Bcr-abl fusion peptide–specific CD4+ T-lymphocyte clones have recently been shown to augment colony formation by chronic myelogenous leukemia (CML) cells in a bcr-abl type-specific and HLA class II–restricted manner without addition of exogenous antigen. These findings suggest that CML cells can naturally process and present endogenous bcr-abl fusion protein to CD4+ T lymphocytes in the context of HLA class II molecules. To verify this possibility, the ability of CML-derived dendritic cells (DCs) to present endogenous bcr-abl fusion protein to bcr-abl fusion peptide–specific CD4+ T-lymphocyte clones was investigated. The bcr-abl b3a2 peptide–specific and HLA-DRB1*0901–restricted CD4+T-lymphocyte clones produced interferon-γ in response to stimulation with monocyte-derived DCs from HLA-DRB1*0901+ patients with b3a2 type CML. In contrast, DCs from patients with HLA-DRB1*0901− or b2a2 type CML and those from healthy individuals did not exert stimulatory activity on bcr-abl–specific CD4+ T-lymphocyte clones. The response of CD4+T-lymphocyte clones to CML-derived mature DCs was higher than that to immature DCs and was inhibited by anti–HLA-DR monoclonal antibody. These data suggest that CML-derived DCs can process and present endogenous bcr-abl fusion protein to CD4+ T lymphocytes.
Introduction
Chronic myelogenous leukemia (CML) is a hematologic malignancy characterized by an initial chronic phase of clonal hematopoiesis with continued differentiation into mature granulocytes.1 Most cases of CML develop to blast crisis, a terminal stage resembling acute leukemia. Because the prognosis of CML blast crisis is very poor, treatment of CML in the chronic phase is essential to achieve cure of this disease. Although treatment with interferon (IFN) is effective for CML, the rate of complete remission achieved by IFN therapy is not sufficient. At present, only bone marrow transplantation can cure this disease, although graft versus host disease and various opportunistic infections are serious complications in the posttransplantation stage. Recently, donor lymphocyte infusion has been found to be effective treatment in patients with CML who relapse after allogeneic bone marrow transplantation.2Although this finding suggests that CML cells are relatively sensitive to cell-mediated immunotherapy, donor lymphocyte infusion is frequently associated with severe graft versus host disease. Therefore, the development of novel cell-mediated immunotherapy for CML that induces graft versus leukemia without graft versus host disease is required. To develop effective immunotherapy for CML, identification of the CML-specific tumor antigens is required.
Translocation between chromosomes 9 and 22 resulting in formation of a chimeric protein of bcr and abl has been detected in more than 95% of cases of CML. One of the 2 major fusion types, b2a2 and b3a2, is produced in most cases of CML. Because bcr-abl chimeric protein is expressed only in CML cells but not in normal cells, the fusion sequence may act as a potential target for a T lymphocyte–mediated immune response to CML. Indeed, it has been possible to generate bcr-abl fusion peptide–specific CD4+ and CD8+T lymphocytes in vitro.3-15 It has been demonstrated that dendritic cells (DCs), which are professional antigen-presenting cells (APCs), are generated from CML cells after treatment with a combination of cytokines.16-18 The generation of cytotoxic T lymphocytes (CTLs) directed against CML cells has also been achieved by stimulation of CD8+ T lymphocytes with CML-derived DCs as APCs.19
A recent report by ten Bosch et al demonstrated that b3a2 peptide–specific and HLA-DRB1*0401–restricted CD4+T-lymphocyte cell lines showed a proliferative response to HLA-DRB1*0401–bearing b3a2+ CML blasts.4 On the other hand, these CD4+ T-lymphocyte cell lines did not respond to HLA-DRB1*0401–bearing b3a2− cells or HLA-DRB1*0401− b3a2 type CML blasts. In our recent study, b3a2 peptide–specific and HLA-DRB1*0901–restricted CD4+T-lymphocyte clones were established and their effect on CML cell growth was investigated.9 The numbers of HLA-DRB1*0901+ b3a2, but not those of b2a2+ or HLA-DRB1*0901−, CML cell colonies appeared to increase when CML cells were cultured with b3a2-specific CD4+T-lymphocyte clones. The effect of b3a2-specific CD4+T-lymphocyte clones on b3a2+ CML cell growth was inhibited by the addition of anti–HLA-DR monoclonal antibody (mAb). These data suggest that bcr-abl fusion protein is processed naturally in CML cells and is recognized by bcr-abl–specific CD4+ T lymphocytes in the context of HLA class II molecules. To verify this possibility, the ability of DCs derived from monocytes from patients with CML to present endogenous bcr-abl fusion protein to CD4+ T lymphocytes was investigated. The results showed that CML-derived mature DCs can process and present endogenous bcr-abl fusion protein to bcr-abl peptide–specific CD4+ T-lymphocyte clones in an HLA class II–restricted manner. The feasibility of cell-mediated immunotherapy of CML using DCs generated from CML cells is discussed on the basis of these results.
Materials and methods
Generation of bcr-abl fusion peptide–specific CD4+T-lymphocyte clones
The bcr-abl fusion peptide–specific and HLA-DR–restricted CD4+ T-lymphocyte clones, MY-1 and TO-1, were established as reported previously.9 Briefly, peripheral blood mononuclear cells (PBMCs) from healthy individuals were suspended in RPMI 1640 supplemented with 10% heat-inactivated human AB serum (referred to hereafter as culture medium) and 10 μg/mL synthetic bcr-abl b3a2 fusion peptide, ATGFKQSSKALQRPVAS, and the cells were then seeded into wells of round-bottom microtiter plates. After 7 days, half of the medium was exchanged for fresh culture medium and a second stimulation was performed by addition of autologous PBMCs treated with mitomycin C as APCs and peptide at a concentration of 10 μg/mL. After a further 7 days, a third stimulation was performed in the same way. Four days after the third stimulation with peptide, human recombinant interleukin-2 (IL-2) (Boehringer-Mannheim, Mannheim, Germany) was added to each well. The growing cells were then transferred to 16 mm–diameter wells, and their proliferative responses were examined. Bulk cells showing a proliferative response to stimulation with bcr-abl fusion peptide were cloned by the limiting dilution method as described previously.20 T-lymphocyte clones were cultured continuously in IL-2–containing culture medium, and mitomycin C–treated autologous PBMCs and bcr-abl fusion peptide were added to the wells every 2 weeks.
Generation of immature and mature DCs from CML cells
DCs derived from monocytes were generated as described previously.21 Briefly, monocyte-enriched PBMC fractions were isolated from patients with CML and healthy individuals using a plastic adherence technique. Informed consent was obtained from all individuals. The plastic-adherent cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 500 U/mL recombinant human IL-4 (Genzyme, Boston, MA), and 800 U/mL recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) (Kirin Brewery, Tokyo, Japan). On day 3 of incubation, half of the medium was exchanged for fresh culture medium supplemented with IL-4 and GM-CSF, and culture was continued. On day 5, half of the medium was exchanged for culture medium supplemented with IL-4 and GM-CSF or for culture medium supplemented with IL-4, GM-CSF, and 100 U/mL recombinant human tumor necrosis factor α (TNF-α) (Dainippon Pharmaceutical, Osaka, Japan). On day 8, the cells cultured with and without TNF-α were harvested and used as CML monocyte-derived mature and immature DCs, respectively. Adherent cells cultured for 8 days in RPMI 1640 supplemented with 10% FCS without exogenous cytokines were used as monocytes.
Flow cytometry of DCs
The surface phenotype of monocytes, immature DCs, and mature DCs generated from CML cells was examined by flow cytometry using the following mAbs: anti-CD1a mAb (Immunotech, Marseilles, France), anti-CD80 mAb (Immunotech), anti-CD83 mAb (Immunotech), anti-CD86 mAb (Immunotech), anti–HLA class I mAb (Pharmingen, San Diego, CA), and anti–HLA-DR mAb (Pharmingen).
Fluorescence in situ hybridization
The percentage of bcr-abl fusion gene–positive cells was determined by fluorescence in situ hybridization (FISH) using Vysis LSI bcr-abl translocation probe (Vysis, Downers Grove, IL) containing a bcr-specific probe labeled with SpectrumGreen and an abl-specific probe labeled with SpectrumOrange in accordance with the manufacturer's protocol. A total of 200 nuclei were counted for the reciprocal translocation between chromosomes 9q34 and 22q11.2.
IFN-γ production by T-lymphocyte clones in response to DCs
For the assays of IFN-γ production, 2 × 106clone cells and 5 × 105 DCs were suspended in 2 mL RPMI 1640 supplemented with 10% FCS and cultured in a 16 mm well. Before coculture, cell debris was removed from a suspension of DCs by Ficoll-Conray gradient centrifugation; more than 95% DCs were viable as determined by the trypan blue exclusion test. In some experiments, sodium azide–free anti–HLA class I mAb, w6/32 (American Type Culture Collection, Rockville, MD), or anti–HLA-DR mAb, L243 (American Type Culture Collection), was added to the culture medium at an optimal concentration (10 μg/mL). After 72 hours, the supernatants were collected and assayed for IFN-γ production by enzyme-linked immunosorbent assay (ELISA) (Endogen, Woburn, MA). For statistical analysis of IFN-γ production, one-way analysis of variance was used to test for overall differences among the groups, followed by the Bonferroni test to compare the separate group means.
Cytotoxicity assays
51Cr release assays were performed as described previously.22 Briefly, 1 × 104 51Cr (NaCrO4) (New England Nuclear, Boston, MA)-labeled target cells suspended in 0.1 mL RPMI 1640 supplemented with 10% FCS were seeded into round-bottom microtiter wells and incubated with or without synthetic peptide for 4 hours. Effector cells suspended in 0.1 mL assay medium were then added to each well. After 5 hours, 0.1 mL supernatant was collected from each well and the percentage specific 51Cr release was calculated as follows: (cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release) × 100. To examine the Ca++ dependency of the cytotoxicity, cytotoxicity assays were performed in the presence of ethyleneglycotetraacetic acid (EGTA) (Sigma, St Louis, MO) at various concentrations and 2 mM MgCl2. To evaluate the role of perforin in CTL-mediated cytotoxicity, effector T lymphocytes were pretreated with an inhibitor of vacuolar type H+-adenosine triphosphatase, concanamycin A (CMA) (Wako Pure Chemical Industries, Osaka, Japan), at various concentrations for 2 hours and then incubated with target cells in the presence of CMA.23
Dependency of peptide concentration on cytotoxicity and IFN-γ production
The b3a2 peptide concentration required to induce cytotoxicity and IFN-γ production by b3a2-specific CD4+ CTL clones was examined as follows. Mature DCs generated from autologous monocytes suspended in 1 mL RPMI 1640 medium supplemented with 10% FCS were incubated with or without b3a2 peptide at various concentrations for 4 hours. After extensive washing, peptide-pulsed and -unpulsed DCs were used as target cells and stimulator cells for cytotoxicity assays and IFN-γ production, respectively, as described above.
Results
IFN-γ production by bcr-abl peptide–specific CD4+T-lymphocyte clones in response to stimulation with synthetic peptides
Proliferation of the bcr-abl (b3a2) peptide–specific CD4+ T-lymphocyte clones, MY-1 and TO-1, in response to stimulation with the b3a2 synthetic peptide has been reported previously. The restriction element of the proliferative response was identified as HLA-DRB1*0901 in experiments using a panel of allogeneic cells and HLA-DRB1*0901–transfected murine L cells.9 Because detection of IFN-γ production was more sensitive than measuring 3H-thymidine uptake to determine CD4+ T-lymphocyte response to antigen stimulation (data not shown), IFN-γ production was measured to determine the T-lymphocyte response to DCs in the present study. As shown in Table1, MY-1 and TO-1 produced IFN-γ in the presence of autologous PBMCs as APCs when these cell lines were stimulated with the b3a2 peptide but not with b2a2 or its physiologic counterpart peptide, bcr b3b4 or abl 1A-a2. The production of IFN-γ by MY-1 and TO-1 was inhibited by adding anti–HLA-DR mAb to the culture medium but not by adding anti–HLA class I mAb. These data indicate that MY-1 and TO-1 produce IFN-γ in a b3a2-specific and HLA-DR (HLA-DRB1*0901)-restricted manner as in the proliferative response.
IFN-γ production by bcr-abl-specific CD4+T-lymphocyte clones in response to stimulation with synthetic peptides
| Clone . | Peptide stimulation . | mAb added . | IFN-γ production, pg/mL* . |
|---|---|---|---|
| MY-1 | None | None | < 10 |
| b3b4 | None | < 10 | |
| 1A-a2 | None | < 10 | |
| b2a2 | None | < 10 | |
| b3a2 | None | 1789 ± 158 | |
| Anti-HLA class I | 1808 ± 132 | ||
| Anti-HLA-DR | 298 ± 28 | ||
| TO-1 | None | None | < 10 |
| b3b4 | None | < 10 | |
| 1A-a2 | None | < 10 | |
| b2a2 | None | < 10 | |
| b3a2 | None | 1815 ± 170 | |
| Anti-HLA class I | 1636 ± 139 | ||
| Anti-HLA-DR | 193 ± 38 |
| Clone . | Peptide stimulation . | mAb added . | IFN-γ production, pg/mL* . |
|---|---|---|---|
| MY-1 | None | None | < 10 |
| b3b4 | None | < 10 | |
| 1A-a2 | None | < 10 | |
| b2a2 | None | < 10 | |
| b3a2 | None | 1789 ± 158 | |
| Anti-HLA class I | 1808 ± 132 | ||
| Anti-HLA-DR | 298 ± 28 | ||
| TO-1 | None | None | < 10 |
| b3b4 | None | < 10 | |
| 1A-a2 | None | < 10 | |
| b2a2 | None | < 10 | |
| b3a2 | None | 1815 ± 170 | |
| Anti-HLA class I | 1636 ± 139 | ||
| Anti-HLA-DR | 193 ± 38 |
The results shown represent the means ± SDs of triplicate experiments.
IFN-γ production by MY-1 and TO-1 in the presence of autologous APCs with and without various synthetic peptides and with and without anti-HLA mAb was determined by ELISA.
Flow cytometric analysis of CML-derived monocytes, immature DCs, and mature DCs
Monocytes isolated from PBMCs of patients with CML in the chronic phase were cultured without cytokine, with IL-4 and GM-CSF, or with IL-4, GM-CSF, and TNF-α. The cells generated were considered as monocytes, immature DCs, and mature DC, respectively. Flow cytometric analysis of CML-derived monocytes, immature DCs, and mature DCs is shown in Figure 1. Monocytes expressed HLA class I and HLA-DR at relatively high levels, whereas expression of DC-associated markers, CD1a, CD80, CD83, and CD86, was undetectable or very low. When monocytes were cultured with IL-4 and GM-CSF for 8 days, expression of CD1a and CD80 was induced and the expression level of HLA class I increased slightly, whereas expression of CD83 and CD86 was unchanged. On the other hand, mature DCs expressed DC-associated markers including CD1a, CD80, CD83, and CD86 at high levels. In addition, expression of HLA class I on mature DCs was higher than that on monocytes.
Flow cytometric analysis of CML-derived monocytes, immature DCs, and mature DCs.
The cell surface expression levels of CD1a, CD80, CD83, CD86, HLA class I, and HLA-DR on monocytes, immature DCs, and mature DCs derived from patients with CML were measured by flow cytometry.
Flow cytometric analysis of CML-derived monocytes, immature DCs, and mature DCs.
The cell surface expression levels of CD1a, CD80, CD83, CD86, HLA class I, and HLA-DR on monocytes, immature DCs, and mature DCs derived from patients with CML were measured by flow cytometry.
IFN-γ production by bcr-abl peptide–specific CD4+T-lymphocyte clones in response to CML-derived DCs
The ability of monocytes, immature DCs, and mature DCs generated from 2 HLA-DRB1*0901+ patients with b3a2 type CML to stimulate b3a2-specific CD4+ T-lymphocyte clones was investigated. Both patients had been treated with IFN-α, but no cytogenetic response was detected. As shown in Table2, IFN-γ was not produced by monocytes, immature DCs, or mature DCs alone. Little IFN-gamma was produced by MY-1 and TO-1 when these clones were cocultured with monocytes, and somewhat more IFN-gamma was produced by the clones when cocultured with immature DCs. But mature DCs exerted an apparent ability to stimulate bcr-abl peptide–specific CD4+T-lymphocyte clones to produce IFN-γ.
IFN-γ production by bcr-abl-specific CD4+T-lymphocyte clones in response to CML-derived monocytes, immature DCs, and mature DCs
| Clone . | Donor, patient no. . | APC . | IFN-γ production, pg/mL* . |
|---|---|---|---|
| None | b3a2 CML, no. 1 | Monocytes | < 10 |
| Immature DCs | < 10 | ||
| Mature DCs | < 10 | ||
| b3a2 CML, no. 2 | Monocytes | < 10 | |
| Immature DCs | < 10 | ||
| Mature DCs | < 10 | ||
| MY-1 | b3a2 CML, no. 1 | Monocytes | < 10 |
| Immature DCs | 183 ± 25 | ||
| Mature DCs | 638 ± 87 | ||
| b3a2 CML, no. 2 | Monocytes | 35 ± 14 | |
| Immature DCs | 256 ± 41 | ||
| Mature DCs | 706 ± 36 | ||
| TO-1 | b3a2 CML, no. 1 | Monocytes | 43 ± 4 |
| Immature DCs | 251 ± 20 | ||
| Mature DCs | 821 ± 121 | ||
| b3a2 CML, no. 2 | Monocytes | < 10 | |
| Immature DCs | 153 ± 16 | ||
| Mature DCs | 688 ± 88 |
| Clone . | Donor, patient no. . | APC . | IFN-γ production, pg/mL* . |
|---|---|---|---|
| None | b3a2 CML, no. 1 | Monocytes | < 10 |
| Immature DCs | < 10 | ||
| Mature DCs | < 10 | ||
| b3a2 CML, no. 2 | Monocytes | < 10 | |
| Immature DCs | < 10 | ||
| Mature DCs | < 10 | ||
| MY-1 | b3a2 CML, no. 1 | Monocytes | < 10 |
| Immature DCs | 183 ± 25 | ||
| Mature DCs | 638 ± 87 | ||
| b3a2 CML, no. 2 | Monocytes | 35 ± 14 | |
| Immature DCs | 256 ± 41 | ||
| Mature DCs | 706 ± 36 | ||
| TO-1 | b3a2 CML, no. 1 | Monocytes | 43 ± 4 |
| Immature DCs | 251 ± 20 | ||
| Mature DCs | 821 ± 121 | ||
| b3a2 CML, no. 2 | Monocytes | < 10 | |
| Immature DCs | 153 ± 16 | ||
| Mature DCs | 688 ± 88 |
IFN-γ production by MY-1 and TO-1 in the presence of monocytes, immature DCs, and mature DCs derived from HLA-DRB1*0901+patients with b3a2 type CML was determined by ELISA. IFN-γ production by monocytes, immature DCs, and mature DCs alone was also determined. The results shown represent the means ± SDs of triplicate experiments.
The values of IFN-γ production by MY-1 and TO-1 cocultured with monocytes, immature DCs, and mature DCs generated from patients with CML were significantly different from each other (P < .01).
Antigen specificity and HLA restriction of IFN-γ production by b3a2 peptide–specific CD4+ T-lymphocyte clones in response to CML-derived DCs
The antigen specificity and HLA restriction of IFN-γ production by b3a2 peptide–specific CD4+ T-lymphocyte clones in response to CML-derived mature DCs was then determined. Table3 shows IFN-γ production by MY-1 and TO-1 cocultured with mature DCs generated from autologous monocytes and monocytes of b2a2 or b3a2 type CML bearing various HLA-DRB1 types. FISH analysis demonstrated that formation of the fusion gene betweenbcr and abl was detected in more than 70% of DCs generated from all CML patients except CML patient no. 3, who was in cytogenetic remission achieved by IFN-α treatment. In DCs generated from CML patient no. 3, fewer than 3% of cells appeared to be positive for the bcr-abl fusion gene. Apparent production of IFN-γ by MY-1 and TO-1 was detected when these clones were cocultured with DCs generated from b3a2 type CML patients who were HLA-DRB1*0901+. On the other hand, the degree of IFN-γ production by MY-1 and TO-1, which were cocultured with DCs generated from the b3a2 type CML patient in cytogenetic remission, b2a2 type CML patients, or HLA-DRB1*0901− CML patients, was very low. Production of IFN-γ by MY-1 and TO-1 cocultured with b3a2 type and HLA-DRB1*0901+ DCs was inhibited by adding anti–HLA-DR but not anti–HLA class I mAb to the culture medium. During culture, most DCs remained alive as determined by microscopic observation and the trypan blue exclusion test. These data strongly suggest that endogenous bcr-abl fusion protein is processed naturally in mature DCs and is presented to bcr-abl peptide–specific CD4+ T lymphocytes in the context of HLA class II molecules.
Antigen specificity and HLA restriction of IFN-γ production by bcr-abl-specific CD4+ T-lymphocyte clones in response to CML-derived mature DCs
| Clone . | DC donor, patient no. . | HLA-DRB1 . | mAb added . | IFN-γ production, pg/mL3-150 . |
|---|---|---|---|---|
| MY-1 | Autologous | 3-1500901/1406 | None | < 10 |
| b3a2 CML, no. 1 | 3-1500901/1201 | None | 636 ± 85 | |
| Anti-HLA class I | 652 ± 68 | |||
| Anti-HLA-DR | 133 ± 18 | |||
| b3a2 CML, no. 2 | 3-1500901/1001 | None | 706 ± 103 | |
| Anti-HLA class I | 721 ± 58 | |||
| Anti-HLA-DR | 159 ± 21 | |||
| b3a2 CML, no. 33-151 | 3-1500901/1502 | None | 87 ± 10 | |
| b3a2 CML, no. 4 | 3-1500405/0803 | None | 52 ± 17 | |
| b3a2 CML, no. 5 | 3-1500803/1405 | None | 62 ± 6 | |
| b2a2 CML, no. 6 | 3-1500901/1302 | None | 51 ± 9 | |
| b2a2 CML, no. 7 | 3-1500901/1101 | None | < 10 | |
| b2a2 CML, no. 8 | 3-1500403/1502 | None | < 10 | |
| TO-1 | Autologous | 3-1500901/0405 | None | 36 ± 14 |
| b3a2 CML, no. 1 | 3-1500901/1201 | None | 821 ± 79 | |
| Anti-HLA class I | 801 ± 51 | |||
| Anti-HLA-DR | 175 ± 28 | |||
| b3a2 CML, no. 2 | 3-1500901/1001 | None | 688 ± 46 | |
| Anti-HLA class I | 672 ± 56 | |||
| Anti-HLA-DR | 128 ± 13 | |||
| b3a2 CML, no. 33-151 | 3-1500901/1502 | None | 66 ± 10 | |
| b3a2 CML, no. 4 | 3-1500405/0803 | None | 52 ± 4 | |
| b3a2 CML, no. 5 | 3-1500803/1405 | None | < 10 | |
| b2a2 CML, no. 6 | 3-1500901/1302 | None | < 10 | |
| b2a2 CML, no. 7 | 3-1500901/1101 | None | 43 ± 4 | |
| b2a2 CML, no. 8 | 3-1500403/1502 | None | < 10 |
| Clone . | DC donor, patient no. . | HLA-DRB1 . | mAb added . | IFN-γ production, pg/mL3-150 . |
|---|---|---|---|---|
| MY-1 | Autologous | 3-1500901/1406 | None | < 10 |
| b3a2 CML, no. 1 | 3-1500901/1201 | None | 636 ± 85 | |
| Anti-HLA class I | 652 ± 68 | |||
| Anti-HLA-DR | 133 ± 18 | |||
| b3a2 CML, no. 2 | 3-1500901/1001 | None | 706 ± 103 | |
| Anti-HLA class I | 721 ± 58 | |||
| Anti-HLA-DR | 159 ± 21 | |||
| b3a2 CML, no. 33-151 | 3-1500901/1502 | None | 87 ± 10 | |
| b3a2 CML, no. 4 | 3-1500405/0803 | None | 52 ± 17 | |
| b3a2 CML, no. 5 | 3-1500803/1405 | None | 62 ± 6 | |
| b2a2 CML, no. 6 | 3-1500901/1302 | None | 51 ± 9 | |
| b2a2 CML, no. 7 | 3-1500901/1101 | None | < 10 | |
| b2a2 CML, no. 8 | 3-1500403/1502 | None | < 10 | |
| TO-1 | Autologous | 3-1500901/0405 | None | 36 ± 14 |
| b3a2 CML, no. 1 | 3-1500901/1201 | None | 821 ± 79 | |
| Anti-HLA class I | 801 ± 51 | |||
| Anti-HLA-DR | 175 ± 28 | |||
| b3a2 CML, no. 2 | 3-1500901/1001 | None | 688 ± 46 | |
| Anti-HLA class I | 672 ± 56 | |||
| Anti-HLA-DR | 128 ± 13 | |||
| b3a2 CML, no. 33-151 | 3-1500901/1502 | None | 66 ± 10 | |
| b3a2 CML, no. 4 | 3-1500405/0803 | None | 52 ± 4 | |
| b3a2 CML, no. 5 | 3-1500803/1405 | None | < 10 | |
| b2a2 CML, no. 6 | 3-1500901/1302 | None | < 10 | |
| b2a2 CML, no. 7 | 3-1500901/1101 | None | 43 ± 4 | |
| b2a2 CML, no. 8 | 3-1500403/1502 | None | < 10 |
IFN-γ production by MY-1 and TO-1 in the presence of autologous mature DCs and mature DCs derived from various patients with CML was determined by ELISA. The results shown represent the means ± SDs of triplicate experiments.
CML patient no. 3 was in complete cytogenetic remission.
Lack of cytotoxicity mediated by bcr-abl peptide–specific CD4+ T-lymphocyte clones against CML-derived DCs
Both MY-1 and TO-1 have previously been shown to be cytotoxic to a b3a2 peptide–loaded autologous B-lymphoblastoid cell line in an HLA-DRB1*0901–restricted manner. On the basis of these findings, the question of whether these CD4+ CTL clones lyse CML-derived DCs was addressed. As shown in Table4, MY-1 and TO-1 did not exert cytotoxicity against b3a2 CML-derived HLA-DRB1*0901+ mature DCs, whereas these clones did lyse b3a2 peptide–loaded HLA-DRB1*0901+ DCs derived from CML cells, as well as peptide-loaded autologous DCs. The possibility that the non-DC population exerted inhibitory effect on CD4+ CTL-mediated cytotoxicity against peptide-unloaded DCs seems unlikely, because the purity of DCs generated from the CML patient used for the experiment shown in Table 4 was about 90% and the contaminating cells were normal lymphocytes.
Cytotoxicity of bcr-abl-specific CD4+T-lymphocyte clones
| Clone . | Target . | Peptide added . | % Specific lysis4-150 . | ||
|---|---|---|---|---|---|
| 10:1 . | 5:1 . | 2.5:1 . | |||
| MY-1 | Autologous DCs | None | 3.3 ± 0.2 | 2.1 ± 0.2 | 0.4 ± 0.1 |
| b3a2 | 74.1 ± 4.8 | 63.9 ± 5.8 | 58.3 ± 3.2 | ||
| b2a2 | 4.0 ± 0.5 | 3.0 ± 0.4 | 0.2 ± 0.2 | ||
| CML DCs | None | 0.4 ± 0.2 | 2.6 ± 0.1 | 0.3 ± 0.2 | |
| b3a2 | 69.4 ± 6.9 | 53.9 ± 3.5 | 49.2 ± 4.1 | ||
| b2a2 | 1.5 ± 0.3 | 2.2 ± 0.5 | 0.3 ± 0.1 | ||
| TO-1 | Autologous DCs | None | 0.4 ± 0 | 0 ± 0.1 | 0 ± 0.5 |
| b3a2 | 63.6 ± 3.0 | 55.2 ± 6.3 | 47.3 ± 4.6 | ||
| b2a2 | 0.3 ± 0 | 0 ± 0 | 0.5 ± 0.5 | ||
| CML DCs | None | 0.4 ± 0.2 | 0 ± 0 | 0 ± 0.3 | |
| b3a2 | 52.9 ± 4.5 | 45.3 ± 6.1 | 36.0 ± 2.6 | ||
| b2a2 | 0.6 ± 0.2 | 0.2 ± 0.6 | 0 ± 0 | ||
| Clone . | Target . | Peptide added . | % Specific lysis4-150 . | ||
|---|---|---|---|---|---|
| 10:1 . | 5:1 . | 2.5:1 . | |||
| MY-1 | Autologous DCs | None | 3.3 ± 0.2 | 2.1 ± 0.2 | 0.4 ± 0.1 |
| b3a2 | 74.1 ± 4.8 | 63.9 ± 5.8 | 58.3 ± 3.2 | ||
| b2a2 | 4.0 ± 0.5 | 3.0 ± 0.4 | 0.2 ± 0.2 | ||
| CML DCs | None | 0.4 ± 0.2 | 2.6 ± 0.1 | 0.3 ± 0.2 | |
| b3a2 | 69.4 ± 6.9 | 53.9 ± 3.5 | 49.2 ± 4.1 | ||
| b2a2 | 1.5 ± 0.3 | 2.2 ± 0.5 | 0.3 ± 0.1 | ||
| TO-1 | Autologous DCs | None | 0.4 ± 0 | 0 ± 0.1 | 0 ± 0.5 |
| b3a2 | 63.6 ± 3.0 | 55.2 ± 6.3 | 47.3 ± 4.6 | ||
| b2a2 | 0.3 ± 0 | 0 ± 0 | 0.5 ± 0.5 | ||
| CML DCs | None | 0.4 ± 0.2 | 0 ± 0 | 0 ± 0.3 | |
| b3a2 | 52.9 ± 4.5 | 45.3 ± 6.1 | 36.0 ± 2.6 | ||
| b2a2 | 0.6 ± 0.2 | 0.2 ± 0.6 | 0 ± 0 | ||
The results shown represent the means ± SDs of triplicate experiments.
Cytotoxicity of MY-1 and TO-1 against autologous mature DCs and HLA-DRB1*0901-bearing mature DCs derived from the patients with b3a2 type CML was determined by 4-hour 51Cr release assays at effector:target ratios of 10:1, 5:1, and 2.5:1.
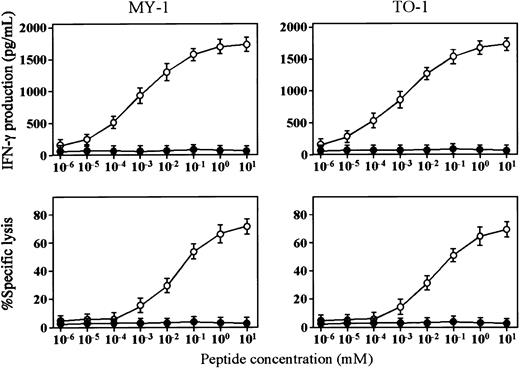
Peptide concentration-dependent IFN-γ production and cytotoxicity mediated by bcr-abl peptide–specific CD4+T-lymphocyte clones
The finding that bcr-abl peptide–specific CD4+CTL clones produced IFN-γ in response to stimulation with CML-derived DCs but did not lyse DCs led us to examine the difference between the optimal concentrations of bcr-abl peptide for IFN-γ production and cytotoxicity. As shown in Figure 2, the minimal concentrations of bcr-abl peptide required for IFN-γ production and cytotoxicity mediated by MY-1 and TO-1 were 10 pM/L and 1 nM/L, respectively. These data suggest that the minimum number of HLA-peptide complexes needed for production of IFN-γ by CD4+ CTLs is lower than that for CD4+CTL-mediated cytotoxicity and that this difference may be one of the reasons for the evidence that bcr-abl peptide–specific CD4+ CTL clones produced IFN-γ in response to CML-derived DCs but did not lyse them.
Peptide concentration-dependent cytotoxicity and IFN-γ production of bcr-abl–specific CD4+ T-lymphocyte clones.
(A) Cytotoxicity of MY-1 and TO-1 against autologous (○) and HLA-DRB1*0901− allogeneic (●) mature DCs preincubated with various concentrations of b3a2 peptide for 4 hours was determined by 51Cr release assays. (B) IFN-γ production by MY-1 and TO-1 stimulated with autologous (○) and HLA-DRB1*0901−allogeneic (●) mature DCs preincubated with various concentrations of b3a2 peptide for 4 hours was determined by ELISA.
Peptide concentration-dependent cytotoxicity and IFN-γ production of bcr-abl–specific CD4+ T-lymphocyte clones.
(A) Cytotoxicity of MY-1 and TO-1 against autologous (○) and HLA-DRB1*0901− allogeneic (●) mature DCs preincubated with various concentrations of b3a2 peptide for 4 hours was determined by 51Cr release assays. (B) IFN-γ production by MY-1 and TO-1 stimulated with autologous (○) and HLA-DRB1*0901−allogeneic (●) mature DCs preincubated with various concentrations of b3a2 peptide for 4 hours was determined by ELISA.
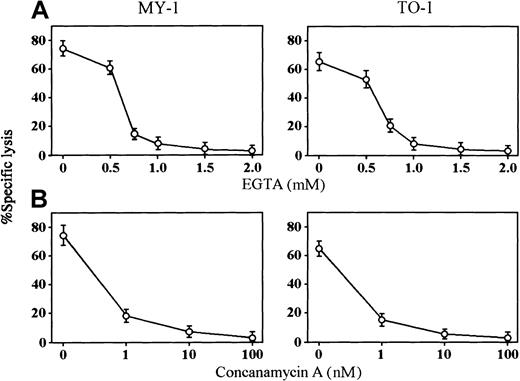
Perforin-mediated cytotoxicity of bcr-abl peptide–specific CD4+ T-lymphocyte clones
The cytolytic mechanisms of MY-1 and TO-1 against peptide-loaded DCs were also investigated. Two major pathways (granule exocytosis, which involves the secretion of perforin and granzymes, and the Fas/Fas ligand system), have been shown to be responsible for CTL-mediated cytotoxicity. Among them, the perforin/granzyme pathway is known to be Ca++ dependent. On the basis of this finding, the cytotoxic activity of bcr-abl peptide–specific CD4+CTL clones was determined in the absence of extracellular Ca++. No cytotoxicity against peptide-loaded DCs was observed in the presence of the Ca++-chelating agent EGTA at concentrations of more than 1 mM (Figure3A), indicating that bcr-abl peptide–specific CD4+ CTL-mediated cytotoxicity against DCs is completely Ca++ dependent.
Perforin-dependent cytotoxicity mediated by bcr-abl–specific CD4+ T-lymphocyte clones.
(A) Effect of EGTA on cytotoxicity mediated by MY-1 and TO-1 against b3a2 peptide–loaded autologous mature DCs was determined at an effector:target cell ratio of 5:1. (B) Effect of CMA on cytotoxicity mediated by MY-1 and TO-1 against b3a2 peptide–loaded autologous mature DCs was determined at an effector:target cell ratio of 5:1.
Perforin-dependent cytotoxicity mediated by bcr-abl–specific CD4+ T-lymphocyte clones.
(A) Effect of EGTA on cytotoxicity mediated by MY-1 and TO-1 against b3a2 peptide–loaded autologous mature DCs was determined at an effector:target cell ratio of 5:1. (B) Effect of CMA on cytotoxicity mediated by MY-1 and TO-1 against b3a2 peptide–loaded autologous mature DCs was determined at an effector:target cell ratio of 5:1.
It has been reported that treatment of CTLs with CMA results in the complete inhibition of perforin-based cytotoxic activity.23 In light of this finding, the significance of granule exocytosis in bcr-abl peptide–specific CD4+CTL-mediated cytotoxicity was examined using CMA. As shown in Figure3B, the cytotoxicity of MY-1 and TO-1 against peptide-loaded DCs was almost completely inhibited by pretreatment of CTL clones with CMA at concentrations of more than 10 nM/L, as has been reported for perforin-dependent CD8+ CTL-mediated cytotoxicity. Taken together with previous results using reverse transcriptase–polymerase chain reaction, which demonstrated expression of perforin and granzyme B messenger RNAs in MY-1 and TO-1,9 these data strongly suggest that granule exocytosis is the main pathway of cytotoxicity mediated by bcr-abl peptide–specific CD4+ CTLs.
Discussion
In the present study, the ability of DCs generated from monocytes of patients with CML to present endogenous bcr-abl fusion protein to bcr-abl peptide–specific CD4+ T-lymphocyte clones was investigated. The findings obtained from the present series of experiments are as follows. First, mature DCs derived from monocytes of patients with CML expressed DC-associated cell surface antigens, including CD1a, CD80, CD83, and CD86, at high levels compared with monocytes and immature DCs, in the same manner as DCs from healthy individuals.24 25 Second, the b3a2-specific and HLA-DRB1*0901–restricted CD4+ T-lymphocyte clones produced IFN-γ in response to stimulation with mature DCs derived from CML cells in a b3a2-specific and HLA-DRB1*0901–restricted manner. Third, although b3a2-specific CD4+ T-lymphocyte clones exerted perforin-dependent cytotoxicity against b3a2 peptide–loaded DCs, they did not lyse HLA-DRB1*0901+ b3a2 type DCs without exogenous antigen. These data suggest that mature DCs derived from CML cells can naturally process endogenous bcr-abl protein and present it to CD4+ T lymphocytes in the context of HLA class II molecules. Our present data also suggest that the threshold levels of HLA class II–peptide complex expression for cytokine production and cytotoxicity mediated by CD4+ T lymphocytes are different.
DCs can develop from peripheral blood monocytes stimulated with GM-CSF and IL-4.26,27 Under these culture conditions, monocytes develop into cells with characteristics of immature DCs. Immature DCs have a high level of endocytic activity but low T lymphocyte stimulatory capacity.28 Immature DCs also have relatively low levels of surface CD80 and CD86, which are important costimulatory molecules for T-lymphocyte activation, compared with mature DCs, and are characterized by the absence of CD83 expression. Addition of TNF-α to immature DCs induces biological change resulting in the loss of endocytic activity and up-regulation of adhesion and costimulatory molecules, including CD80, CD83, and CD86.28 In the present study, monocytes from patients with CML in the chronic phase appeared to develop into immature and mature DCs following treatment with GM-CSF and IL-4 and with GM-CSF, IL-4, and TNF-α, respectively, in the same manner as monocytes from healthy individuals. FISH analysis demonstrated clearly that most DCs generated in the present study originated from CML cells. The stimulatory activity of mature DCs derived from CML monocytes to bcr-abl–specific CD4+T-lymphocyte clones appeared to be higher than that of immature DCs. This might be due to the augmented expression of costimulatory molecules, such as CD80, CD83, and CD86, in mature DCs. It is also possible that synthesis of bcr-abl fusion protein increases in maturing DCs and that the capacity for processing and presenting endogenous bcr-abl protein to HLA class II molecules increases in mature DCs compared with monocytes and immature DCs. The mechanisms of the different endogenous antigen processing and presentation between immature and mature DCs should be clarified in a further study.
It is noteworthy from the present study that mature DCs derived from monocytes of patients with CML can naturally process and present endogenous bcr-abl fusion protein to CD4+ T lymphocytes in the context of HLA class II molecules. The possibility that DCs captured exogenous bcr-abl fusion protein that had been released from dead CML cells seems unlikely because most DCs were alive during culture and bcr-abl–specific CD4+ T-lymphocyte clones did not respond to the culture supernatant of DCs derived from CML cells (data not shown). In general, endogenous antigens are degraded in the cytoplasm into oligopeptides, a fraction of which is transported into the endocytoplasmic reticulum by the transporter associated with antigen presentation. In the endoplasmic reticulum, these peptides bind to newly synthesized MHC class I molecules and the resulting complexes are transported to the cell surface where they are recognized by CD8+ T lymphocytes. On the other hand, exogenous antigens are processed into peptides capable of binding to MHC class II molecules in an endocytic compartment and are presented to CD4+ T lymphocytes. However, it has recently been shown that the MHC class II pathway can process and present endogenous antigens as well as exogenous antigens.29-31 For example, virus-infected cells are recognized by CD4+ T lymphocytes in a viral antigen-specific and MHC class II–restricted manner.32-36 It has also been reported that tumor cells transfected with syngeneic MHC class II genes could present endogenously synthesized tumor-associated protein-derived peptides in the context of MHC class II molecules to CD4+ T lymphocytes.37,38 Colony formation of CML cells has been shown to be augmented by b3a2 peptide–specific CD4+T-lymphocyte clones in a b3a2-specific and HLA-DR–restricted manner.9 ten Bosch and colleagues have reported that bcr-abl (b3a2) peptide–specific CD4+ T lymphocytes could recognize b3a2 fusion protein expressing blasts from a patient with CML blast crisis in an HLA-DRB1*0401–restricted manner.4These authors also reported that CD4+ T-lymphocyte clones specific for the bcr-abl (b2a2) fusion peptide responded to an autologous B-lymphoblastoid cell line transfected with invariant chain complementary DNA in which the HLA class II–associated invariant chain peptide was replaced by a b2a2 fusion oligonucleotide sequence.39 Taken together with previous data, our present findings strongly suggest that DCs generated from monocytes from patients with CML can process and present endogenously synthesized bcr-abl fusion protein to CD4+ T lymphocytes.
Although HLA-DRB1*0901–restriced CD4+ T-lymphocyte clones specific for b3a2 peptide, MY-1 and TO-1, lysed b3a2 peptide–loaded autologous DCs and HLA class II–matched allogeneic DCs generated from CML cells, peptide-unloaded DCs derived from HLA-DRB1*0901+patients with b3a2 type CML were not lysed by these CTL clones. Because cytotoxicity mediated by MY-1 and TO-1 appeared to be Ca++dependent and was inhibited by CMA, an inhibitor of perforin-dependent cytotoxicity, the granule exocytosis mechanism seemed to be the main pathway of cytotoxicity mediated by MY-1 and TO-1. In a murine system using various mutant and knockout mice, it has been demonstrated that granule exocytosis is dominant in CD8+ CTL-mediated cytotoxicity, whereas the main pathway of cytotoxicity mediated by CD4+ CTLs is the Fas/Fas ligand system.40-43In contrast, we have clearly demonstrated that human CD4+CTLs specific for herpes simplex virus as well as allogeneic antigens exerted cytotoxic activity via the perforin-dependent pathway.44 45 Taken together, granule exocytosis seems to be an important pathway for CD4+ CTL-mediated cytotoxicity to CML cells.
IFN-γ production and cytotoxicity of the CD4+ CTL clones, MY-1 and TO-1, to DCs are mediated undoubtedly by recognition of bcr-abl fusion peptide in the context of HLA-DRB1*0901 molecules via T-cell receptor/CD3 complexes, because both responses appeared to be b3a2-specific and restricted by HLA-DRB1*0901. MY-1 and TO-1 produced IFN-γ but did not exert cytotoxicity in response to stimulation with HLA-DR–matched DCs generated from b3a2 type CML cells. The present study suggests that the minimum number of b3a2 peptide/HLA-DR complexes required for mediating cytotoxicity is higher than that for IFN-γ production and that the threshold for intracellular signaling via T-cell receptor/CD3 complexes for perforin-dependent cytotoxicity mediated by CD4+ CTLs is higher than that for IFN-γ production. It has been reported that leukemia cells are resistant to perforin-mediated cytotoxicity, possibly due to the reduced binding of perforin to the surface of leukemia cells.46 According to this finding, the reduced binding of perforin to the surface of CML cells might also be one of the causes of resistance of DCs generated from CML cells to perforin-dependent cytotoxity mediated by CD4+ CTLs.
The present data increase our understanding of the role of CD4+ T lymphocytes and DCs in the anti-CML immune response. Our previous report showing that bcr-abl peptide–specific CD4+ T lymphocytes augmented colony formation by CML cells9 suggests that stimulation of bcr-abl–specific CD4+ T lymphocytes may exacerbate the disease. On the other hand, the critical role of CD4+ T lymphocytes in induction of antitumor immunity has been demonstrated in human in vitro as well as murine in vivo and in vitro systems.47,48CD4+ T lymphocytes activated by antigen stimulation provide a helper function for induction of CD8+ CTLs through the release of cytokines. In addition, interactions between CD40 ligand and CD40 on the CD4+ T lymphocytes and DCs, respectively, appear critical in activating the DCs to present antigens to and costimulate the priming of CD8+ CTL precursors.49 Taken together, DCs generated from CML cells, which can present endogenous bcr-abl fusion protein to both CD4+ and CD8+ T lymphocytes in the context of HLA class II and class I, respectively, seem to play a crucial role in generating an anti-CML immune response. Accordingly, immunotherapy using DCs derived from CML cells might be an effective treatment for CML.
We thank Drs Junko Torii and Eiji Sada for their help with statistical analyses.
Supported by grants from the Ministry of Education, Science, Sports and Culture of Japan; the Naito Foundation; and the Sagawa Cancer Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masaki Yasukawa, the First Department of Internal Medicine, Ehime University School of Medicine, Shigenobu, Ehime 791-0295, Japan; e-mail: yasukawa@m.ehime-u.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal