Aminobisphosphonates, potent derivatives of bisphosphonates, are frequently used for the treatment of conditions such as osteoporosis and bone metastases that are characterized by excessive osteoclastic bone resorption. Using T-cell receptor (TCR) transfer studies, we show that recognition of antigenic aminobisphosphonates that are known to stimulate human γδ T cells in vitro and in vivo (potency: risedronate > alendronate > pamidronate) requires expression of the Vγ2Vδ2 TCR and is thus Vγ2Vδ2 TCR–dependent. Myeloma cells or monocytes pulsed with risedronate and then washed rendered these target cells sensitive to lysis by a Vγ2Vδ2 T-cell clone or cell line. These results suggest that Vγ2Vδ2 TCR–dependent recognition leading to direct cytolysis of aminobisphosphonate-sensitized osteoclast or tumor targets may be a mechanism whereby aminobisphosphonate treatment of cancers metastatic to bone decreases osteoclastic activity and tumor burden and also may account for the decreased osteoclastic activity associated with successful treatment of osteoporosis.
Introduction
Bisphosphonates are antiresorptive drugs effectively used to treat osteoporosis, multiple myeloma, and metastatic breast and prostate cancer, in which patients have excessive bone resorption, leading to pathologic fractures. Chemically, bisphosphonates are synthetic analogs of endogenous pyrophosphate.1,2 Their side-chain composition contributes to the relative potencies of bisphosphonates.3 One mechanism whereby these drugs inhibit bone resorption is by directly inhibiting osteoclast function.4 However, indirect mechanisms also may exist. Two recent studies have shown that aminobisphosphonates can expand the most abundant population of γδ T cells in the human peripheral blood, Vγ2Vδ2 T cells (alternate nomenclature, Vγ9Vδ2), in vivo and in vitro, raising the possibility that aminobisphosphonates may induce γδ T cells to mediate antitumor and antiresorptive activity.5 6 However, these studies did not address the role of the T-cell receptor (TCR) in aminobisphosphonate recognition and whether these drugs in soluble form activate γδ T cells to kill tumor targets subsequently or sensitize these targets to γδ T-cell–mediated lysis by binding to the target cell surface.
The structural similarities between aminobisphosphonates and defined alkylamine and prenyl pyrophosphate γδ T-cell antigens prompted us to more closely investigate γδ T-cell–mediated recognition of aminobisphosphonates. Using TCR transfer studies, we show here that reactivity to aminobisphosphonates requires expression of the Vγ2Vδ2 TCR and that tumor cells and monocytes can be sensitized for Vγ2Vδ2 T-cell–mediated lysis by pretreatment with risedronate, an aminobisphosphonate. These data suggest a new mechanism whereby treatment of multiple myeloma and osteoporosis with aminobisphosphonates may decrease tumor burden and bone resorption.
Study design
Derivation of γδ T-cell lines, γδ T-cell clones, and monocytes
The Vγ2Vδ2 T-cell lines and clone isoamyl 5.C7 were isolated from human peripheral blood mononuclear cells (PBMCs) as previously described.7 Fresh monocytes were derived from PBMCs obtained from healthy volunteers by Ficoll-Paque density centrifugation, followed by plastic adherence and harvesting with a rubber policeman.
TCR transfectants
DBS43, Vγ2Vδ2 and 27/3.62, Vγ1Vδ1 TCR transfectants, respectively, were made as described8 by transfecting TCR− J.RT3-T3.5 cells with TCRγ and TCRδ complementary DNAs.
Antigens and cell lines
Risedronate (Actonel) and etidronate (Didronel) were obtained from Procter and Gamble (Cincinnati, OH), alendronate (Fosamax) from Merck (West Point, PA), and pamidronate (Aredia) from Novartis (Basel, Switzerland). Myeloma cell line U266B1 was obtained from American Type Culture Collection (Rockville, MD).
Cytokine release assays
Stimulation of γδ T-cell lines or of the Vγ2Vδ2 TCR transfectant was performed as previously described.7 The SD of the triplicate determination was less then 10% of the mean. Interferon (IFN)-γ and tumor necrosis factor (TNF)-α were analyzed by enzyme-linked immunosorbent assay (Pharmingen, San Diego, CA) after 24 hours of antigenic stimulation.
Flow cytometry
Three-color flow cytometric analyses for intracellular cytokine were performed using Cytofix/Cytoperm Plus (with GolgiStop) (Pharmingen) to determine IFN-γ and TNF-α production of γδ T cells at the single-cell level. GolgiStop was added for the last 4 hours during antigenic stimulation to the γδ T-cell lines for 17 hours. Cells were stained for surface expression of TCRγδ by alexafluor (AF)-conjugated anti-γδ TCR (TCRδ1) and Cy-conjugated anti-CD3. Cells were fixed and permeabilized with Cytofix/Cytoperm solution and were stained with either anti–IFN-γ or anti–TNF-α or an isotype-matched monoclonal antibody (mAb) (Pharmingen) conjugated to phycoerythrin. Samples were analyzed in a FACScan flow cytometer using FlowJo software (Becton Dickinson, Palo Alto, CA).
Cytotoxicity assay
Cytotoxic activity was performed as described, using the classical 51Cr release assay.7
Results and discussion
Aminobisphosphonates induced IL-2 and IFN-γ secretion from aminobisphosphonate-derived γδ T-cell lines and γδ T-cell expansion in PBMCs
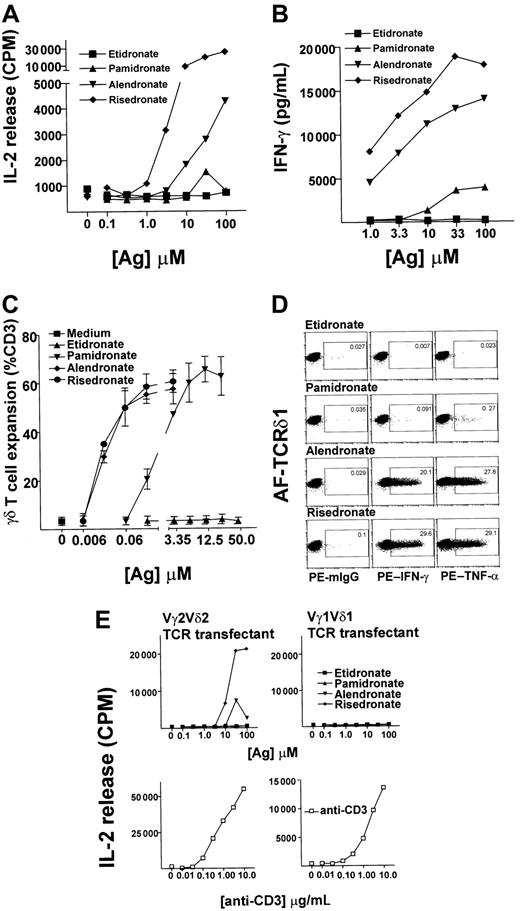
To examine the effect of antigen specificity on the Vγ2Vδ2 T-cell–mediated cytokine responses to bisphosphonates with different side chains, we performed cytokine release assays using a Vγ2Vδ2 T-cell line as an effector population and 3 aminobisphosphonates (risedronate, alendronate, pamidronate) or a bisphosphonate (etidronate) as antigens. Compared with pamidronate, 10- to 100-fold less alendronate and 100- to 1000-fold less risedronate was needed to achieve a half-maximal IL-2 (Figure 1A) or IFN-γ (Figure 1B) release from the γδ T-cell lines. There was no significant IL-2 or IFN-γ release in the absence of antigens or with etidronate stimulation, even with concentrations much higher than physiologic levels, suggesting that the amino group is necessary for antigenic activity. Fresh PBMCs exposed to these aminobisphosphonates showed a similar pattern of reactivity when γδ T-cell expansion was analyzed after incubation for 10 days, with γδ T cells expanding up to 12-fold in response to as little as 20 nM aminobisphosphonate (Figure 1C). Single-cell analysis of cytokine production by intracellular staining of IFN-γ and TNF-α, using 3-color flow cytometric analyses to gate on γδ T cells, confirmed that γδ T cells were the source of cytokine in these experiments (Figure1D).
(A,B) Aminobisphosphonate-derived γδ T cells respond to aminobisphosphonates but not to bisphosphonates.
Risedronate was used to derive T-cell lines consisting of 80% to 90% γδ T cells (coexpressing Vγ2 and Vδ2 TCR chains; data not shown). These T-cell lines were rested for 3 weeks after the first stimulation and then restimulated for 24 hours with increasing concentrations of antigen, and collected supernatants were tested for IL-2 secretion by HT-2 cell proliferation and for IFN-γ secretion by enzyme-linked immunosorbent assay. (C) Antigenic aminobisphosphonates risedronate, alendronate, and pamidronate stimulated Vγ2Vδ2 T-cell expansion. Dose response is shown of aminobisphosphonates and of etidronate in stimulation of γδ T-cell expansion from 2 random leukopack PBMC preparations, as analyzed by flow cytometry. (D) Single-cell analysis of IFN-γ and TNF-α production by γδ T cells. Single-cell cytokine analysis performed by intracellular staining of IFN-γ and TNF-α using 3-color flow cytometric analyses is shown. A representative risedronate-derived line was restimulated with 10 μM concentration of aminobisphosphonates or etidronate for 17 hours. About 20% of cells in this line were monocytes or non-γδ T-cell lymphocytes; none of these stained positive for intracellular cytokines (data not shown). The number in the small box indicates the percent of γδ T cells that produced cytokines. (E) IL-2 release by TCR transfectants in response to aminobisphosphonates or etidronate. Vγ2Vδ2 and Vγ1Vδ1 TCR transfectants were stimulated with increasing concentrations of aminobisphosphonates or etidronate (upper panel) and with anti-CD3 mAb (OKT3) (lower panel) for 24 hours, and IL-2 release was measured by HT-2 cell proliferation.
(A,B) Aminobisphosphonate-derived γδ T cells respond to aminobisphosphonates but not to bisphosphonates.
Risedronate was used to derive T-cell lines consisting of 80% to 90% γδ T cells (coexpressing Vγ2 and Vδ2 TCR chains; data not shown). These T-cell lines were rested for 3 weeks after the first stimulation and then restimulated for 24 hours with increasing concentrations of antigen, and collected supernatants were tested for IL-2 secretion by HT-2 cell proliferation and for IFN-γ secretion by enzyme-linked immunosorbent assay. (C) Antigenic aminobisphosphonates risedronate, alendronate, and pamidronate stimulated Vγ2Vδ2 T-cell expansion. Dose response is shown of aminobisphosphonates and of etidronate in stimulation of γδ T-cell expansion from 2 random leukopack PBMC preparations, as analyzed by flow cytometry. (D) Single-cell analysis of IFN-γ and TNF-α production by γδ T cells. Single-cell cytokine analysis performed by intracellular staining of IFN-γ and TNF-α using 3-color flow cytometric analyses is shown. A representative risedronate-derived line was restimulated with 10 μM concentration of aminobisphosphonates or etidronate for 17 hours. About 20% of cells in this line were monocytes or non-γδ T-cell lymphocytes; none of these stained positive for intracellular cytokines (data not shown). The number in the small box indicates the percent of γδ T cells that produced cytokines. (E) IL-2 release by TCR transfectants in response to aminobisphosphonates or etidronate. Vγ2Vδ2 and Vγ1Vδ1 TCR transfectants were stimulated with increasing concentrations of aminobisphosphonates or etidronate (upper panel) and with anti-CD3 mAb (OKT3) (lower panel) for 24 hours, and IL-2 release was measured by HT-2 cell proliferation.
The potent stimulatory activity of risedronate was somewhat surprising because compounds with ring structures that are not catabolized have previously been shown to be poorly stimulatory for γδ T cells.9,10 However, the antigenic activity of aminobisphosphonates in our study correlated with their antiresorptive potency (risedronate > alendronate > pamidronate) and with their ability to inhibit the activity of farnesyl pyrophosphate synthase, an enzyme that catalyzes a rate-limiting step in the mevalonate pathway of cholesterol synthesis.11 These data confirm and extend previously published data showing that the aminobisphosphonates alendronate, pamidronate, and ibandronate induce γδ T-cell expansion, while the bisphosphonates etidronate and clodronate do not.6
Recognition of amino bisphosphonates was Vγ2Vδ2 TCR–mediated
To examine if Vγ2Vδ2 T-cell recognition of aminobisphosphonates was mediated by their TCR, either Vγ2Vδ2 or Vγ1Vδ1 TCRs were reconstituted in a TCR-deficient Jurkat T-cell line by transfection with appropriate TCR γ and δ complementary DNAs, and the transfected cells were tested for their ability to release IL-2 in response to bisphosphonates. Both Vγ2Vδ2 TCR–reconstituted Jurkat cells and Vγ1Vδ1 TCR–reconstituted Jurkat cells produced IL-2 in response to anti-CD3 mAb in a dose-dependent manner, suggesting that the reconstituted TCRs could transduce signals in both transfectants. However, only Vγ2Vδ2+ and not the Vγ1Vδ1+ TCR transfectants produced IL-2 in response to risedronate and alendronate in a dose-dependent manner (Figure 1D). In a separate experiment, we observed a very low dose-dependent response by the Vγ2Vδ2+ TCR transfectant in response to pamidronate, suggesting that recognition of pamidronate is Vγ2Vδ2 TCR–dependent (data not shown). However, because these TCR transfectants are up to 100-fold less sensitive than native γδ T cells in IL-2 release assays, we believe that the response to pamidronate, the weakest aminobisphosphonate studied here, was below the detection level of the assay in most experiments. Taken together, these data show that recognition of aminobisphosphonates is Vγ2Vδ2 TCR–dependent.
Aminobisphosphonates sensitized tumor cells and fresh monocytes to lysis by Vγ2Vδ2 T cells
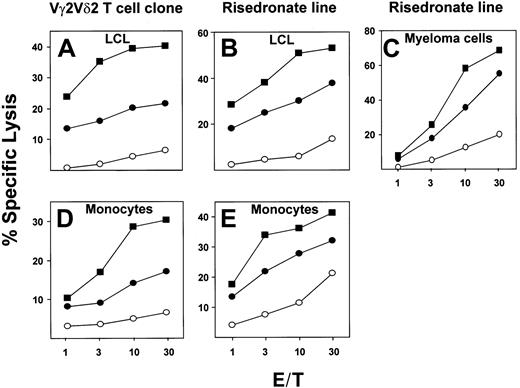
Using a Vγ2Vδ2 T-cell clone or a risedronate-derived Vγ2Vδ2 T-cell line as effectors, we examined the sensitivity to lysis of an Epstein-Barr virus (EBV)–transformed lymphoblastoid cell line (LCL), U266B1 myeloma cells, or fresh monocytes either pretreated with 10 μM risedronate, and then washed extensively, or in the continuous presence of 10 μM risedronate in a 4-hour cytotoxicity assay. When exposed to risedronate, target cells were lysed 3- to 30-fold more efficiently by either the Vγ2Vδ2 T-cell clone (isoamyl 5.C7) or γδ T-cell line (risedronate-derived) as compared with mock-treated targets. Surprisingly, pretreatment of the target cells with risedronate, followed by extensive washing, resulted in up to a 2-fold increase in sensitivity to lysis as compared with continuous antigen exposure (Figure 2A-E). No difference in toxicity or spontaneous release of label was observed. This result was unexpected because previous studies have shown that alkylamine and prenyl pyrophosphate antigens cannot be pulsed onto targets and must be present throughout the assay period to stimulate γδ T cells.9 12 The most likely explanation for this comparative decrease in cytolysis in the continuous presence of soluble antigen is that γδ T cells can kill each other under these conditions and that soluble antigen can compete with target cell–associated antigen, resulting in decreased target cell lysis (data not shown). These results suggest that risedronate, perhaps due to its high potency, in contrast to lower-potency alkylamine and prenyl pyrophosphate Vγ2Vδ2 T-cell antigens, can stably associate with target cell surfaces at levels high enough to render these targets extremely sensitive to lysis by Vγ2Vδ2 T cells.
Cytotoxic lysis of an EBV-transformed lymphoblastoid cell line (LCL), fresh peripheral blood monocytes, or myeloma cell lines by a Vγ2Vδ2 T-cell clone or a Vγ2Vδ2 T-cell line.
Lysis is shown of targets EBV-transformed LCLs (A,B) or fresh peripheral blood monocytes (D, E) or myeloma cell line U266B1 (C) that were either mock-treated (○), pretreated with 10 μM risedronate for 3 hours, and washed 3 times (▪) or were exposed to 10 μM risedronate during assays (●). Effectors were a Vγ2Vδ2 T-cell clone (A,D) or a Vγ2Vδ2 T-cell line (B,C,E).
Cytotoxic lysis of an EBV-transformed lymphoblastoid cell line (LCL), fresh peripheral blood monocytes, or myeloma cell lines by a Vγ2Vδ2 T-cell clone or a Vγ2Vδ2 T-cell line.
Lysis is shown of targets EBV-transformed LCLs (A,B) or fresh peripheral blood monocytes (D, E) or myeloma cell line U266B1 (C) that were either mock-treated (○), pretreated with 10 μM risedronate for 3 hours, and washed 3 times (▪) or were exposed to 10 μM risedronate during assays (●). Effectors were a Vγ2Vδ2 T-cell clone (A,D) or a Vγ2Vδ2 T-cell line (B,C,E).
Treatment with aminobisphosphonates of cancer metastatic to bone results in a decrease in metastatic events and pathological fractures.13 Aminobisphosphonates also are used widely for the treatment and prevention of osteoporosis. Aminobisphosphonates are preferentially bound to skeletal sites of bone resorption, resulting in concentrations in the millimolar range, much higher than the nanomolar concentrations found in blood.14 Our data showing that blood monocytes and myeloma cells can be sensitized to γδ T-cell–mediated lysis by pretreatment with micromolar concentrations of risedronate (Figure 2) suggest that osteoclasts and myeloma cells that resorb bone containing aminobisphosphonates could be subject to elimination by cytotoxic Vγ2Vδ2 T cells present in the bone marrow. Alternatively, these aminobisphosphonate-laden myeloma cells and osteoclasts may induce γδ T cells to secrete IFN-γ, a known inhibitor of tumor cell growth and bone resorption.15Thus, VγVδ2 T cells, via TCR-dependent recognition of tumor- and osteoclast-bound aminobisphosphonates, may be key mediators of the antitumor and antiosteoporotic effects associated with aminobisphosphonate treatment.
We thank Dr Michael B. Brenner for helpful discussions and Lin Li for technical assistance.
Supported by grants from the National Institutes of Health and the Arthritis Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jack F. Bukowski, Department of Medicine, Brigham and Women's Hospital, Smith Bldg, Rm 526D, One Jimmy Fund Way, Boston, MA 02115; e-mail: jbukowski@rics.bwh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal