcr/Abl mRNA expression in STI571-treated animals (S1 compared with P1 and P3; S6 compared with P2). In fact, the level of Bcr/Abl mRNA expression in the S6 animal rivaled that observed in a population of growth factor–independent 32D cells (a myeloid hematopoietic cell line) transduced with the same P210BCR/ABL retrovirus (Figure 6, right). Bcr/Abl mRNA expression was also readily detected in hematopoietic cells isolated from secondary recipients of STI571-treated mice, sometimes at levels higher than the original primary animals (lane 5 compared with lane 9; lane 7 compared with lane 11).
Bcr/Abl mRNA and protein expression is readily detected in hematopoietic tissues from mice treated with STI571 or placebo, and in recipients of secondary BM transplants from STI571-treated animals.
Total RNA was prepared from placebo-treated (P) or STI571-treated (S) animals and analyzed by Northern blot by means of a P210BCR/ABL–specific probe. At right are total RNA samples of blood (B) or BM (M) from secondary BM transplant recipients (indicated by number) from the donor animals STI571 no. 4 (lanes 8,9) or STI571 no. 6 (lanes 10-12). As a control, total RNA from the myeloid cell line 32D transduced with either P210 (B/A) or retroviral vector alone (neo) is shown at far right (lanes 13,14). Ethidium bromide (EtBr)–stained total RNA samples from spleen and peripheral blood of the secondary BM transplant recipients are shown (lower panel) to demonstrate equal RNA loading among the groups of samples. The position of P210BCR/ABL mRNA is shown at the arrow.
Bcr/Abl mRNA and protein expression is readily detected in hematopoietic tissues from mice treated with STI571 or placebo, and in recipients of secondary BM transplants from STI571-treated animals.
Total RNA was prepared from placebo-treated (P) or STI571-treated (S) animals and analyzed by Northern blot by means of a P210BCR/ABL–specific probe. At right are total RNA samples of blood (B) or BM (M) from secondary BM transplant recipients (indicated by number) from the donor animals STI571 no. 4 (lanes 8,9) or STI571 no. 6 (lanes 10-12). As a control, total RNA from the myeloid cell line 32D transduced with either P210 (B/A) or retroviral vector alone (neo) is shown at far right (lanes 13,14). Ethidium bromide (EtBr)–stained total RNA samples from spleen and peripheral blood of the secondary BM transplant recipients are shown (lower panel) to demonstrate equal RNA loading among the groups of samples. The position of P210BCR/ABL mRNA is shown at the arrow.
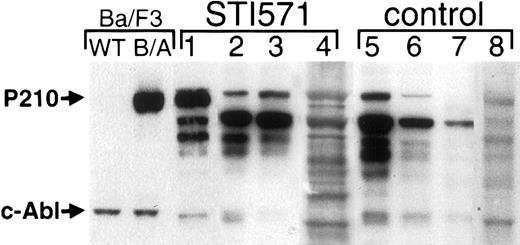
Peripheral blood and BM cells from STI571-treated and control animals were also analyzed for Bcr/Abl protein expression by immunoblot. Untreated mice exhibited a range of Bcr/Abl protein expression, often at the lower threshold of detection by Western blot (Figure7, lanes 6 and 7). As observed in the mRNA expression analysis (Figure 6), some STI571-treated animals expressed Bcr/Abl at levels similar to untreated animals, but there was a trend for Bcr/Abl protein to be more readily detectable in STI571-treated mice, and the highest levels were observed in STI571-treated mice (Figure 7, lane 1, and data not shown). For example, the BM cells shown in lane 1 of Figure 7 were isolated from a mouse with a WBC of only 9000/μL after 85 days of STI571 treatment. Despite this excellent hematologic response to STI571, BM Bcr/Abl protein expression was quite robust in this animal, rivaling that observed in a population of P210BCR/ABL growth factor–independent Ba/F3 cells (Figure 7, left). These results suggest that in murine hematopoietic cells there is a broad range of Bcr/Abl expression that may result in leukemogenesis and that STI571 treatment, in some cases, may contribute to a biologic selection yielding enhanced Bcr/Abl expression.
Hematopoietic cells from some STI571-treated mice express increased levels of Bcr/Abl protein.
Protein lysates from blood and BM cells from either STI571-treated (left, lanes 1-4) or STI571-untreated (right, lanes 5-8) mice were analyzed by anti-Abl immunoblot. As a control, lysates from wild type (WT) Ba/F3 or a population of growth factor–independent Ba/F3 cells expressing P210BCR/ABL (B/A) are shown at left. The position of Bcr/Abl and endogenous c-Abl are shown at the arrows.
Hematopoietic cells from some STI571-treated mice express increased levels of Bcr/Abl protein.
Protein lysates from blood and BM cells from either STI571-treated (left, lanes 1-4) or STI571-untreated (right, lanes 5-8) mice were analyzed by anti-Abl immunoblot. As a control, lysates from wild type (WT) Ba/F3 or a population of growth factor–independent Ba/F3 cells expressing P210BCR/ABL (B/A) are shown at left. The position of Bcr/Abl and endogenous c-Abl are shown at the arrows.
STI571 treatment is often associated with oligoclonal leukemia by Southern blot analysis of mice with the CML-like myeloproliferative disorder
With increased efficiency of retroviral transduction of murine BM cells,7 leukemia in the murine BM retroviral transduction and transplantation model of CML is polyclonal by Southern blot analysis.7,13 Because STI571 treatment resulted in increased expression of Bcr/Abl in some cases, it was conceivable that STI571 treatment suppressed or eliminated low-expressing hematopoietic clones. To address this possibility, genomic DNA from peripheral blood granulocytes, BM, or spleen from STI571-treated and STI571-untreated mice was analyzed for the number of unique BCR/ABL proviral integrations, each reflecting a distinct leukemic clone. DNA was digested with the restriction enzyme BglII, which cuts once in the proviral sequence recognized by the radiolabeled DNA probe (the neomycin cDNA) and again at the next BglII site encountered in murine genomic DNA. Therefore, each leukemic clone will generate a unique BglII fragment by Southern blot analysis.13 Consistent with previous results,13 animals not treated with STI571 (Figure 8, lanes 1-5) showed a range of 7 to 11 unique clones (average of 9 ± 0.7). In contrast, hematopoietic cells from STI571-treated animals (Figure 8, lanes 6-9) showed a marked reduction in the number of leukemic clones, with a range of 3 to 7 clones (average of 4.25 ± 0.9). Interestingly, the STI571-treated animal with the highest number of clones (lane 8) had received the drug for the shortest amount of time (24 days), dying from leukemia on day 34. Nonetheless, even this animal had fewer leukemic clones by Southern blot (7) than the average untreated mouse, with 2 clones predominating. In contrast, the other STI571-treated animals with the most striking oligoclonal leukemia (lanes 6, 7, and 9), consisting of only 3 or 4 leukemic clones, had been treated for an average of 83 days before analysis. None of these differences could be ascribed to differences in retroviral transduction efficiency since STI571-treated and STI571-untreated mice had received the same P210BCR/ABL-transduced BM cells. These results suggest that, in this murine model of CML, STI571 treatment may profoundly suppress, or even eradicate, certain Bcr/Abl leukemic clones over time, but that some clones persist, leading to an oligoclonal pattern of leukemia.
A trend for oligoclonal leukemia in STI571-treated mice.
Genomic DNA was prepared from peripheral blood granulocytes from either STI571-treated (right, lanes 6-9) or STI571-untreated (left, lanes 1-5) mice, digested with the restriction enzyme BglII, and then analyzed by Southern blot by means of a neomycin probe. There is only a single BglII site within the proviral sequence recognized by the neomycin probe, with the second BglII site located in adjacent genomic DNA. Therefore, each leukemic clone will have a unique BglII fragment recognized by the probe.13 As controls, an oligoclonal lymphoid tumor cell line established from a mouse with the Bcr/Abl–induced ALL-like illness (lane 10) and BM cells from a syngeneic healthy mouse (lane 11) are shown at right.
A trend for oligoclonal leukemia in STI571-treated mice.
Genomic DNA was prepared from peripheral blood granulocytes from either STI571-treated (right, lanes 6-9) or STI571-untreated (left, lanes 1-5) mice, digested with the restriction enzyme BglII, and then analyzed by Southern blot by means of a neomycin probe. There is only a single BglII site within the proviral sequence recognized by the neomycin probe, with the second BglII site located in adjacent genomic DNA. Therefore, each leukemic clone will have a unique BglII fragment recognized by the probe.13 As controls, an oligoclonal lymphoid tumor cell line established from a mouse with the Bcr/Abl–induced ALL-like illness (lane 10) and BM cells from a syngeneic healthy mouse (lane 11) are shown at right.
Discussion
Since its development a decade ago, the murine BM retroviral transduction and transplantation model of CML has proved a powerful research tool. Investigators have used this model to explore hematopoietic targets of BCR/ABL, evaluate the impact of Bcr/Abl mutants on leukemogenesis, and determine the role of discrete signaling pathways on the spectrum of Bcr/Abl-induced leukemia. This study demonstrates that this murine CML model is also a powerful tool for the investigation of novel therapeutic agents against Bcr/Abl-induced leukemia. One limitation of the traditional murine CML-like myeloproliferative disorder has been that all animals die within 3 to 4 weeks owing to a fulminant granulocytosis. Our findings demonstrate that the murine CML-like illness, which until now has been uniformly fatal, is reversible with STI571, a promising new addition to the therapeutic armamentarium against Ph+leukemias.29,40 41 Although no CML mice were cured with this drug, most STI571-treated animals exhibited clinical and hematologic features reminiscent of CML in chronic phase, with a marked reduction in splenomegaly and granulocytosis.
The durable response of most animals to STI571 afforded the opportunity to examine the impact of this drug on some known downstream signaling molecules of Bcr/Abl. Compared with cells from placebo-treated mice, hematologic cells isolated from STI571-treated animals demonstrated a general decrease in the number and intensity of tyrosine-phosphorylated proteins by immunoblot (Figure 4), probably owing to STI571-induced inhibition of Bcr/Abl tyrosine kinase activity.17,20,21Consistent with this hypothesis, there was also decreased constitutive tyrosine phosphorylation of Crk-L and Shc, well-established targets of Bcr/Abl,22-28 in leukemic cells from STI571-treated mice. Interestingly, Shc and Crk-L nonetheless remained among the most prominently tyrosine-phosphorylated molecules in STI571-treated P210-expressing hematopoietic cells (Figure 4, right), perhaps owing to preserved activation of other signaling pathways unaffected by Bcr/Abl. Constitutive activation of STAT5, a consistent feature of Bcr/Abl-expressing cell lines and primary cells from CML patients,31-35 was markedly reduced in STI571-treated blood granulocytes and BM cells compared with control P210 cells (Figure 5A). Moreover, compared with control animals, hematopoietic cells from STI571-treated CML mice also demonstrated a constitutive decrease in cis gene expression, a STAT5 target gene (Figure5B). STAT5 is important for the mouse CML-like myeloproliferative disorder12 and has been demonstrated to play a role in Bcr/Abl-dependent antiapoptotic signals.38,42 43 Although it is unknown whether STI571 induces apoptosis of Bcr/Abl hematopoietic target cells in vivo, these results suggest that the effects of STI571 on Bcr/Abl-induced STAT5 activation deserves further study in human STI571-treated Ph+ leukemias.
With increasing availability and duration of STI571 therapy in humans, the subject of STI571 resistance will probably become a subject of intense interest and study. Although the majority of mice in our study exhibited an excellent hematologic response to STI571 treatment, approximately 25% of mice never experienced a significant hematologic response to STI571 but had a prolonged survival compared with placebo-treated mice. Antiphosphotyrosine immunoblot analysis of hematopoietic cells from these STI571-nonresponsive mice usually showed a moderate increase in phosphotyrosine-containing proteins, intermediate between STI571-responsive and control animals (Figure 4, lane 9). These same animals also often had some of the highest levels of Bcr/Abl mRNA expression (Figure 6, S6). These results suggest the possibility that there may be a threshold level of Bcr/Abl expression that can be readily inhibited by a given dose of STI571 and that animals in our group that initially expressed the highest levels of Bcr/Abl were the most initially resistant to treatment. However, there were also exceptions to this paradigm, with some STI571-nonresponsive mice having antiphosphotyrosine immunoblot profiles similar to STI571-responsive animals and fairly average Bcr/Abl expression (data not shown). An alternative explanation for STI571 nonresponsiveness could be an altered pattern of intracellular signaling pathway activation; however, we found no consistent differences in extracellular-related kinase or Akt activation between STI571-responsive and STI571-nonresponsive mice by phospho-specific immunoblot (data not shown). These results suggest that the issue of STI571 resistance may be complex and possibly multifactorial and will best be addressed in the future by analyzing the large number of human subjects participating in STI571 clinical trials.
Because the incidence of STI571 failure was relatively low in our study, a relatively large number of mice were available for analysis after many weeks of STI571 treatment. These animals also provided some important insights into the biologic effects of prolonged STI571 treatment. For example, although relatively high Bcr/Abl expression was noted in some of the animals demonstrating primary resistance to STI571, animals on treatment for 60 to 90 days consistently had the most readily detectable Bcr/Abl protein expression by immunoblot (Figure 7 and data not shown). In fact, the highest Bcr/Abl protein expression observed in this study was from a mouse in this group with a WBC of only 14 000/μL and minimal splenomegaly, which expressed Bcr/Abl at levels similar to a cytokine-independent hematopoietic factor–dependent cell line. This observation suggested the possibility of an STI571-associated biologic selection for increased Bcr/Abl expression. Southern blot analysis of STI571-treated and STI571-untreated mice provided some indirect evidence to support this hypothesis. Consistent with previous results,13 blood and BM cells from untreated leukemic mice demonstrated multiple unique proviral integrations, generally in the range of 7 to 12 per animal (Figure 8), reflecting the contribution of multiple unique leukemic clones to the CML-like myeloproliferative disorder. In contrast, STI571-treated animals demonstrated a marked decrease in the number of leukemic clones by Southern blot, with several mice exhibiting 1 or 2 dominant clones, and 1 or 2 minor ones. These results suggest that STI571 treatment might have eradicated or severely suppressed certain leukemic clones, which may account for the heterogeneity of the hematologic response to STI571 in our study (Figure 3). An alternative explanation is that owing to the improved survival of STI571-treated mice, certain leukemic clones were allowed to naturally predominate over others, independent of a direct effect of STI571 treatment. Previous studies using retrovirally marked murine stem cells have shown that hematopoiesis can become oligoclonal several months after reconstitution.44 Although we cannot rule out such a possibility in our study, one STI571-treated mouse with a moderate response to STI571 (WBC, 39 000/μL at day 89) demonstrated polyclonal leukemia (data not shown), suggesting that prolonged survival alone did not always correlate with the development of oligoclonal murine CML.
The secondary BM transplantation experiments also provided some indirect evidence for an STI571-associated biologic selection for higher Bcr/Abl-expressing leukemic clones. The CML-like myeloproliferative disorder can be transplanted to secondary recipients in some cases (data not shown)7,13; however, the rapid, nearly 100% transplantability of the CML-like illness from primary CML animals treated with STI571 was striking. These results suggest that STI571 treatment might enrich the leukemic clone population for clones most competent for re-establishing the CML-like disease. Interestingly, one secondary transplant recipient that did not develop CML had received BM cells from an STI571-treated leukemic animal that had failed therapy and that had therefore received the drug for a relatively short time. Several mice with secondary CML from STI571-treated primary CML animals received STI571 therapy, representing the second time the leukemic cells had been treated with the compound. In 2 cases, the animals failed to respond to STI571, dying from the CML-like myeloproliferative disorder in the setting of hind-limb paralysis (Table 2). The latter is a manifestation of the ALL-like disease involving paraspinous lymph nodes that has been observed in this Bcr/Abl murine model.13 The ALL-like illness can coexist with the CML-like disease under conditions in which the CML-like illness has been crippled,9,11 12 raising the possibility that STI571 was still able to exert some antileukemic effect in these previously treated cells. In 2 other cases, however, STI571 therapy was successful in treating secondary CML arising from an STI571-treated primary CML animal, with 1 mouse achieving a normal WBC up to 135 days of STI571 therapy (data not shown). Therefore, in some cases, leukemic cells previously treated with STI571 were still sensitive to the drug during a subsequent course of therapy. Data from ongoing human clinical STI571 trials should provide important insights into the mechanisms of STI571 resistance and into whether STI571 will prove effective in repeated courses of therapy.
In conclusion, the murine BM retroviral transduction and transplantation model of CML is a powerful tool for the evaluation of novel therapeutic agents against Bcr/Abl-induced leukemias. Prolonged administration of therapeutic agents is feasible in this animal model and, in the case of STI571, has allowed us to extend the traditional murine CML model to a clinical disease more closely resembling human chronic phase CML. This murine chronic phase CML model may be a useful tool in the study of CML progression and in exploring the interplay between the immune system and Bcr/Abl-expressing hematopoietic cells. Perhaps some of our findings in STI571-treated CML mice will prove useful for investigators involved in human STI571 Bcr/Abl trials and will broaden our understanding of the signaling mechanisms underlying STI571 sensitivity and resistance in vivo.
We thank Huamei Xu and Shumin Zhang for technical assistance, Elisabeth Buchdunger and Shaoguang Li for helpful discussions, and Richard Gaynor for critical review of the manuscript.
Supported by National Institutes of Health grant RO1 CA61764 and the Leukemia Association of North Central Texas.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert L. Ilaria Jr, Simmons Cancer Center, UT Southwestern Medical Center, 5323 Harry Hines Blvd, MC 8593, Dallas, TX 75390-8593; e-mail: rilari@mednet.swmed.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal