Transcription factors of the nuclear factor of activated T cells (NFAT) family are thought to regulate the expression of a variety of inducible genes such as interleukin-2 (IL-2), IL-4, and tumor necrosis factor-α. However, it remains unresolved whether NFAT proteins play a role in regulating transcription of the interferon- γ (IFN-γ) gene. Here it is shown that the transcription factor NFAT1 (NFATc2) is a major regulator of IFN-γ production in vivo. Compared with T cells expressing NFAT1, T cells lacking NFAT1 display a substantial IL-4–independent defect in expression of IFN-γ mRNA and protein. Reduced IFN-γ production by NFAT1−/−× IL-4−/− T cells is observed after primary in vitro stimulation of naive CD4+ T cells, is conserved through at least 2 rounds of T-helper cell differentiation, and occurs by a cell-intrinsic mechanism that does not depend on overexpression of the Th2-specific factors GATA-3 and c-Maf. Concomitantly, NFAT1−/−× IL-4−/− mice show increased susceptibility to infection with the intracellular parasiteLeishmania major. Moreover, IFN-γ production in a murine T-cell clone is sensitive to the selective peptide inhibitor of NFAT, VIVIT. These results suggest that IFN-γ production by T cells is regulated by NFAT1, most likely at the level of gene transcription.
Introduction
In response to antigen stimulation, cells of the immune system initiate the expression of activation-induced genes through the coordinate action of transcription factors at gene regulatory elements. The nuclear factor of activated T cells (NFAT), originally identified as a nuclear complex binding to the antigen-response element of the IL-2 gene,1 has been implicated in the regulation of various inducible genes, particularly those encoding cytokines and cell surface receptors.2Sequence inspection, in vitro binding assays, and overexpression studies have identified potential binding sites for NFAT in the promoters and enhancers of numerous genes, including those encoding interleukin-2 (IL-2), IL-3, IL-4, IL-5, tumor necrosis factor α (TNF-α), granulocyte macrophage–colony-stimulating factor (GM-CSF), CD40L, FasL, CD25, b-type natriuretic peptide, and the adipocyte-specific protein aP2.2-5 In general, NFAT is thought to act as a positive regulator of gene expression. For instance, IL-2 production is profoundly compromised in patients unable to generate functional NFAT complexes,6 and peptides based on the calcineurin-docking site of NFAT proteins, which specifically inhibit the activation of NFAT, impair the production of IL-2 and other cytokines in vitro.7
To date, 4 calcium-regulated members of the NFAT family (NFAT1-4; also known as NFATc1-4 and hereafter abbreviated NFAT), with distinct but overlapping tissue distributions, have been identified.8-15 A fifth protein, NFAT5/TonEBP,16,17 is regulated by osmotic shock. NFAT3 is expressed predominantly outside the immune system, whereas NFAT1 (NFATp, NFATc2), NFAT2 (NFATc, NFATc1), and NFAT4 (NFATx, NFATc3) can be found in immune cells including T cells, B cells, natural killer (NK) cells, macrophages, and mast cells.2,10,18-21Although NFAT proteins are expressed in multiple cell types, their regulation has been extensively studied in T and B cells, where they are present as phosphoproteins in the cytoplasm of resting cells. After cell stimulation, 4 consecutive steps lead to the activation of NFAT: dephosphorylation by the calcium-dependent phosphatase calcineurin, a process inhibited by the immunosuppressive drugs cyclosporin A and FK50622-26; translocation into the nucleus18,23,27; binding to specific DNA elements in the regulatory regions of target genes, which occurs in physical or functional cooperation with other transcription factors including AP-1 (Fos/Jun), c-Maf (Maf), and GATA-family proteins2,4,22,28-30; and interaction with known or putative coactivator proteins such as p300, CBP, and NIP-45.31-33
After antigen stimulation, CD4+ T cells differentiate into at least 2 types of effector cells that differ as to the pattern of cytokines they produce on restimulation.34-36 Th1 cells are defined by the production of interferon-γ (IFN-γ) and mediate predominantly cellular immune responses, whereas the signature cytokines of Th2 cells—IL-4, IL-5, and IL-13—are involved in allergic reactions and immune responses against helminthic parasites. In a naive precursor cell, Th1 or Th2 cell differentiation is initiated when prolonged stimulation through the T-cell receptor occurs together with activation of the IL-12/STAT4 or IL-4/STAT6 signaling pathways, respectively.34,36-40 The downstream events in the differentiation process are not completely elucidated, but they involve cell-specific chromatin remodeling.41 The pathophysiologic significance of Th1/Th2 imbalance has been documented in recent years for a number of diseases in humans and mice, and Th1/Th2 balance can be affected by the expression levels of various intracellular proteins including NFAT1, NFAT2, and NFAT4; JNK1 and JNK2; BCL-6; Maf; GATA-3; and the recently identified T box transcription factor T-bet.42-52
We and others42-44,53 have previously shown that T cells isolated from NFAT1-deficient mice have a strong bias to differentiate into Th2 cells. Detailed analysis traced this phenotype to the fact that NFAT1−/− T cells produce increased levels of IL-4 relative to NFAT1+/+ cells as a result of prolonged expression of IL-4 mRNA.44 Naive NFAT1−/− T cells, differentiated and restimulated in vitro under unskewed conditions, also transcribed significantly less IFN-γ mRNA than naive NFAT1+/+ T cells44; however, in this case the secondary effects of IL-4 overproduction made it difficult to determine whether the loss of NFAT1 directly affected the expression of IFN-γ and other cytokines. In particular, IL-4 and the Th2-specific factors Maf and GATA-3, besides promoting Th2 differentiation, are known to down-regulate the expression of IFN-γ and possibly other Th1 cytokines35,36,50 51; thus, increased IL-4 production by NFAT1−/− cells would have a confounding effect. Therefore, a complete analysis of cytokine expression in NFAT1-deficient mice required additional investigation.
To eliminate the skewing effects caused by IL-4 overexpression in NFAT1−/− mice, we bred these mice to IL-4−/− mice54 and compared cytokine expression by NFAT1−/− IL-4−/− versus NFAT1+/+ IL-4−/− T cells. Here we show that, in the absence of endogenous overproduction of IL-4, Th2 differentiation in NFAT1−/− cells is essentially normal. In contrast, CD4+ T cells lacking NFAT1 display a pronounced, cell-intrinsic, IL-4–independent defect in IFN-γ production that most likely reflects a requirement for NFAT1 in the acute phase of transcription of the IFN-γ gene. This is confirmed by our demonstration that IFN-γ production in the murine T-cell clone CL.7W2 is sensitive to VIVIT, a selective peptide inhibitor of NFAT.7 Our results provide strong evidence for an in vivo role of NFAT1 in IFN-γ gene transcription.
Materials and methods
Mice
NFAT1−/− and NFAT1+/+mice,42 on a mixed C57BL/6 and 129Sv/J background, were bred to IL-4−/− mice54 (Jackson Laboratories, Bar Harbor, ME) to generate NFAT1−/−IL4−/− double-knockout and NFAT1+/+IL-4−/− control mice. All mice were maintained in pathogen-free conditions in barrier facilities at the Center for Animal Resources and Comparative Medicine, Harvard Medical School, and were used at age 8 to 12 weeks.
Cell culture conditions and reagents
Cell culture was performed in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (FCS), L-glutamine, streptomycin–penicillin, nonessential amino acids, sodium pyruvate, vitamins, HEPES (all from GIBCO, Gaithersburg, MD), and 5 × 10−5 M 2-mercaptoethanol (Sigma, St Louis, MO). Single-cell suspensions were prepared from lymph node (inguinal, axillary, brachial, cervical) and spleen cells. CD4+ T cells were purified using magnetic beads (Dynabeads L3T4; Dynal, Lake Success, NY). In selected experiments, CD4+ T cells were further purified by cell sorting in Mel-14hiCD4+ T cells, as described.44 All experiments were performed in parallel using equal numbers of cells from NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/− mice.
For primary stimulation, purified CD4+ T cells (1 × 106/mL) were stimulated in vitro for the indicated time points with 1 μg/mL plate-bound anti-CD3 (2C11; Pharmingen, San Diego, CA) or the combination of 10 ng/mL IL-12 (courtesy of M. O'Donnell, Genetics Institute, Cambridge, MA) and 50 ng/mL IL-18 (gift of M. Su, Vertex Pharmaceuticals, Cambridge, MA). In vitro differentiation assays were performed as described.44Briefly, purified CD4+ T cells (1 × 106/mL) were stimulated with 1 μg/mL plate-bound anti-CD3 alone (default conditions) or in the presence of 5 ng/mL IL-12 or the indicated amounts of IL-4 (IL-4 was added as supernatant from the cell line I3L6 transfected with a constitutively expressed murine IL-4 cDNA55). All cultures were supplemented with 20 U/mL IL-2 (Collaborative Biomedical Products, Bedford, MA) after 24 hours, and fresh media (30% of the initial volume) was added after 48 hours of stimulation. After 4 days, cells were extensively washed, rested for 24 to 48 hours in IL-2 (20 U/mL), counted, and restimulated at 1 × 106 cells/mL with 1 μg/mL plate-bound anti-CD3 for the indicated time points.
For mixing experiments, NFAT1+/+ IL-4−/− or NFAT1−/− IL-4−/− CD4+ T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) before differentiation, as described.56 57 Briefly, 1 × 107 cells/mL were incubated with 10 μM CFSE for 8 minutes at room temperature. To stop labeling, one volume of cold FCS was added, and cells were washed 3 times with culture media. Cells were then differentiated as described above, either alone or in mixed cultures together with unlabeled cells.
Transfections
Plasmid DNA (0.75 μg/106 cells) was introduced in Cl.7W2 cells58 by electroporation in serum-free medium in 0.4-cm cuvettes with settings of 250 V and 950 μF, using a Bio-Rad Gene Pulser II (Hercules, CA). The day after transfection, cells were stimulated for intracellular cytokine staining as indicated.
Enzyme-linked immunosorbent assay and intracellular cytokine staining
Culture supernatants of cells activated as described above were collected at 24 hours (secondary stimulation) or 48 hours (primary stimulation), and cytokine levels for IFN-γ, IL-2, and IL-5 were analyzed by enzyme-linked immunosorbent assay (ELISA) using standard protocols and monoclonal antibodies specific for murine (m) IFN-γ, mIL-2, and mIL-5 (courtesy of A. Clausell, Pharmingen). Calculated values are expressed as mean ± SEM.
For intracellular cytokine staining, differentiated T cells or Cl.7W2 cells were stimulated with 20 nM phorbol 12-myristate 13-acetate (PMA) and 1 μM ionomycin for 4 to 5 hours, the last 2 hours in the presence of 10 μg/mL Brefeldin A (Sigma). After harvest, cells were washed with cold phosphate-buffered saline (PBS), fixed for 10 minutes in 4% paraformaldehyde, washed twice with PBS, and permeabilized with PBS/FCS containing 0.5% saponin (Sigma). Staining was performed for 30 minutes at room temperature with allophycocyanin- or phycoerythrin-conjugated control immunoglobulin and anti–mIFN-γ antibody (Pharmingen) diluted in 0.5% saponin buffer. After staining, cells were washed twice with 0.5% saponin buffer and once with PBS and then were analyzed on a flow cytometer (FACScalibur and FACScan; Becton Dickinson Immunocytometry Systems, San Jose, CA).
RNase protection assay and Northern blot analysis
At the indicated time points after stimulation, cells were harvested and total cellular RNA was immediately extracted (Ultraspec, Houston, TX). Cytokine mRNA levels were analyzed by RNase protection assay using the RiboQuant multiprobe kit (Pharmingen) following the instructions of the manufacturer. Briefly, equal amounts of target RNA (1-5 μg) were hybridized overnight to a [32P]-labeled RNA probe that had been synthesized in vitro from a multicytokine template set, after which free probe and other single-stranded RNA were digested with RNases. Protected fragments were purified and resolved on a 6% denaturing polyacrylamide gel and visualized by autoradiography. Cytokine transcripts were identified by the lengths of the respective fragments. RNA loading was estimated by measuring intensities of protected fragments encoding 2 housekeeping genes, L32 andGAPDH, included in the multitemplate set as internal controls.
For Northern blot analysis, total RNA was separated by gel electrophoresis (10 μg/lane) and transferred to a nylon membrane (Nytran; Schleicher & Schuell, Keene, NH). cDNA fragments for Northern hybridization were purified from plasmids containing the full-length murine c-Maf30 and human GATA-359 cDNAs, respectively.
Infections with Leishmania major
L major LRC-L137 clone V121 promastigotes60 were cultured in semidefined medium 7961 supplemented with 10% FCS (Seromed, Berlin, Germany) to stationary phase at 26°C. The cells were harvested and washed twice with PBS and finally resuspended in PBS at 108parasites/mL. Thirty microliters of this suspension was injected into the left hind footpad. Footpad thickness was recorded at the indicated times using a caliper. The difference to the uninfected foot was calculated and plotted as the mean ± SD.
Parasite burden in draining lymph nodes was estimated by limiting-dilution analysis. Single-cell suspensions of lymph node cells were serially diluted by a factor of 3.18 in 4 replicates in semidefined medium 79. Parasite growth was recorded microscopically after 4 to 6 days of culture. Total number of parasites per lymph node was calculated from the highest dilution at which a minimum of 1 of 4 replicates still contained parasites and the total number of lymph node cells in suspension.
Results
All the experiments described in this article were performed using IL-4–deficient T cells that either expressed or lacked the transcription factor NFAT1. For simplicity, therefore, the cells are occasionally referred to as NFAT1−/− or NFAT1+/+, with the common IL-4−/−background assumed.
NFAT1 regulates IFN-γ expression through an IL-4–independent mechanism in T cells
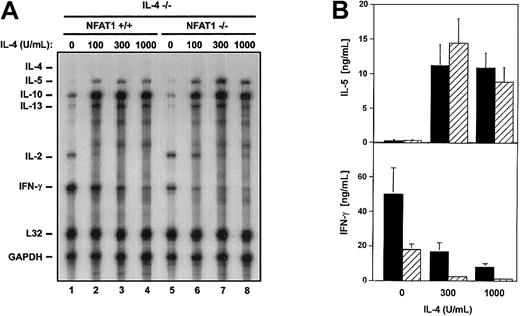
To assess the effect of NFAT1 on cytokine production in the absence of the confounding effects of IL-4, we purified CD4+ T cells from NFAT1+/+IL-4−/− and NFAT1−/− IL-4−/−spleen and lymph node cells and differentiated them in vitro with plate-bound anti-CD3, in the absence of added cytokines or antibodies (default conditions). Four days after stimulation, cells were washed, rested, and restimulated with plate-bound anti-CD3, after which levels of cytokine transcripts were quantified by RNase protection assay (Figure 1A). Under these conditions, both NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/− T cells expressed barely detectable levels of mRNAs encoding the Th2 cytokines IL-5 and IL-13 and, as expected, no mRNA encoding IL-4 (Figure 1A, left panel). This result confirmed our earlier conclusion that Th2 differentiation in NFAT1−/−cells was completely dependent on IL-4 because it was inhibited by inclusion of a neutralizing anti–IL-4 antibody.44 53 In the same experiment, NFAT1−/− IL-4−/− T cells expressed considerably lower levels of mRNA encoding GM-CSF and the Th1 cytokine IFN-γ than NFAT1+/+IL-4−/− T cells (Figure 1A). In contrast, there was no consistent difference in the expression of IL-2, FasL, and TNF-α between NFAT1−/− IL-4−/− and NFAT1+/+ IL-4−/− T cells (Figure 1A-B, right panel). Analysis of supernatants of restimulated cells by ELISA confirmed that IFN-γ production by NFAT1−/−IL-4−/− T cells was profoundly compromised (Figure 1B, left panel). These experiments suggested that NFAT1 acted independently of IL-4 to regulate IFN-γ and possibly GM-CSF expression during T-cell differentiation.
Reduced IFN-γ production by NFAT1−/−IL-4−/− T-helper cells.
(A) NFAT1−/− IL-4−/− T cells show diminished levels of IFN-γ mRNA. CD4+ T cells from NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/−mice were cultured for 4 days with anti-CD3 and IL-2, rested for 48 hours, and restimulated with anti-CD3. Differentiation and restimulation were performed under neutral conditions, without the addition of antibodies and cytokines other than IL-2. Six hours after restimulation, total cellular RNA was isolated and analyzed by RNase protection assay for transcript levels of the indicated cytokines. (B) NFAT1−/− IL-4−/− T cells show diminished production of IFN-γ protein. NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/− CD4+ T cells were cultured as in panel A. Supernatants were collected 24 hours after restimulation, and levels of IFN-γ and IL-2 were determined by ELISA. Values are expressed as the means ± SEM of 9 (IFN-γ) or 3 (IL-2) independent experiments.
Reduced IFN-γ production by NFAT1−/−IL-4−/− T-helper cells.
(A) NFAT1−/− IL-4−/− T cells show diminished levels of IFN-γ mRNA. CD4+ T cells from NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/−mice were cultured for 4 days with anti-CD3 and IL-2, rested for 48 hours, and restimulated with anti-CD3. Differentiation and restimulation were performed under neutral conditions, without the addition of antibodies and cytokines other than IL-2. Six hours after restimulation, total cellular RNA was isolated and analyzed by RNase protection assay for transcript levels of the indicated cytokines. (B) NFAT1−/− IL-4−/− T cells show diminished production of IFN-γ protein. NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/− CD4+ T cells were cultured as in panel A. Supernatants were collected 24 hours after restimulation, and levels of IFN-γ and IL-2 were determined by ELISA. Values are expressed as the means ± SEM of 9 (IFN-γ) or 3 (IL-2) independent experiments.
As shown previously, Th2 skewing of NFAT1−/− T cells is preceded by prolonged expression of the IL-4 gene in response to the differentiation-inducing stimulation. However, because of the endogenous overproduction of IL-4, other potential mechanisms affecting T-helper cell differentiation in NFAT1−/− cells could not be tested in these experiments. We examined the effect of IL-4 on the differentiation of IL-4−/− T cells that expressed or lacked NFAT1. CD4+ T cells were purified from NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/− mice and were stimulated as described above, in the absence or presence of IL-4 (0 to 1000 U/mL). Inclusion of IL-4 induced comparable Th2 differentiation in both the NFAT1+/+IL-4−/− and the NFAT1−/−IL-4−/− T cells, characterized by increased IL-5, IL-10, and IL-13 mRNA and down-regulation of IFN-γ mRNA (Figure2A). A concomitant increase in IL-5 secretion and down-regulation of IFN-γ secretion was also observed (Figure 2B). Notably, considerably lower levels of IL-4 sufficed for almost total extinction of IFN-γ production in NFAT1−/−IL-4−/− relative to NFAT1+/+IL-4−/− cells (compare lanes 2 and 6 in Figure 2A and black and hatched bars in Figure 2B, lower panel).
IL-4 promotes Th2 differentiation of NFAT1−/− IL-4−/− T cells.
(A) IL-4 treatment up-regulates mRNAs encoding Th2 cytokines and down-regulates IFN-γ mRNA in both NFAT1−/−IL-4−/− (▨) and NFAT1+/+IL-4−/− (▪) cells. CD4+ T cells from NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/− mice were cultured for 4 days with anti-CD3 and IL-2 in the presence of IL-4 (0-1000 U/mL), rested for 48 hours, and restimulated with anti-CD3. Six hours after restimulation, total cellular RNA was isolated and analyzed by RNase protection assay for transcript levels of the indicated cytokines. (B) IL-4 treatment up-regulates IL-5 production and down-regulates IFN-γ production in both NFAT1−/− IL-4−/− and NFAT1+/+ IL-4−/− cells. NFAT1+/+IL-4−/− and NFAT1−/− IL-4−/−CD4+ T cells were cultured as in panel A. Supernatants were collected 24 hours after restimulation, and levels of IFN-γ and IL-5 were determined by ELISA. Values are expressed as the means ± SEM of 3 independent experiments.
IL-4 promotes Th2 differentiation of NFAT1−/− IL-4−/− T cells.
(A) IL-4 treatment up-regulates mRNAs encoding Th2 cytokines and down-regulates IFN-γ mRNA in both NFAT1−/−IL-4−/− (▨) and NFAT1+/+IL-4−/− (▪) cells. CD4+ T cells from NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/− mice were cultured for 4 days with anti-CD3 and IL-2 in the presence of IL-4 (0-1000 U/mL), rested for 48 hours, and restimulated with anti-CD3. Six hours after restimulation, total cellular RNA was isolated and analyzed by RNase protection assay for transcript levels of the indicated cytokines. (B) IL-4 treatment up-regulates IL-5 production and down-regulates IFN-γ production in both NFAT1−/− IL-4−/− and NFAT1+/+ IL-4−/− cells. NFAT1+/+IL-4−/− and NFAT1−/− IL-4−/−CD4+ T cells were cultured as in panel A. Supernatants were collected 24 hours after restimulation, and levels of IFN-γ and IL-5 were determined by ELISA. Values are expressed as the means ± SEM of 3 independent experiments.
NFAT1 regulates IFN-γ expression by a cell-intrinsic mechanism independent of the subset-specific factors GATA-3 and Maf
As a transcription factor, NFAT1 is known to induce the expression of numerous cytokines and cell surface molecules,2 some of which could potentially affect the development of IFN-γ–producing cells during T-cell differentiation. To examine the involvement of soluble or membrane-bound factors that might influence IFN-γ production, we performed mixing experiments in which NFAT1−/− IL-4−/− and NFAT1+/+IL-4−/− T cells were differentiated either alone or together. To distinguish the 2 types of T cells in the mixed populations, we labeled one of the populations with the fluorescent dye CFSE.57 After differentiation, IFN-γ production by NFAT1−/− IL-4−/− and NFAT1+/+IL-4−/− T cells was independently quantified, either by restimulation followed by intracellular cytokine staining (Figure3A) or by separating differentiated cells by cell sorting, restimulating the separated populations, and measuring IFN-γ by ELISA assays of cell supernatants (Figure 3B). As shown in Figure 3, NFAT1−/− IL-4−/− T cells consistently produced less IFN-γ than NFAT1+/+IL-4−/− T cells, regardless of whether they were labeled with CFSE and whether they were cultured alone or together with the other cell type. This result suggested that NFAT1 promotes IFN-γ production by differentiated T cells through a cell-intrinsic mechanism rather than indirectly by inducing soluble or membrane-bound factors.
NFAT1 promotes IFN-γ production through a cell-intrinsic mechanism.
Both panels show that NFAT1−/− IL-4−/− T cells produce less IFN-γ, regardless of whether or not they are mixed with NFAT1+/+ IL-4−/− T cells. (A) Intracellular staining for IFN-γ–producing cells. CD4+ T cells from NFAT1+/+ IL-4−/− and NFAT1−/− IL-4−/− mice were left unlabeled or were labeled with the fluorescent tracker dye CFSE, then differentiated by culturing them separately or as a 1:1 mixture with anti-CD3 and IL-2 for 4 days. After restimulation for 4 hours with PMA and ionomycin, IFN-γ production was analyzed at a single-cell level by intracellular cytokine staining, without further separation of the mixed-cell populations. Numbers on the right refer to the percentage of cells in each quadrant, relative to the total number of NFAT1+/+ IL-4−/− (italics) or NFAT1−/− IL-4−/− (nonitalics) cells. Note that cell division during the differentiation period can be followed over 6 to 7 generations by the decrease of CFSE fluorescence intensity. Note also that CFSE labeling does not cause significant changes in the proportion of cells expressing IFN-γ. (B) Levels of secreted IFN-γ. Cells were treated as in panel A, except that mixed populations were separated by FACS sorting immediately before they were restimulated. Twenty-four hours after restimulation, IFN-γ secreted into the supernatants was analyzed by ELISA. (left) Cells were cultured separately. Again, note that CFSE labeling does not cause significant changes in levels of IFN-γ production. (right) Cells were cultured as mixed populations and separated by sorting; the labeled populations are indicated. Note that cells in the mixed cultures, which underwent the sorting procedure, showed a considerable decrease in their levels of IFN-γ production regardless of whether they expressed or lacked NFAT1 (compare the scale of the y-axis in the left and right panels). Nevertheless, the relative decrease in IFN-γ expression by NFAT1−/− IL-4−/− versus NFAT1+/+ IL-4−/− T cells was maintained even after cell sorting (right panel).
NFAT1 promotes IFN-γ production through a cell-intrinsic mechanism.
Both panels show that NFAT1−/− IL-4−/− T cells produce less IFN-γ, regardless of whether or not they are mixed with NFAT1+/+ IL-4−/− T cells. (A) Intracellular staining for IFN-γ–producing cells. CD4+ T cells from NFAT1+/+ IL-4−/− and NFAT1−/− IL-4−/− mice were left unlabeled or were labeled with the fluorescent tracker dye CFSE, then differentiated by culturing them separately or as a 1:1 mixture with anti-CD3 and IL-2 for 4 days. After restimulation for 4 hours with PMA and ionomycin, IFN-γ production was analyzed at a single-cell level by intracellular cytokine staining, without further separation of the mixed-cell populations. Numbers on the right refer to the percentage of cells in each quadrant, relative to the total number of NFAT1+/+ IL-4−/− (italics) or NFAT1−/− IL-4−/− (nonitalics) cells. Note that cell division during the differentiation period can be followed over 6 to 7 generations by the decrease of CFSE fluorescence intensity. Note also that CFSE labeling does not cause significant changes in the proportion of cells expressing IFN-γ. (B) Levels of secreted IFN-γ. Cells were treated as in panel A, except that mixed populations were separated by FACS sorting immediately before they were restimulated. Twenty-four hours after restimulation, IFN-γ secreted into the supernatants was analyzed by ELISA. (left) Cells were cultured separately. Again, note that CFSE labeling does not cause significant changes in levels of IFN-γ production. (right) Cells were cultured as mixed populations and separated by sorting; the labeled populations are indicated. Note that cells in the mixed cultures, which underwent the sorting procedure, showed a considerable decrease in their levels of IFN-γ production regardless of whether they expressed or lacked NFAT1 (compare the scale of the y-axis in the left and right panels). Nevertheless, the relative decrease in IFN-γ expression by NFAT1−/− IL-4−/− versus NFAT1+/+ IL-4−/− T cells was maintained even after cell sorting (right panel).
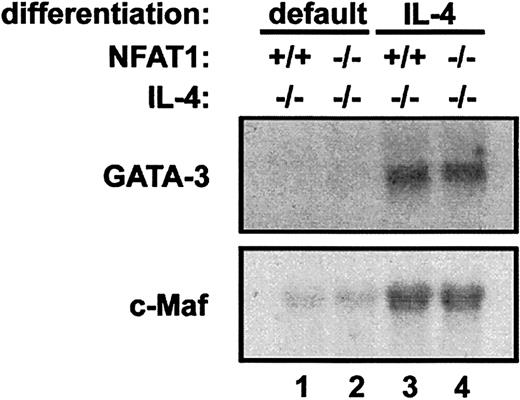
We asked whether reduced IFN-γ production in differentiated NFAT1−/− IL-4−/− T cells was secondary to increased expression of Maf or GATA-3. In addition to promoting Th2 differentiation, overexpression of these transcription factors in T cells results in IL-4–independent inhibition of IFN-γ production.50 51 NFAT1−/−IL-4−/− and NFAT1+/+ IL-4−/− T cells were differentiated in the absence or presence of IL-4 (1000 U/mL), and levels of Maf and GATA-3 were assessed by Northern and Western blotting. As expected, Maf and GATA-3 transcripts (Figure4) and GATA-3 protein (data not shown) were undetectable in T cells cultured without IL-4 (Figure 4, lanes 1 and 2) but were present in T cells differentiated along the Th2 pathway by exposure to IL-4 (Figure 4, lanes 3 and 4). However, there was no difference in Maf and GATA-3 expression in NFAT1−/−IL-4−/− versus NFAT1+/+ IL-4−/−T cells (Figure 4, compare lanes 1 and 3 to lanes 2 and 4, respectively). Thus, the decrease in IFN-γ production by differentiated NFAT1−/− IL-4−/− T cells is not mediated through increases in the expression levels of Maf or GATA-3.
Decreased production of IFN-γ by NFAT1−/− IL-4−/− T cells cannot be explained by overexpression of Maf or GATA-3.
CD4+ T cells from NFAT1+/+IL-4−/− and NFAT1−/− IL-4−/−mice were cultured for 4 days with anti-CD3 and IL-2, either under neutral conditions (lanes 1 and 2) or in the presence of 1000 U/mL IL-4 to induce Th2 differentiation (lanes 3 and 4). After harvesting the cells, total RNA was extracted, and Northern blot analysis was performed as described in “Materials and methods.” Ethidium bromide staining of the 28S bands confirmed equal loading of RNAs isolated from NFAT1−/− IL-4−/− and NFAT1+/+IL-4−/− cells (data not shown). Results of 1 of 2 identical experiments are shown.
Decreased production of IFN-γ by NFAT1−/− IL-4−/− T cells cannot be explained by overexpression of Maf or GATA-3.
CD4+ T cells from NFAT1+/+IL-4−/− and NFAT1−/− IL-4−/−mice were cultured for 4 days with anti-CD3 and IL-2, either under neutral conditions (lanes 1 and 2) or in the presence of 1000 U/mL IL-4 to induce Th2 differentiation (lanes 3 and 4). After harvesting the cells, total RNA was extracted, and Northern blot analysis was performed as described in “Materials and methods.” Ethidium bromide staining of the 28S bands confirmed equal loading of RNAs isolated from NFAT1−/− IL-4−/− and NFAT1+/+IL-4−/− cells (data not shown). Results of 1 of 2 identical experiments are shown.
NFAT1 is required for optimal IFN-γ production by naive T-helper cells and throughout T-cell differentiation
The presence of IL-12 strongly induces Th1 differentiation and is necessary for optimal IFN-γ production by effector T cells.62 The experiments reported above involved purified CD4+ T cells and thus did not reflect IL-12–dependent mechanisms. We therefore asked whether the inclusion of IL-12 during differentiation would rescue high levels of IFN-γ production in NFAT1−/− IL-4−/− relative to NFAT1+/+ IL-4−/− T cells. In the same experiment, we asked whether the IFN-γ defect of these cells was limited to a restricted time window or was maintained through several rounds of differentiation involving repeated restimulations. To avoid the potentially confounding effects of CD4+ memory or NK1.1 T cells, we used naive T-cell populations (CD4+Mel-14hi) of NFAT1−/− IL-4−/−and NFAT1+/+ IL-4−/− cells. Cells were cultured in the absence or presence of IL-12 for 2 consecutive rounds of differentiation involving a total of 3 stimulations—the primary stimulation (depicted as number 1 in Figure5), the secondary stimulation (number 2) occurring after the first round of differentiation, and the tertiary stimulation (number 3) occurring after the second round of differentiation. After each stimulation, supernatants (Figure 5) and total RNA (data not shown) were analyzed for IFN-γ expression.
NFAT1 is required for optimal IFN-γ production by T cells at all stages of T-cell differentiation.
NFAT1 is required for optimal IFN-γ production by naive and differentiated T-helper cells. NFAT1+/+IL-4−/− and NFAT1−/− IL-4−/−CD4+ T cells were sorted by FACS for high expression of Mel-14 and were cultured with plate-bound anti-CD3 and IL-2 for 2 consecutive rounds of differentiation, in the absence (top panels) or presence (bottom panels) of IL-12. Before differentiation (stimulation 1, left panel) and after the first (stimulation 2, middle panels) and second (stimulation 3, right panels) rounds of differentiation, cells were stimulated for 48 hours (stimulation 1) or 24 hours (stimulations 2 and 3) with plate-bound anti-CD3, after which IFN-γ was analyzed in the supernatants of the cells. Note that y-axis scales representing IFN-γ production are different in all panels.
NFAT1 is required for optimal IFN-γ production by T cells at all stages of T-cell differentiation.
NFAT1 is required for optimal IFN-γ production by naive and differentiated T-helper cells. NFAT1+/+IL-4−/− and NFAT1−/− IL-4−/−CD4+ T cells were sorted by FACS for high expression of Mel-14 and were cultured with plate-bound anti-CD3 and IL-2 for 2 consecutive rounds of differentiation, in the absence (top panels) or presence (bottom panels) of IL-12. Before differentiation (stimulation 1, left panel) and after the first (stimulation 2, middle panels) and second (stimulation 3, right panels) rounds of differentiation, cells were stimulated for 48 hours (stimulation 1) or 24 hours (stimulations 2 and 3) with plate-bound anti-CD3, after which IFN-γ was analyzed in the supernatants of the cells. Note that y-axis scales representing IFN-γ production are different in all panels.
Strikingly, a marked reduction in IFN-γ production by NFAT1−/− IL-4−/− relative to NFAT1+/+ IL-4−/− cells was already apparent after the first in vitro stimulation of naive T cells (Figure 5, left panel). The NFAT1−/− IL-4−/− T cells remained capable of differentiation because culture in the absence of IL-12 led to successively increased IFN-γ production in both NFAT1+/+ IL-4−/− and NFAT1−/−IL-4−/− cells (Figure 5, top panels: note changes in y-axis scales). In both populations, inclusion of IL-12 in the differentiation cultures greatly potentiated IFN-γ production by the differentiated cells (Figure 5, bottom panels), indicating that NFAT1−/− IL-4−/− T cells remained responsive to IL-12. However, NFAT1−/−IL-4−/− T cells consistently produced considerably lower levels of IFN-γ than NFAT1+/+ IL-4−/−cells, despite repeated restimulation in either the absence or presence of IL-12. Thus, NFAT1 is required for optimal production of IFN-γ not only in a restricted time window, but throughout the differentiation of CD4+ T cells.
Decreased resistance of NFAT1−/−IL-4−/− mice to infection with L major
Efficient production of IFN-γ is critical for resistance to infections with intracellular pathogens, such as L major. To test whether the reduced IFN-γ production by NFAT1−/−IL-4−/− T cells had biologically relevant consequences in vivo, we infected NFAT1−/− IL-4−/− and NFAT1+/+ IL-4−/− mice with L majorin one hind footpad. As expected from both their IL-4 null and their C57BL/6 and 129/SvJ genetic backgrounds,63 both mouse strains were considerably resistant to the infection and eventually controlled the disease (Figure 6A). However, compared to footpads of NFAT1+/+IL-4−/− mice, footpads of NFAT1−/−IL-4−/− mice showed a slight but significant increase in size during the course of the infection (Figure 6A). This was paralleled by an approximately 10-fold increase in the number of parasites (Figure 6B; note logarithmic scale), and a 2-fold increase in the number of lymphocytes (data not shown), in the draining lymph nodes of NFAT1−/− animals 11 weeks after infection. These results suggested that resistance to infection with L majorwas reduced in NFAT1−/− IL-4−/− mice compared to IL-4−/− mice and is consistent with reduced IFN-γ production by NFAT1−/− IL-4−/−relative to IL-4−/− CD4+ T cells.
Decreased resistance of NFAT1−/−IL-4−/− mice to infection with
L major. (A) NFAT1−/−IL-4−/− and NFAT1+/+ IL-4−/−female mice (10 mice per group) were infected with promastigotes ofL major in the left hind footpad. Footpad thickness was recorded at the indicated times using a metric caliper, and the lesion size was calculated as the difference between the injected and the uninjected footpads. Values are shown as means ± SD. (B) NFAT1−/− IL-4−/− and NFAT1+/+IL-4−/− mice (3 mice per group) were infected with promastigotes of L major as in panel A. Eleven weeks after infection, the number of parasites in the draining popliteal lymph nodes was determined as described in “Material and methods.” Values are shown as means ± SD. KO, knockout mice; DKO, double-knockout mice.
Decreased resistance of NFAT1−/−IL-4−/− mice to infection with
L major. (A) NFAT1−/−IL-4−/− and NFAT1+/+ IL-4−/−female mice (10 mice per group) were infected with promastigotes ofL major in the left hind footpad. Footpad thickness was recorded at the indicated times using a metric caliper, and the lesion size was calculated as the difference between the injected and the uninjected footpads. Values are shown as means ± SD. (B) NFAT1−/− IL-4−/− and NFAT1+/+IL-4−/− mice (3 mice per group) were infected with promastigotes of L major as in panel A. Eleven weeks after infection, the number of parasites in the draining popliteal lymph nodes was determined as described in “Material and methods.” Values are shown as means ± SD. KO, knockout mice; DKO, double-knockout mice.
NFAT1 is required for the acute phase of IFN-γ gene expression
The experiments above suggested that NFAT1 is required for the acute phase of IFN-γ gene expression in naive T cells. To test this hypothesis further, we asked whether freshly isolated, undifferentiated NFAT1−/− IL-4−/− T cells would show impaired IFN-γ production in response to stimuli that do not involve activation of NFAT proteins (Figure 7A). The stimuli chosen were IL-12 and IL-18, cytokines that activate STAT and NFκB proteins, respectively, but are not known to activate NFAT.35,36 64 As shown in Figure 7A, NFAT1−/− IL-4−/− T cells stimulated by anti-CD3 for 48 hours produced substantially lower levels of IFN-γ than NFAT1+/+ IL-4−/− T cells. In contrast, IFN-γ production in response to combined stimulation with IL-12 and IL-18 was not impaired but was slightly enhanced in NFAT1−/− IL-4−/− relative to NFAT1+/+ IL-4−/− T cells. Thus, NFAT1 appears to be required for acute expression of the IFN-γ gene in T cells through a specific CD3-mediated pathway, whereas an alternative, IL-12– and IL-18–dependent pathway remains unaffected.
NFAT1 regulates the acute phase of IFN-γ production in T-helper cells.
(A) Reduced IFN-γ production by freshly isolated NFAT1−/− IL-4−/− T-helper cells in response to stimulation with anti-CD3 but not IL-12/IL-18. NFAT1+/+IL-4−/− (▪) and NFAT1−/−IL-4−/− (▨) CD4+ T cells were stimulated either with plate-bound anti-CD3 (left panel) or a combination of IL-12 and IL-18 (right panel) for 48 hours, after which IFN-γ was analyzed in the supernatants of the cells. Shown is a representative of 3 (left panel) or 5 (right panel) independent experiments. (B) The selective NFAT inhibitor GFP-VIVIT suppresses IFN-γ production by a murine T-cell clone. Cl.7W2 cells were transfected with plasmids encoding either GFP or GFP-VIVIT. The next day the cells were either left unstimulated or were stimulated for 5 hours with PMA and ionomycin. IFN-γ production was analyzed at a single-cell level by intracellular cytokine staining. Dot blots from 1 of 3 similar experiments are shown. Thresholds for IFN-γ production were set using the unstimulated control. Note that each panel shows transfected (GFP-positive) and nontransfected (GFP-negative) cells of the same sample.
NFAT1 regulates the acute phase of IFN-γ production in T-helper cells.
(A) Reduced IFN-γ production by freshly isolated NFAT1−/− IL-4−/− T-helper cells in response to stimulation with anti-CD3 but not IL-12/IL-18. NFAT1+/+IL-4−/− (▪) and NFAT1−/−IL-4−/− (▨) CD4+ T cells were stimulated either with plate-bound anti-CD3 (left panel) or a combination of IL-12 and IL-18 (right panel) for 48 hours, after which IFN-γ was analyzed in the supernatants of the cells. Shown is a representative of 3 (left panel) or 5 (right panel) independent experiments. (B) The selective NFAT inhibitor GFP-VIVIT suppresses IFN-γ production by a murine T-cell clone. Cl.7W2 cells were transfected with plasmids encoding either GFP or GFP-VIVIT. The next day the cells were either left unstimulated or were stimulated for 5 hours with PMA and ionomycin. IFN-γ production was analyzed at a single-cell level by intracellular cytokine staining. Dot blots from 1 of 3 similar experiments are shown. Thresholds for IFN-γ production were set using the unstimulated control. Note that each panel shows transfected (GFP-positive) and nontransfected (GFP-negative) cells of the same sample.
To further determine the role of NFAT in the acute phase of IFN-γ gene expression, we tested whether IFN-γ production in the murine T-cell clone Cl.7W258 could be inhibited by the selective peptide inhibitor of NFAT, VIVIT (Figure 7B, Table1). The VIVIT peptide specifically suppresses the activation of NFAT in stimulated T cells by binding with high affinity to its binding site in calcineurin.7Plasmids encoding green fluorescent protein (GFP) and the fusion protein GFP-VIVIT were introduced into Cl.7W2 cells, and a day later the cells were stimulated with PMA and ionomycin. Five hours after stimulation, IFN-γ production was analyzed by intracellular cytokine staining, which permitted direct comparison of IFN-γ production by transfected (GFP-positive) and nontransfected (GFP-negative) cells at a single cell level in the same sample. Transfection efficiency for GFP (5.3%) and GFP-VIVIT (5.7%) was comparable (mean of 3 experiments). Expression of GFP alone did not affect IFN-γ production by stimulated Cl.7W2 cells (Figure 7B, left lower panel, and data not shown). In contrast, cells expressing GFP-VIVIT produced considerably less IFN-γ, in comparison with both the nontransfected cells of the same sample and the cells transfected with GFP alone (Figure 7B; compare upper right quadrants in right and left lower panels). Because GFP-VIVIT is estimated to have an IC50 for inhibition of approximately 300 to 500 nM7 26 and is effective only when expressed at relatively high concentrations, we considered for quantification only the cells with GFP fluorescence intensities greater than 100 (upper right quadrants of dot blots in Figure 7B). The inhibitory effect of GFP-VIVIT on IFN-γ production in this cell population was reflected in the decreased number of IFN-γ–producing cells (decrease of 61.2%, 74.7%, and 75% in experiments 1, 2, and 3, respectively) and in the mean fluorescence intensity of these cells (decrease of 55.5%, 69.3%, and 27.3% in experiments 1, 2, and 3 respectively) (Table 1). The product of these 2 numbers is a measure of total IFN-γ production by the GFP- or GFP-VIVIT–expressing cells (Table 1, last column). The ratios, which are 82.7%, 92.2%, and 81.9% in experiments 1, 2, and 3, respectively, measure the ability of VIVIT to inhibit IFN-γ production and therefore measure the dependence of IFN-γ production on NFAT. We conclude that the NFAT inhibitor VIVIT markedly inhibits IFN-γ production by stimulated T cells, proving that IFN-γ gene transcription requires the activation of NFAT.
The NFAT inhibitor GFP-VIVIT suppresses IFN-γ production by T cells
| . | GFP . | GFP-VIVIT . | % inhibition by VIVIT . | ||||
|---|---|---|---|---|---|---|---|
| % IFN-γ–pos . | MFI . | % pos × MFI . | % IFN-γ–pos . | MFI . | % pos × MFI . | % pos × MFI . | |
| Experiment 1 | 61.1 | 243.9 | 14 893 | 23.7 | 108.6 | 2 573 | 82.7 |
| Experiment 2 | 58.9 | 251.4 | 14 797 | 14.9 | 77.3 | 1 155 | 92.2 |
| Experiment 3 | 33.2 | 86.6 | 2 871 | 8.3 | 63.0 | 520 | 81.9 |
| . | GFP . | GFP-VIVIT . | % inhibition by VIVIT . | ||||
|---|---|---|---|---|---|---|---|
| % IFN-γ–pos . | MFI . | % pos × MFI . | % IFN-γ–pos . | MFI . | % pos × MFI . | % pos × MFI . | |
| Experiment 1 | 61.1 | 243.9 | 14 893 | 23.7 | 108.6 | 2 573 | 82.7 |
| Experiment 2 | 58.9 | 251.4 | 14 797 | 14.9 | 77.3 | 1 155 | 92.2 |
| Experiment 3 | 33.2 | 86.6 | 2 871 | 8.3 | 63.0 | 520 | 81.9 |
Table summarizing the information of 3 independent experiments as shown in Figure 7B. For each experiment, the first column shows the percentage of IFN-γ–producing cells in the cell populations expressing high levels of GFP and GFP-VIVIT (number of cells in upper right quadrant divided by number of cells in upper and lower right quadrants), and the second column shows the mean fluorescence intensity (MFI) of IFN-γ staining in the cells in the upper right quadrant. Note that only cells expressing high levels of GFP and GFP-VIVIT (fluorescence cutoff 100) are considered for reasons described in “Results.” The product of these 2 numbers is shown in the last column and is a measure of total IFN-γ production by the GFP- or GFP-VIVIT–expressing cells. Because the transfection efficiencies for GFP and GFP-VIVIT were comparable, the ratio of the numbers in the last column is a measure of the ability of VIVIT to inhibit IFN-γ production. The same calculations performed on the untransfected (GFP-negative) cells showed no inhibition by VIVIT.
Discussion
In this study, we show that T cells lacking NFAT1 display a substantial, cell-intrinsic defect in IFN-γ gene expression independent of the down-regulatory effects of IL-4, GATA-3, and Maf. Naive CD4+ T cells from NFAT1−/−IL-4−/− mice show a marked impairment of IFN-γ production after primary in vitro stimulation with anti-CD3, a defect that continues to be apparent throughout 2 rounds of subsequent differentiation in vitro. The defect is not restricted to CD4+ T cells; it is also observed after primary in vitro stimulation of CD8+ T cells (F.J.G.-C., A.K., and A.R., unpublished results, 1999). IFN-γ production in freshly isolated NFAT1−/− IL-4−/− T cells is normal when they are stimulated with IL-12 and IL-18, demonstrating that NFAT1−/− IL-4−/− T cells are capable of producing IFN-γ under conditions that do not involve the acute activation of NFAT. Moreover, IFN-γ production in normal (NFAT-expressing) T cells is susceptible to the selective NFAT inhibitor GFP-VIVIT. We also show that the effect of NFAT1 on IFN-γ production is likely to be biologically relevant: despite their highly resistant genetic background (IL-4-null, C57BL/6, and 129/SvJ), enhanced susceptibility of NFAT1−/− IL-4−/−mice to infection with the intracellular parasite L majorwas clearly detectable (Figure 6). The fact that NFAT1−/−IL-4−/− mice were eventually able to control the disease is not surprising considering reports showing that mice on a resistant background treated with IFN-γ– and IL-4–neutralizing antibodies were only temporarily susceptible to the infection.65 It should be emphasized that the previously shown enhanced susceptibility of NFAT1−/− (IL-4+/+) mice to L major could be explained by their IL-4 overproduction and Th2 differentiation,44 which in the present study is excluded. Whereas a role of additional, yet unknown, factors is possible, a causal association to the reduced production of IFN-γ by NFAT1−/− IL-4−/− T cells appears to be likely. These results provide the first evidence for the involvement of an NFAT transcription factor in IFN-γ gene expression in vivo and extend other studies that demonstrated binding and transactivation of IFN-γ promoter constructs by NFAT in vitro and in cell lines.66-69
NFAT1 regulates acute transcription of the IFN-γ gene
Cytokine gene expression may be divided into 2 phases, a differentiative stage characterized by chromatin remodeling and demethylation of cytokine gene loci, in which T cells become “transcriptionally competent” to express certain cytokine genes,41,56 and a phase of acute gene transcription by the differentiated T cells. The competence phase of IFN-γ gene transcription is complex. During Th1 differentiation, T-cell receptor– and IL-12/STAT4-dependent signaling pathways are highly interconnected, such that the final levels of differentiated, IFN-γ producing Th1 cells reflect a balance between permissive factors such as STAT4, JNK2, and T-bet38,40,48,52 versus repressive factors such as STAT6, GATA-3, and Maf.50,51,70 In contrast, the acute transcriptional phase is less complex: differentiated Th1 cells produce IFN-γ in response to activation through at least 2 independent signaling pathways involving the T-cell receptor and the IL-12 and IL-18 receptors, respectively.36,64,71 72 As discussed in the following sections, our results implicate a role for NFAT1 in the acute phase of IFN-γ gene transcription.
As a transcription factor activated by T-cell–receptor stimulation, NFAT1 is likely to contribute to the T-cell–receptor pathway, but not the STAT4–IL-12/NFκB–IL-18 pathway, of acute IFN-γ induction. That is, NFAT1 might directly regulate IFN-γ gene transcription in the same way that it is thought to participate directly in transcription of the IL-2, IL-4, and TNF-α genes.2 This hypothesis is supported by our observation that T cells lacking NFAT1 are not compromised in their ability to differentiate into IFN-γ–secreting cells but that they display markedly reduced IFN-γ gene transcription at all stages of differentiation in response to acute stimulation with anti-CD3 (Figure 5). Moreover, IFN-γ production in anti-CD3–stimulated T cells is highly sensitive to CsA (data not shown) and to the selective NFAT inhibitor GFP-VIVIT (Figure7B, Table 1), again consistent with the hypothesis that NFAT1 acts during the acute stage of IFN-γ gene transcription. NFAT1 might bind directly to DNA elements in promoter/enhancer regions of the IFN-γ gene, or it might rapidly up-regulate secondary transcription factor(s) required for IFN-γ gene transcription.
Our results do not rule out that NFAT1 also regulates IFN-γ gene expression through a second, developmental mechanism. However, we have excluded certain mechanisms by which NFAT1 might promote transcriptional competence (as distinct from acute transcription) of the IFN-γ gene. First, as our mixing experiments indicate, NFAT1 does not induce the expression of cytokines or other soluble factors necessary for high-level IFN-γ production by differentiated T cells (Figure 3). Furthermore, our specific differentiation conditions represent an IL-12–free system, ruling out potential effects of NFAT1 on IL-12, IL-12 receptors, or other IL-12–related mechanisms. Second, NFAT1−/− IL-4−/− T cells do not show perceptible differences, compared to wild-type cells, in chromatin remodeling of the IFN-γ gene during peripheral differentiation. The mature DNase I hypersensitivity pattern over 11 kb of the IFN-γ gene developed identically in NFAT1−/− IL-4−/−and NFAT1+/+ IL-4−/− cells (A.K., S. Agarwal, and A.R., unpublished results, 1998). Finally, NFAT1 did not affect the levels of the Th2-specific transcription factors GATA-3 and Maf (Figure 4), nor was there any difference in expression of the Th1-specific factors ERM73 or T-bet52 in differentiated NFAT1−/− IL-4−/− versus NFAT1+/+ IL-4−/− T cells (X. Yu, A.K., and A.R., unpublished results, 2000).
Potential NFAT-dependent regulatory elements in the IFN-γ gene
DNA elements through which NFAT1 might regulate IFN-γ gene transcription have been extensively studied but not yet definitively identified. Analysis of the human IFN-γ promoter identified NFAT sites at −280 and −160 relative to the transcription start site.66-69 The −280 NFAT site and a previously identified intronic site may bind both NFAT and Rel, whereas the −160 site has the characteristics of a composite NFAT-AP-1 site.68,69 In chromatin immunoprecipitation assays, which measure the ability of a transcription factor to bind a regulatory element in intact cells in vivo, the IFN-γ promoter is clearly capable of binding NFAT.83 However in transient reporter assays, mutation of either of the 2 NFAT sites only marginally reduced (approximately 2-fold) the overall inducibility of the IFN-γ promoter, and mutation of both sites had only a small additional effect.66,68,69Deletion or mutation of the −280 NFAT site considerably diminished, but did not abolish, calcineurin sensitivity of the IFN-γ promoter.66 68 Together these results indicate that although NFAT binds strongly and selectively to the proximal IFN-γ promoter region in vivo, the activity of the isolated promoter is only weakly dependent on NFAT in transient reporter assays.
The most proximal region of the IFN-γ promoter (nucleotides −108 to −40) is thought to play a major role in regulating IFN-γ promoter activity.74-76 This region contains 2 strong regulatory elements that bind a variety of transcription factors, including AP-1, ATF/CREB, and GATA proteins. In unstimulated cells, the proximal regulatory element (−40 to −70) binds CREB and ATF-1, whereas the distal element (−78 to −100) binds GATA proteins and AP-1; a weak NFAT site identified at −100 is of questionable functional importance because it is not conserved in the mouse gene.69,74,75 In stimulated cells, the composition of factors binding to the proximal element shifts from CREB and ATF-1 to Jun and ATF-2,75suggesting that the CsA sensitivity of the −108 to −40 region69,74,75 reflects the known CsA sensitivity of p38 and JNK kinases, which activate Jun and ATF2.77,78 Indeed, p38 MAP kinase has been implicated in the signaling pathway leading to acute transcription of the IFN-γ gene.79
In transgenic mouse models, neither the proximal nor the distal elements fully recapitulates the behavior of the endogenous IFN-γ gene; the proximal element shows no activity in CD8 cells and only weak activity in CD4 cells, whereas the activity of the distal element is not up-regulated by differentiation in the presence of IL-12.76 Similarly, a larger fragment of the IFN-γ promoter shows high constitutive activity in Th2 cells (K. Murphy, personal communication). Together these results suggest strongly that IFN-γ gene transcription is regulated by distal elements in addition to the proximal promoter. A precedent is provided by the GM-CSF and IL-3 genes, which have weak NFAT-dependent promoters but strong distal enhancers.80 81 The strong NFAT dependence and CsA sensitivity of IFN-γ gene transcription could be explained either by a major involvement of the −280 NFAT site in the context of endogenous gene expression or by the presence of a distal NFAT-responsive element, which resembles the IL-3 and GM-CSF enhancers in containing multiple binding sites for NFAT. Presumably, other distal regulatory elements control the acute responsiveness of the IFN-γ gene to IL-12 and IL-18.
NFAT1 may act coordinately to promote Th1 differentiation and suppress Th2 differentiation
The role of NFAT1 in T-helper cell differentiation has been analyzed in several in vivo models using NFAT1 knockout mice. Targeted disruption of the NFAT1 gene in mice results in a Th2 bias,43,44,53 though this is fairly mild and poorly apparent in 1 of 3 mouse strains tested.82 The mechanism of this Th2 bias remains to be understood, but it is IL-4 dependent (Kiani et al,44 Viola et al,53 and this study), and we have postulated that it might reflect the absence of a NFAT1-regulated negative feedback loop that normally terminates the antigen-induced expression of IL-4.44 The impressive allergic phenotype of mice lacking both NFAT1 and NFAT4 suggests that these 2 NFAT proteins act in concert to down-regulate Th2 responses in vivo.45
In the present study, we have identified a second role for NFAT1—activation of IFN-γ and Th1 responses. It is interesting that both IL-4 suppression and IFN-γ activation shift the Th1/Th2 balance in favor of Th1 responses, confirming a role for NFAT1 in biasing the immune response toward the Th1 phenotype in normal mice. The relative contributions of IL-4 down-regulation and IFN-γ up-regulation to NFAT1-mediated control of T-helper cell differentiation still must be determined. Potentially, however, NFAT1 could mediate both effects through a single underlying mechanism, for example by inducing a secondary transcription factor that concurrently up-regulates IFN-γ expression and down-regulates IL-4 expression.
We thank A. Abbas, F. Alt, L. Glimcher, K. Murphy, and S. Orkin for plasmids and other reagents; S. Agarwal and J. Aramburu for helpful suggestions; F. Macian for help with densitometry; R. Grewal for technical assistance; and T. Bernickel for animal husbandry.
Supported by National Institutes of Health grants R01 CA-42471 and P01 AI-35297 (A.R.) and by Deutsche Forschungsgemeinschaft grants KI 605/1-1 (A.K.), KI 605/2-1 (A.K., G.E.), and AE 16/1-3 (T.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anjana Rao, Department of Pathology, Harvard Medical School, The Center for Blood Research, 200 Longwood Ave, Boston, MA 02115; e-mail: arao@cbr.med.harvard.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal