The functional importance of platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) in platelets is unclear. Because PECAM-1 represents a newly assigned immunoglobulin–ITIM superfamily member expressed on the surface of platelets, it was hypothesized that it may play an important regulatory role in modulating ITAM-bearing receptors such as collagen (GP)VI receptor and FcγRIIA. To examine the functional role of PECAM-1 in regulating platelet-collagen interactions, 2 different approaches were applied using recombinant human PECAM-1–immunoglobulin chimeras and platelets derived from PECAM-1–deficient mice. Stimulation of platelets by collagen-, (GP)VI-selective agonist, collagen-related peptide (CRP)–, and PECAM-1–immunoglobulin chimera induced tyrosine phosphorylation of PECAM-1 in a time- and dose-dependent manner. Activation of PECAM-1 directly through the addition of soluble wild-type PECAM-1–immunoglobulin chimera, but not mutant K89A PECAM-1–immunoglobulin chimera that prevents homophilic binding, was found to inhibit collagen- and CRP-induced platelet aggregation. PECAM-1–deficient platelets displayed enhanced platelet aggregation and secretion responses on stimulation with collagen and CRP, though the response to thrombin was unaffected. Under conditions of flow, human platelet thrombus formation on a collagen matrix was reduced in a dose-dependent manner by human PECAM-1–immunoglobulin chimera. Platelets derived from PECAM-1–deficient mice form larger thrombi when perfused over a collagen matrix under flow at a shear rate of 1800 seconds−1 compared to wild-type mice. Collectively, these results indicate that PECAM-1 serves as a physiological negative regulator of platelet-collagen interactions that may function to negatively limit growth of platelet thrombi on collagen surfaces.
Introduction
Platelet adhesion to subendothelial matrix proteins plays a central role in both hemostatic and thrombotic processes.1 Collagen represents an important thrombogenic component of the subendothelium, with collagen types I, III, and VI identified as major components of the vessel wall. Platelet-collagen interactions are mediated by several platelet surface receptors, including integrin α2β1, (GP)VI, (GP)IV, and a 65-kd receptor for collagen type I.1 Of these, integrin α2β1 is thought to play a major role in collagen binding to platelets, whereas (GP)VI appears to be important in platelet activation through its constitutive association with a receptor-associated signaling molecule, FcR γ-chain.1-3 Platelet adhesion to immobilized collagen is a complex process and involves multiple receptors depending on the flow conditions.1-3 At high wall shear rates, platelets initially tether to immobilized collagen through an indirect mechanism involving the platelet adhesion receptor, glycoprotein (GP)Ib/IX/V complex, and collagen-bound von Willebrand factor (vWF). Once tethered, the collagen receptors, integrin α2β1, and (GP)VI are able to bind and synergistically induce activation of integrin αIIbβ3, leading to irreversible platelet adhesion, spreading, and subsequent thrombus growth.
Biochemical and functional studies in human and mouse platelets have demonstrated that (GP)VI physically couples collagen stimulation to the phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM)-bearing FcR γ-chain.4-8 Phosphorylation of the putative ITAM tyrosine residue of FcR γ-chain leads to recruitment and activation of p72Syk protein-tyrosine kinase. Once activated, p72Syk is able to phosphorylate SLP-76 and phospholipase Cγ2, thereby initiating activation-dependent signaling pathways resulting in the release of granule contents and platelet aggregation. (GP)VI-dependent activation pathways can be dissected in vitro by using the (GP)VI-selective agonist, collagen-related peptide (CRP), which exclusively binds (GP)VI through a glycine-proline-hydroxyproline (Gly-Pro-Hyp) recognition sequence within the collagen triple helical structure. This synthetic CRP peptide consists of a GCP*(GPP*)10GCP*G (where P* denotes hydroxyproline) repeat sequence with the N- and C-terminal cysteine residues cross-linked to form a quaternary structure that resembles the conformational structure of collagen essential for its activity.9 10 In this form, CRP serves as a highly potent platelet agonist.
Although considerable progress has been made concerning the molecular mechanisms by which platelets are activated, little is known about inhibitory mechanisms that regulate platelet adhesion under physiological blood flow conditions. A potential candidate inhibitory receptor is platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31). We have previously demonstrated that the PECAM-1 cytoplasmic domain contains consensus sequences typical of immunoreceptor tyrosine-based inhibitory motifs (ITIM) that, when phosphorylated, serve as docking sites for SH2-containing protein-tyrosine phosphatases.11-13 The PECAM-1 ITIM motifs have been shown to recruit and activate protein-tyrosine phosphatases SHP-1 and SHP-2 in response to a variety of stimuli, including integrin αIIbβ3-mediated platelet aggregation.11-13 More recently, we have demonstrated that the PECAM-1 cytoplasmic domain is capable of mediating an inhibitory signal to dampen ITAM-mediated B-cell antigen receptor activation and requires its putative ITIM motifs and protein-tyrosine phosphatases, SHP-1 and SHP-2, to deliver this inhibitory signal.14
To test whether PECAM-1 functions as a physiological regulator of collagen-mediated platelet activation, we applied 2 different experimental approaches. First, a recombinant human PECAM-1–immunoglobulin chimera containing the complete extracellular domain of PECAM-1 was used to selectively activate PECAM-1, thereby mimicking homophilic ligand interactions. In the second approach, platelets derived from PECAM-1–deficient mice were used to examine collagen-induced platelet activation. By applying these distinct but complementary approaches, we examined the role of PECAM-1 in collagen- and CRP-induced platelet aggregation and secretion and during platelet thrombus formation on type I fibrillar collagen under physiological flow conditions. These studies have defined a key role for PECAM-1 in negatively regulating collagen-induced platelet activation.
Materials and methods
Materials and antibodies
The synthetic CRP peptide GCP*(GPP*)10GCP*G (where P* denotes a hydroxyproline amino acid residue) was cross-linked through N- and C-terminal cysteine residues as previously described.9 Type I acid-soluble collagen was purchased from Chrono-Log (Havertown, PA). FcγRIIA-specific monoclonal antibody (IV.3) was kindly donated by Dr Clark Anderson (The Ohio State University, Columbus). c7E3 Fab fragment (Reopro) was purchased from Centocor B. V. (Leiden, The Netherlands). Aggrastat (tirofiban, MSD) was purchased from Merck Sharp & Dohme (New South Wales, Australia). Fab fragments were generated using immobilized papain according to manufacturer's (Pierce, Rockford, IL) instructions. After overnight dialysis in phosphate-buffered saline, pH 7.4, these fragments were analyzed by SDS-PAGE under reducing and nonreducing conditions. The functional integrity of IV.3 Fab fragments was confirmed by measuring their ability to block FcγRIIA–receptor-mediated platelet activation induced by heparin-induced thrombocytopenia–thrombosis antibodies. Fluorescent probe DiOC6 was purchased from Molecular Probes (Eugene, OR). Horseradish peroxidase (HRP)-conjugated anti-phosphotyrosine monoclonal antibody 4G10 was purchased from Upstate Biotechnology (Lake Placid, NY). PECAM-1 knockout mice were a gift from Dr Tak Mak (Amgen Institute, Toronto, Ontario, Canada).15 PECAM-1–deficient mice were housed under pathogen-free conditions at the University of Adelaide Animal SPF facility (Adelaide, Australia) under National Health and Medical Research Council guidelines and approved animal protocols. The phenotype of the PECAM-1–deficient mice was confirmed by flow cytometric analysis of peripheral blood elements.15 Recombinant human wild-type PECAM-1–immunoglobulin chimera, recombinant human K89A PECAM-1–immunoglobulin chimera, polyclonal anti-PECAM-1 antibody, SEW16, and anti-human PECAM-1.3 monoclonal IgG1 antibody were a gift from Prof Peter Newman (Blood Research Institute, Milwaukee, WI) and have been previously described.16 The mutant K89A PECAM-1–immunoglobulin chimera has been previously characterized.17 PGE1 prostaglandin, cyanogen bromide-activated Sepharose beads, sodium orthovanadate, leupeptin, phenylmethylsulfonyl fluoride, human thrombin, and type I fibrillar collagen were purchased from Sigma Chemical (St Louis, MO). [3H]5-Hydroxytryptamine was purchased from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom).
Platelet aggregation studies
Human whole blood was obtained from consenting donors who had not taken any antiplatelet medication within the preceding 2 weeks. Platelet-rich plasma (PRP) or washed platelets were prepared as previously described.11 Aggregation studies were performed in siliconized glass cuvettes at 37°C with constant stirring at 1000 rpm in a Paton 2-channel aggregometer module (Paton Scientific, Adelaide, Australia). Platelet aggregation was initiated by the addition of collagen (10 μg/mL) or CRP (2 μg/mL) and was detected by changes in light transmission. In some studies, platelets were pre-incubated with either recombinant human wild-type PECAM-1–immunoglobulin chimera (0-100 μg/mL), recombinant human K89A PECAM-1–immunoglobulin chimera (0-100 μg/mL), or human IgG (0-100 μg/mL) for 10 minutes at 37°C.
Wild-type C57/BL6 and PECAM-1–deficient C57/BL6 age- and sex-matched mice were halothane asphyxiated and bled by cardiac puncture into 0.1 vol. 3.8% (vol/vol) trisodium citrate. Anticoagulated whole blood was then centrifuged at 200g for 15 minutes at room temperature and PRP isolated. Platelet counts of PRP were performed and normalized (0.5 × 106 platelets/μL) for platelet cell number by dilution with Ringer citrate–dextrose buffer (108 mM NaCl, 38 mM KCl, 1.7 mM NaHCO3, 21.2 mM sodium citrate, 27.8 mM glucose, and 1.1 mM MgCl2, pH 7.4). Collagen (30 μg/mL) and CRP (10 μg/mL) -induced aggregation were performed as described above.
5-Hydroxytryptamine platelet secretion assay
PECAM-1+/+ and PECAM-1−/− murine PRP was loaded with 0.5 μCi/mL 5-[3H]HT for 1 hour at 37°C. Platelets were isolated and washed as described above and stimulated with CRP (5 μg/mL) or thrombin (1 U/mL) for 2 minutes. Platelets were pelleted by centrifugation at 13 000 rpm for 5 minutes, and the level of 5-[3H]HT released into the supernatant was determined by scintillation spectrometry. 5-Hydroxytryptamine (5-HT) release is expressed as a percentage of total tissue content after subtraction of release under nonstimulated conditions.
Analysis of mural platelet adhesion and thrombus formation under flow
Platelet adhesion and platelet thrombus formation under flow was performed as previously described.18 19 Rectangular glass microcapillary tubes (dimensions 0.1 × 1.0 × 100 mm, H×W×L) (microslides; Vitro Dynamics, Rockaway, NJ) were coated with type I fibrillar collagen (2.5 mg/mL) overnight at 4°C. In some experiments, citrated human whole blood was pre-incubated with FcγRIIA-blocking antibody IV.3 Fab (10 μg/mL) and recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) for 10 minutes. Blood was perfused through collagen-coated microslides at a wall shear rate of 150 seconds−1 and 600 seconds−1 for 5 minutes. Nonadherent cells were removed by perfusion of modified Tyrode buffer (10 mM HEPES, 12 mM NaHCO3, pH 7.4, 137 mM NaCl, 2.7 mM KCl, 5 mM glucose) through the microcapillary tubes, and adherent erythrocytes were removed through lysis with 1% (vol/vol) ammonium oxalate. Thrombi were lysed in 1% (vol/vol) Triton X-100 and collected for analysis of platelet lactate dehydrogenase content (U/mL) using the Unimate 3 lactate dehydrogenase assay kit (Hoffman-LaRoche, Basel, Switzerland). The accuracy of this technique for quantitation of platelet content was confirmed by confocal imaging of duplicated thrombi samples.
In studies examining murine thrombus formation under flow, platelets were fluorescently labeled by incubating citrated whole blood with the fluorescent probe, DiOC6 (1 μM) for 10 minutes at room temperature. Blood was perfused through the collagen-coated microcapillary tubes at a wall shear rate of 1800 seconds−1 for 5 minutes. Thrombi were imaged by confocal microscopy (1 μm sections) (×100; Leica TCS SP, Heidelberg, Germany) to allow end-point analysis of thrombus volume. In studies examining the time-course of thrombus growth, confocal sections (1 μm) were imaged at 0.5, 1.0, 1.5, 2.0, 3.0, 4.0, and 5.0 minutes. Thrombus volume was determined by quantifying the surface area of each section using the image analysis software package ImageTool (University of Texas Health and Science Center of San Antonio) multiplied by the z height. Thrombi were reconstructed in 3-D using Voxblast (Vaytek, Fairfield, IA). All confocal images were taken from planes that were the same distance from the inlet of collagen-coated microcapillary tube.
Immunoprecipitation and immunoblotting
After platelet stimulation, reactions were terminated by the addition of an equal volume of Triton lysis buffer (15 mM HEPES, pH 7.4, containing 145 mM NaCl, 0.1 mM MgCl2, 10 mM EGTA, 2 mM sodium orthovanadate, 0.2 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1% [vol/vol] Triton X-100). Cell suspensions were mixed on a nutator for 1 hour at 4°C, then centrifuged at 13 000 rpm for 15 minutes at 4°C. Triton-soluble supernatants were separated and precleared twice with 50 μL 50% Protein G-Sepharose beads by mixing for 15 minutes at 4°C. Precleared supernatants were incubated overnight with 10 μg anti–PECAM-1.3 monoclonal IgG1antibody followed by the addition of 50 μL 50% Protein G-Sepharose beads for 1 hour at 4°C. Protein–antibody complexes were washed 5 times with immunoprecipitation buffer (50 mM Tris, pH 7.4, containing 150 mM NaCl and 1% [vol/vol] Triton X-100), eluted in 30 μL SDS reducing buffer, and boiled for 10 minutes. Eluted proteins were electrophoresed on a 10% SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membrane by semidry Western blotting. PVDF membranes were blocked by incubation for 1 hour at room temperature in blocking buffer (20 mM Tris, pH 7.4, containing 3% [wt/vol] bovine serum albumin and 0.05% [vol/vol] Tween 20), then probed with either HRP-conjugated 4G10 anti-phosphotyrosine antibody (1 μg/mL), 4G10, or polyclonal anti-human PECAM-1 antibody, SEW16 (1 μg/mL) for 2 hours at room temperature. Membranes were washed for more than 1 hour with Tris-buffered saline (TBS; 20 mM Tris, pH 7.4, containing 150 mM NaCl and 0.05% [vol/vol] Tween 20); where appropriate, membranes were incubated with HRP-conjugated secondary antibody diluted 1:10 000 in TBS. Membranes were then washed for more than 1 hour with TBS and developed with the enhanced chemiluminescence detection system.
Statistical analysis
Significant differences were detected using Student ttest and one-way analysis of variance, using the Prism software program (GraphPAD Software for Science, San Diego, CA).
Results
Aggregation-dependent and -independent mechanisms of PECAM-1 tyrosine phosphorylation
Human platelets contain at least 2 known ITAM-associated receptors including FcγRIIA with an intrinsic ITAM motif in its cytoplasmic domain and the collagen receptor (GP)VI, constitutively associated with the ITAM-bearing signaling molecule, FcR-γ chain.20-22In the context of platelets, PECAM-1 represents a newly assigned immunoglobulin–ITIM-bearing receptor that may serve a negative regulatory role to dampen the activation-dependent pathways induced by collagen receptors, including (GP)VI and an additional cross-linking receptor, FcγRIIA. In our initial studies, we examined whether the activation of protein tyrosine kinase-dependent signaling pathways by collagen and CRP induces tyrosine phosphorylation of the PECAM-1 cytoplasmic domain. As shown in Figure1A, stimulation of platelets with soluble collagen resulted in the time-dependent tyrosine phosphorylation of PECAM-1, which was maximal at 2 minutes. PECAM-1 tyrosine phosphorylation was observed over a range of collagen concentrations (1-20 μg/mL) (data not shown). These characteristics of induction of tyrosine phosphorylation of PECAM-1 by collagen are similar to those recently reported by Cicmil et al.23 Using CRP to stimulate the (GP)VI-coupled signaling pathway, tyrosine phosphorylation of PECAM-1 was evident by 30 seconds of stimulation (Figure 1B). These results suggest that the activation of platelets by collagen or by CRP induces tyrosine phosphorylation of PECAM-1. An important issue is whether (GP)VI-induced PECAM-1 tyrosine phosphorylation is downstream of integrin αIIbβ3 activation. Given that there has been some controversy in the literature about the mechanisms of induction of PECAM-1 tyrosine phosphorylation, we decided to use a number of αIIbβ3 blockers to examine whether tyrosine phosphorylation of PECAM-1 occurred in an aggregation-dependent and -independent mechanism.11 23 We and others have used RGDW (Arg-Gly-Asp-Trp) as an integrin αIIbβ3 blocker with different effects. In these experiments, washed platelets were pre-incubated and stirred in an aggregometer cuvette for 10 minutes at 37°C in the presence or absence of either 0.5 mM RGDW peptide, 20 μg/mL c7E3 Fab (Reopro), or 500 nM Aggrastat before CRP stimulation. In our initial studies, we observed that RGDW had minimal effect on reducing the tyrosine phosphorylation of PECAM-1 on CRP-induced platelet aggregation (Figure 1C). This was in contrast to c7E3 Fab and Aggrastat inhibitors that showed a major reduction in the tyrosine phosphorylation of PECAM-1 but did not completely block the tyrosine phosphorylation of PECAM-1 on CRP-induced platelet aggregation (Figure 1C). The RDGW CRP-treated samples showed the presence of microaggregates, but not in the c7E3 Fab and Aggrastat-treated samples, indicating the potency of inhibitors, c7E3 Fab fragment (an inhibitor of integrins αIIbβ3 and αvβ3), and Aggrastat (an inhibitor specific for integrin αIIbβ3). These results indicate the importance of integrin αIIbβ3activation and aggregation in the induction of the tyrosine phosphorylation of PECAM-1; however, the residual tyrosine phosphorylation indicates a (GP)VI-induced aggregation-independent mechanism of tyrosine phosphorylation of PECAM-1.
Aggregation-dependent and -independent mechanisms of induction of PECAM-1 tyrosine phosphorylation.
PECAM-1 was immunoprecipitated from human platelets under resting conditions or after stimulation with TRAP (7 μM); (A) collagen (10 μg/mL) (0-6 minutes); (B) CRP (2 μg/mL) (0-6 minutes); and (C) no stimulation, 2 μg/mL CRP alone for 2 minutes, 2 μg/mL CRP + 0.5 mM RGDW, 2 μg/mL CRP + 20 μg/mL c7E3 Fab (Reopro), or 2 μg/mL CRP + 500 nM Aggrastat. All αIIbβ3 blockers were pre-incubated with washed platelets for 10 minutes before CRP stimulation for 2 minutes at 37°C with stirring. Proteins were separated on SDS-PAGE and immunoblotted for anti-phosphotyrosine content using an HRP-conjugated 4G10 anti-phosphotyrosine antibody. The presence of PECAM-1 antigen was confirmed by reprobing with polyclonal anti–PECAM-1 antibody, SEW16.
Aggregation-dependent and -independent mechanisms of induction of PECAM-1 tyrosine phosphorylation.
PECAM-1 was immunoprecipitated from human platelets under resting conditions or after stimulation with TRAP (7 μM); (A) collagen (10 μg/mL) (0-6 minutes); (B) CRP (2 μg/mL) (0-6 minutes); and (C) no stimulation, 2 μg/mL CRP alone for 2 minutes, 2 μg/mL CRP + 0.5 mM RGDW, 2 μg/mL CRP + 20 μg/mL c7E3 Fab (Reopro), or 2 μg/mL CRP + 500 nM Aggrastat. All αIIbβ3 blockers were pre-incubated with washed platelets for 10 minutes before CRP stimulation for 2 minutes at 37°C with stirring. Proteins were separated on SDS-PAGE and immunoblotted for anti-phosphotyrosine content using an HRP-conjugated 4G10 anti-phosphotyrosine antibody. The presence of PECAM-1 antigen was confirmed by reprobing with polyclonal anti–PECAM-1 antibody, SEW16.
Recombinant human PECAM-1–immunoglobulin chimera induces tyrosine phosphorylation of PECAM-1
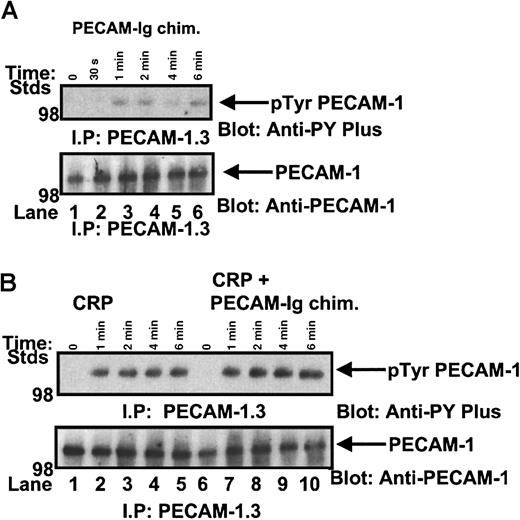
To define the signaling properties of the recombinant human PECAM-1–immunoglobulin chimera, we performed dose-response and time-course experiments of recombinant human PECAM-1–immunoglobulin chimera stimulation of IV.3-treated washed platelets under stirred conditions in the aggregometer. Platelets were lysed, PECAM-1 was immunoprecipitated, and anti-phosphotyrosine content was determined by Western blotting. As shown in Figure2A, we observed a weak induction in the tyrosine phosphorylation of PECAM-1, which was evident by 1 minute of stimulation.
Recombinant human PECAM-1–immunoglobulin chimera can induce tyrosine phosphorylation of PECAM-1.
(A) IV.3-treated washed platelets were stirred in the presence of 40 μg/mL recombinant human PECAM-1–immunoglobulin chimera for 0 to 6 minutes. Platelet lysates were precleared with Protein G–Sepharose beads, PECAM-1 immunoprecipitated, separated by SDS-PAGE, and immunoblotted for anti-phosphotyrosine content using an HRP-conjugated 4G10 anti-phosphotyrosine antibody. The presence of PECAM-1 antigen was confirmed by reprobing with polyclonal anti–PECAM-1 antibody SEW16. (B) PECAM-1 was immunoprecipitated from human platelets under resting conditions or after stimulation with CRP (2 μg/mL) (0-6 minutes) and 2 μg/mL CRP + 40 μg/mL recombinant human PECAM-1–immunoglobulin chimera (0-6 minutes). Proteins were separated on SDS-PAGE and immunoblotted for anti-phosphotyrosine content using an HRP-conjugated 4G10 anti-phosphotyrosine antibody. The presence of PECAM-1 antigen was confirmed by reprobing with polyclonal anti-PECAM-1 antibody SEW16.
Recombinant human PECAM-1–immunoglobulin chimera can induce tyrosine phosphorylation of PECAM-1.
(A) IV.3-treated washed platelets were stirred in the presence of 40 μg/mL recombinant human PECAM-1–immunoglobulin chimera for 0 to 6 minutes. Platelet lysates were precleared with Protein G–Sepharose beads, PECAM-1 immunoprecipitated, separated by SDS-PAGE, and immunoblotted for anti-phosphotyrosine content using an HRP-conjugated 4G10 anti-phosphotyrosine antibody. The presence of PECAM-1 antigen was confirmed by reprobing with polyclonal anti–PECAM-1 antibody SEW16. (B) PECAM-1 was immunoprecipitated from human platelets under resting conditions or after stimulation with CRP (2 μg/mL) (0-6 minutes) and 2 μg/mL CRP + 40 μg/mL recombinant human PECAM-1–immunoglobulin chimera (0-6 minutes). Proteins were separated on SDS-PAGE and immunoblotted for anti-phosphotyrosine content using an HRP-conjugated 4G10 anti-phosphotyrosine antibody. The presence of PECAM-1 antigen was confirmed by reprobing with polyclonal anti-PECAM-1 antibody SEW16.
This is not surprising given that in previous immunological studies, it is observed that for inhibitory signaling to occur, co-aggregation mechanisms with simultaneous engagement of the ITAM-containing receptor with the ITIM-containing receptor leads to synergy with increased tyrosine phosphorylation of the inhibitory coreceptor, recruitment of protein-tyrosine phosphates (PTPs), and negative modulation of ITAM-dependent signaling cascades.24 By applying this concept to platelets, we compared the tyrosine phosphorylation profiles of PECAM-1 following a time course of CRP stimulation alone and with simultaneous engagement of collagen (GP)VI receptor and PECAM-1 with CRP peptide and recombinant human PECAM-1–immunoglobulin chimera. In these experiments, FcγRIIA activation was blocked by IV.3 pretreatment of platelets. As shown in Figure 2B, the simultaneous engagement of collagen (GP)VI receptor and PECAM-1 led to a synergistic increase in the level of tyrosine phosphorylation of PECAM-1 over time compared to CRP stimulation alone. As we have reported previously,11 12 recruitment and activation of protein-tyrosine phosphatases, such as SHP-1 and SHP-2, occur under conditions of induction of tyrosine phosphorylation of PECAM-1. In this study, we observed SHP-2 PTP recruitment by tyrosine phosphorylated PECAM-1 (data not shown).
Regulation of collagen- and CRP-induced platelet aggregation by PECAM-1–PECAM-1 interactions
To explore the functional importance of PECAM-1 interactions on collagen and collagen-related triple-helical peptide-induced platelet aggregation, a recombinant human PECAM-1–immunoglobulin chimeric protein encompassing the 6 extracellular immunoglobulin domains of human PECAM-1 and the Fc portion of human IgG was used as a physiological PECAM-1 homophilic ligand. As controls, a mutant K89A form of recombinant human PECAM-1–immunoglobulin chimera was included that prevents homophilic PECAM-1 interactions, and human IgG was used to ensure there was no contribution by FcγRIIA activation. Further, in all these experiments, platelets were pretreated with IV.3 Fab fragments to block FcγRIIA activation. Previous studies have validated the use of a recombinant PECAM-1–immunoglobulin chimeric protein for functional studies in preference to anti-PECAM-1 antibodies because antibody recognition may not necessarily mimic a natural ligand interaction.16 To examine the effect of PECAM-1 interactions in modulating collagen-induced platelet aggregation, washed platelets or PRP (data not shown) were pre-incubated with either recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) in the presence of IV.3 Fab (10 μg/mL) followed by the induction of collagen-induced platelet aggregation. As shown in Figure3A, recombinant human PECAM-1–immunoglobulin chimera (white bars) induced a moderate dose-dependent decrease in collagen-induced platelet aggregation (22% ± 2.1% at 100 μg/mL recombinant human PECAM-1–immunoglobulin; P < .0001). This decrease in collagen-induced platelet aggregation was consistently observed over a range of collagen concentrations (40-100 μg/mL;P < .0001). In control experiments, no effect was observed even at 100 μg/mL recombinant human K89A PECAM-1–immunoglobulin chimera (black bars) or human IgG (gray bars) (Figure 3B). Using a similar approach, we examined the effect of PECAM-1 interactions in modulating CRP-induced platelet aggregation. As shown in Figure 4A, a significant dose-dependent reduction (46% ± 1.7% at 100 μg/mL recombinant human PECAM-1-immunoglobulin; P < .0001) (white bars) in CRP-induced platelet aggregation after the induction of PECAM-1–PECAM-1 interactions was observed. This decrease in CRP-induced platelet aggregation was consistently observed over a range of CRP concentrations (20-100 μg/mL; P < .0001). In control experiments, no inhibitory effect was observed with 100 μg/mL recombinant human K89A PECAM-1–immunoglobulin chimera (black bars) or human IgG (gray bars) (Figure 4B).
Effect of PECAM-1 interactions in collagen-induced platelet aggregation.
(A) Washed platelets were pre-incubated with FcγRIIA-blocking antibody IV.3 Fab (10 μg/mL) followed by recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) for 5 minutes with stirring. Platelet aggregation was initiated by the addition of collagen (5 μg/mL), and the percentage change in light transmission was monitored. Aggregation traces from 1 of 3 representative experiments are shown. (B) Washed platelets were pre-incubated with increasing doses of recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) (white bars) or mutant K89A human PECAM-1–immunoglobulin chimera (0-100 μg/mL) (black bars) or human IgG (0-100 μg/mL) (gray bars) before stimulation with collagen (5 μg/mL). These results are expressed as mean ± SD of percentage of controls (0 μg/mL recombinant protein) from 3 separate experiments with different blood donors. **Significantly differentP < .0001, according to Student unpaired ttest from 3 separate experiments.
Effect of PECAM-1 interactions in collagen-induced platelet aggregation.
(A) Washed platelets were pre-incubated with FcγRIIA-blocking antibody IV.3 Fab (10 μg/mL) followed by recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) for 5 minutes with stirring. Platelet aggregation was initiated by the addition of collagen (5 μg/mL), and the percentage change in light transmission was monitored. Aggregation traces from 1 of 3 representative experiments are shown. (B) Washed platelets were pre-incubated with increasing doses of recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) (white bars) or mutant K89A human PECAM-1–immunoglobulin chimera (0-100 μg/mL) (black bars) or human IgG (0-100 μg/mL) (gray bars) before stimulation with collagen (5 μg/mL). These results are expressed as mean ± SD of percentage of controls (0 μg/mL recombinant protein) from 3 separate experiments with different blood donors. **Significantly differentP < .0001, according to Student unpaired ttest from 3 separate experiments.
Effect of PECAM-1 interactions in collagen (GP)VI-selective ligand, CRP-induced platelet aggregation.
Washed human platelets were pre-incubated with FcγRII-blocking antibody IV.3 Fab (10 μg/mL) followed by recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) for 5 minutes with stirring. Platelets were then stimulated with collagen-related peptide (2 μg/mL). Platelet aggregation was monitored by changes in light transmission. The ordinate represents percentage changes in light transmission. Data are representative of 3 experiments. (B) Percentage CRP-induced platelet aggregation after pre-incubation with increasing doses of recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) (white bars) or mutant K89A human PECAM-1–immunoglobulin chimera (0-100 μg/mL) (black bars) or human IgG (0-100 μg/mL) (gray bars) represented by mean ± SD of the mean of 3 separate experiments with different blood donors. **Significantly differentP < .0001, according to Student unpaired ttest from 3 separate experiments.
Effect of PECAM-1 interactions in collagen (GP)VI-selective ligand, CRP-induced platelet aggregation.
Washed human platelets were pre-incubated with FcγRII-blocking antibody IV.3 Fab (10 μg/mL) followed by recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) for 5 minutes with stirring. Platelets were then stimulated with collagen-related peptide (2 μg/mL). Platelet aggregation was monitored by changes in light transmission. The ordinate represents percentage changes in light transmission. Data are representative of 3 experiments. (B) Percentage CRP-induced platelet aggregation after pre-incubation with increasing doses of recombinant human PECAM-1–immunoglobulin chimera (0-100 μg/mL) (white bars) or mutant K89A human PECAM-1–immunoglobulin chimera (0-100 μg/mL) (black bars) or human IgG (0-100 μg/mL) (gray bars) represented by mean ± SD of the mean of 3 separate experiments with different blood donors. **Significantly differentP < .0001, according to Student unpaired ttest from 3 separate experiments.
PECAM-1–deficient platelets demonstrate enhanced collagen- and CRP-induced platelet aggregation and secretion responses
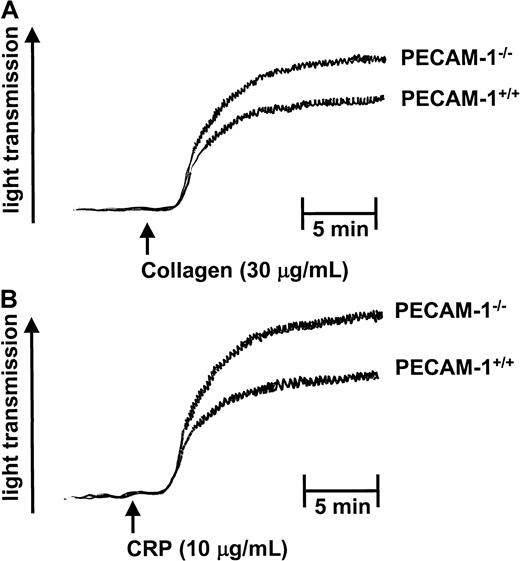
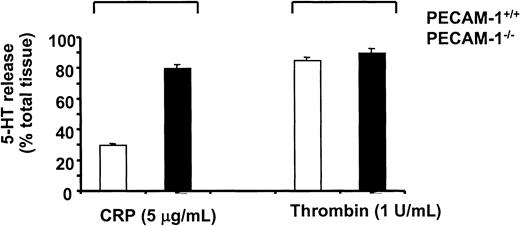
To further investigate the role of PECAM-1 in platelet function, we examined the collagen- and CRP-induced platelet aggregation responses of age- and sex-matched wild-type (PECAM-1+/+) and PECAM-1−/− platelets. As shown in Figure5, PECAM-1–deficient platelets showed enhancement in both collagen- and CRP-induced platelet aggregation responses. In these experiments, aggregation in response to soluble collagen (30 μg/mL) was not as robust with mouse platelets as with human platelets. However, this enhancement in platelet aggregation was observed for both collagen- and CRP-induced platelet aggregation over a wide range of doses (20-100 μg/mL collagen and 5-30 μg/mL CRP) (data not shown). This increase in aggregation in the absence of PECAM-1 did not result in global enhancement in platelet function because adenosine diphosphate-induced platelet aggregation was normal, consistent with previous reports15 (data not shown). Evaluation of platelet secretion responses using a 5-HT secretion assay revealed that PECAM-1–deficient platelets showed enhanced secretion with CRP but not thrombin stimulation (Figure6). Collectively, these results suggest that PECAM-1 serves to negatively regulate the ITAM-bearing pathway mediated by collagen (GP)VI-FcR γ-chain platelet interactions.
PECAM-1–deficient platelets demonstrate enhanced collagen- and CRP-induced platelet aggregation responses.
PRP from PECAM-1+/+ and PECAM-1−/− mice was stimulated with collagen (30 μg/mL) and CRP (10 μg/mL) under stirring conditions, and aggregation was monitored by changes in light transmission. The ordinate represents percentage changes in light transmission. Data are representative of 3 experiments.
PECAM-1–deficient platelets demonstrate enhanced collagen- and CRP-induced platelet aggregation responses.
PRP from PECAM-1+/+ and PECAM-1−/− mice was stimulated with collagen (30 μg/mL) and CRP (10 μg/mL) under stirring conditions, and aggregation was monitored by changes in light transmission. The ordinate represents percentage changes in light transmission. Data are representative of 3 experiments.
PECAM-1–deficient platelets demonstrate enhanced secretion on CRP but not thrombin stimulation.
Control PECAM-1+/+ (white bars) and PECAM-1–deficient (black bars) murine platelets were loaded with 5-HT and stimulated with CRP (5 μg/mL) or thrombin (1 U/mL) for 2 minutes. Platelets were pelleted by centrifugation at 13 000 rpm for 5 minutes, and 5-HT secretion into the medium was determined by scintillation spectrometry. Results are expressed as a percentage of the total tissue content after subtraction of release under basal conditions and are representative of 3 experiments.
PECAM-1–deficient platelets demonstrate enhanced secretion on CRP but not thrombin stimulation.
Control PECAM-1+/+ (white bars) and PECAM-1–deficient (black bars) murine platelets were loaded with 5-HT and stimulated with CRP (5 μg/mL) or thrombin (1 U/mL) for 2 minutes. Platelets were pelleted by centrifugation at 13 000 rpm for 5 minutes, and 5-HT secretion into the medium was determined by scintillation spectrometry. Results are expressed as a percentage of the total tissue content after subtraction of release under basal conditions and are representative of 3 experiments.
Regulation of human platelet thrombus formation on collagen under flow by PECAM-1–PECAM-1 interactions
To investigate the functional importance of PECAM-1 in regulating thrombus formation on a collagen matrix under flow conditions, we performed in vitro flow studies as described in “Materials and methods.” Whole blood was pretreated with varying concentrations of recombinant human PECAM-1–immunoglobulin chimera in the presence of FcγRIIA-blocking antibody IV.3 Fab (10 μg/mL) for 10 minutes, before perfusion through collagen-coated microslides at wall shear rates of 150 seconds−1 and 600 seconds−1. As shown in Figure 7, pre-incubation of human PECAM-1–immunoglobulin chimera induced a dose-dependent reduction in platelet thrombus formation at a wall shear rate of 150 seconds−1. This effect was also observed at a wall shear rate of 600 seconds−1 (data not shown). This reduction could not be attributed to complexed IgG alone because pre-incubation of whole blood with up to 100 μg/mL human IgG had no effect on platelet thrombus formation (data not shown).
PECAM-1 interactions negatively regulate thrombus formation on collagen under flow.
Whole human blood was pre-incubated with FcγRIIA-blocking antibody IV.3 Fab (10 μg/mL), followed by recombinant human PECAM-1–immunoglobulin chimera alone (0-100 μg/mL) for 10 minutes, before perfusion through collagen-coated microslides at a wall shear rate of 150 seconds−1. Nonadherent cells were removed by washing with Tyrode buffer, and adherent erythrocytes were lysed with 1% (vol/vol) ammonium oxalate. Adherent platelets were lysed in 1% (vol/vol) Triton X-100 and collected for analysis of platelet lactate dehydrogenase (U/mL). In control studies, up to 100 μg/mL human IgG had no effect on thrombus formation on collagen under flow (data not shown).
PECAM-1 interactions negatively regulate thrombus formation on collagen under flow.
Whole human blood was pre-incubated with FcγRIIA-blocking antibody IV.3 Fab (10 μg/mL), followed by recombinant human PECAM-1–immunoglobulin chimera alone (0-100 μg/mL) for 10 minutes, before perfusion through collagen-coated microslides at a wall shear rate of 150 seconds−1. Nonadherent cells were removed by washing with Tyrode buffer, and adherent erythrocytes were lysed with 1% (vol/vol) ammonium oxalate. Adherent platelets were lysed in 1% (vol/vol) Triton X-100 and collected for analysis of platelet lactate dehydrogenase (U/mL). In control studies, up to 100 μg/mL human IgG had no effect on thrombus formation on collagen under flow (data not shown).
PECAM-1–deficient platelets exhibit enhanced thrombus formation on collagen under physiological conditions of flow
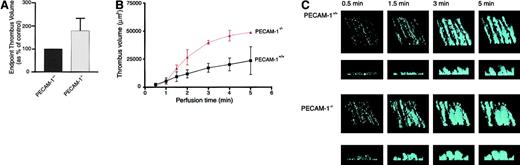
To further investigate the role of PECAM-1 in modulating platelet thrombus formation on immobilized collagen, in vitro flow studies were performed with blood obtained from either wild-type (PECAM-1+/+) or PECAM-1–deficient (PECAM-1−/−) mice. In initial studies, blood was perfused through collagen-coated microcapillary tubes at a wall shear rate of 1800 seconds−1, and thrombi were imaged after 5 minutes of blood perfusion by confocal microscopy. Volumetric analysis of individual thrombi revealed that platelets from PECAM-1–deficient mice formed significantly larger thrombi (178% ± 54%;P < .05) than wild-type platelets (Figure8A). To determine whether this increase in thrombus volume was attributed to differences in thrombus growth or stability, real-time analysis of thrombus formation was performed. For these studies, growing thrombi were imaged by confocal microscopy at various time points during blood perfusion (0.5, 1.0, 1.5, 2.0, 3.0, 4.0, and 5.0 minutes). As demonstrated in Figure 8B, the increase in thrombus volume observed in PECAM-1−/− platelets occurred in a time-dependent manner. This increase in thrombus volume in the absence of PECAM-1 appeared to result from enhanced thrombus growth because at no stage were thrombi from either PECAM-1+/+ or PECAM-1−/− platelets observed to be unstable (Figure 8C). Collectively, these results demonstrate that PECAM-1 serves as a negative physiological regulator of thrombus formation on immobilized collagen by inhibiting thrombus growth.
PECAM-1–deficient platelets form larger thrombi on a collagen matrix under physiological flow conditions.
Whole blood obtained from PECAM-1+/+ control mice or PECAM-1–deficient (PECAM-1−/−) mice were labeled with a fluorescent probe, DiOC6 (1 μM) for 10 minutes and perfused through collagen-coated (2.5 mg/mL) microcapillary tubes at a wall shear rate of 1800 seconds−1. (A) Thrombi were imaged after 5 minutes of blood flow by confocal microscopy (×100; 1-μm sections), and thrombus volume was determined by quantifying the surface area of each section using the image analysis software package ImageTool (University of Texas Health and Science Center at San Antonio) multiplied by the z height. These results are expressed as a percentage of PECAM-1+/+ controls (mean ± SEM;P < .05; n = 15). (B, C) Thrombi were imaged at 0.5-, 1.0-, 1.5-, 2.0-, 3.0-, 4.0-, and 5.0-minute time points of blood perfusion by confocal microscopy (×100; 1-μm sections), and thrombus volume was analyzed (B). Results presented in panel B show calculated thrombus volume versus perfusion time for PECAM-1+/+(■) (solid line) and PECAM-1−/− (▴) (dashed line) platelets represented as the mean ± SEM from an experiment performed using the blood of 4 individual mice. (C) Representative thrombi were reconstructed in 3-D using Voxblast image analysis software package (Vaytek). The upper panel represents an oblique view of the thrombi, illustrating thrombi surface coverage, and the lower panel represents a cross-section of the thrombi, illustrating thrombi height. All confocal images were taken from planes equidistant from the microcapillary inlet.
PECAM-1–deficient platelets form larger thrombi on a collagen matrix under physiological flow conditions.
Whole blood obtained from PECAM-1+/+ control mice or PECAM-1–deficient (PECAM-1−/−) mice were labeled with a fluorescent probe, DiOC6 (1 μM) for 10 minutes and perfused through collagen-coated (2.5 mg/mL) microcapillary tubes at a wall shear rate of 1800 seconds−1. (A) Thrombi were imaged after 5 minutes of blood flow by confocal microscopy (×100; 1-μm sections), and thrombus volume was determined by quantifying the surface area of each section using the image analysis software package ImageTool (University of Texas Health and Science Center at San Antonio) multiplied by the z height. These results are expressed as a percentage of PECAM-1+/+ controls (mean ± SEM;P < .05; n = 15). (B, C) Thrombi were imaged at 0.5-, 1.0-, 1.5-, 2.0-, 3.0-, 4.0-, and 5.0-minute time points of blood perfusion by confocal microscopy (×100; 1-μm sections), and thrombus volume was analyzed (B). Results presented in panel B show calculated thrombus volume versus perfusion time for PECAM-1+/+(■) (solid line) and PECAM-1−/− (▴) (dashed line) platelets represented as the mean ± SEM from an experiment performed using the blood of 4 individual mice. (C) Representative thrombi were reconstructed in 3-D using Voxblast image analysis software package (Vaytek). The upper panel represents an oblique view of the thrombi, illustrating thrombi surface coverage, and the lower panel represents a cross-section of the thrombi, illustrating thrombi height. All confocal images were taken from planes equidistant from the microcapillary inlet.
Discussion
These studies demonstrate a key role for PECAM-1 in negatively regulating collagen-induced platelet activation. Using 2 distinct but complementary approaches, we have examined the functional role of PECAM-1 in the context of collagen- and CRP-induced platelet aggregation and secretion and platelet thrombus formation under flow conditions. Our study demonstrates that in both human and mouse models, PECAM-1 acts as a negative regulator of both collagen and CRP-induced platelet aggregation (Figures 3, 4, and 5), CRP-induced dense granule secretion (Figure 6) and platelet thrombus formation on collagen under flow (Figures 7 and 8). Our study provides a key role for a naturally occurring inhibitory receptor, PECAM-1 that serves as a negative regulator of platelet-collagen interactions under physiological flow conditions (Figure 8A-C).
Previous studies in B cells have demonstrated that PECAM-1 is able to inhibit ITAM-mediated receptor activation events.14Platelets contain 2 ITAM-bearing receptors, the (GP)VI-associated FcR γ-chain and the FcγRIIA. Because the function of FcγRIIA in platelets is unclear, we took steps to exclude the possible compounding effect of this receptor. In the first instance, we used an antibody (IV.3) to block IgG binding to FcγRIIA in studies examining the effect of the PECAM-1–immunoglobulin chimera on human platelets. In the second instance, we used a mutant K89A form of recombinant PECAM-1–immunoglobulin chimera, and human IgG were used as controls to demonstrate that these proteins had no effect on platelet function. Furthermore, we used mouse platelets that lack the human genetic equivalent of the FcγRIIA while retaining the FcR γ-chain. In each case, we showed that PECAM-1 is capable of mediating an inhibitory signal that down-regulates collagen-induced signaling events, suggesting that this effect was through the (GP)VI-associated FcR γ-chain and not through FcγRIIA.
Although PECAM-1 was originally identified as expressed in platelets more than 10 years ago, its functional importance has been unclear. In previous studies, it was shown on platelet activation and spreading that PECAM-1 redistributes toward the platelet granulomere and that a subset of PECAM-1 remains at points of platelet–platelet contact.25 These PECAM-1 molecules at sites of platelet contact did not appear to play a role in platelet cohesion, as evidenced by the fact that Glanzmann thrombasthenia platelets do not aggregate despite having a full complement of PECAM-1.25Because no patients have been reported with qualitative or quantitative defects in PECAM-1, the availability of primary PECAM-1–deficient platelets provided us with an opportunity to directly test its involvement in platelet function. Initial assessment of PECAM-1–deficient mice demonstrated that megakaryocyte and platelet production were normal and that no abnormality was detected in adenosine diphosphate-induced platelet aggregation.15PECAM-1–deficient mice display a prolonged bleeding time that has been attributed to a vascular defect rather than a platelet abnormality because irradiation of PECAM-1–deficient mice and reconstitution of the hematopoietic cell compartment containing PECAM-1–positive platelets did not correct the prolonged bleeding time.26
Our study demonstrates that in the absence of PECAM-1, platelets are hyperresponsive to collagen and (GP)VI-selective agonist CRP but not to thrombin. These findings suggest that PECAM-1 primarily acts as a regulator of a tyrosine kinase-dependent pathway and not a G-protein–coupled pathway (Figures 5, 6). Although our studies provide evidence that PECAM-1 acts as a negative regulator of ITAM-associated collagen (GP)VI-FcR γ-chain dependent pathway, studies are under way to define the mechanism of negative regulation of collagen-induced platelet activation. Based on our previous studies11-14 in the context of B lymphocytes, we propose the following model. Co-engagement of platelet-collagen receptor adhesion and activation with PECAM-1 is followed by recruitment of the protein-tyrosine phosphatases SHP-1 and SHP-2 by tyrosine-phosphorylated PECAM-1. Formation of these signaling complexes leads to down-modulation of collagen (GP)VI-FcR γ-chain tyrosine kinase signaling events that culminate in dense granule platelet secretion and aggregation. Previous studies have demonstrated that the recruitment and activation of protein-tyrosine phosphatases SHP-1 and SHP-2 by activated ITIM-bearing receptors leads to dephosphorylation of substrates such as protein-tyrosine kinases, ITAM motifs, and linker for activation of T cells, all of which are components of ITAM-associated activation pathways.27
A recent study by Pasquet et al28 demonstrates that platelets derived from moth-eaten viable mice with a catalytically inactive protein-tyrosine phosphatase, SHP-1 (which retains approximately 20% normal activity), show hypo-responsiveness rather than hyper-responsiveness to (GP)VI-induced signaling and may, in fact, potentiate activation through (GP)VI. These data could be complicated by the residual 20% normal SHP-1 activity; therefore, further studies on me/me mice, which are completely devoid of SHP-1, will be required to define the role of SHP-1 in (GP)VI-induced signaling events. These studies appear to conflict with a potential role for SHP-1 in PECAM-1–inhibitory signaling to down-modulate platelet collagen interactions. An alternative hypothesis we have proposed is that PECAM-1 may exert a negative regulatory role on (GP)VI-induced signaling by SHP-2 and not SHP-1. SHP-2 has been implicated in both positive and negative signaling pathways, depending on the cell type and the signaling complexes formed.29 Additional studies are required to more precisely define this inhibitory signaling mechanism in platelets.
Our studies have also shown an important role for PECAM-1 in regulating thrombus formation on immobilized collagen under flow. Adhesion to collagen under rapid blood flow conditions requires an initial vWF-GPIb/IX/V–mediated platelet tethering that then allows integrin α2β1 to bind and mediate stable adhesion to collagen.30 Once the platelet is immobilized, (GP)VI is able to interact with the collagen matrix and, together with integrin α2β1, to initiate intracellular signaling pathways to activate integrin αIIbβ3. At present, it is unknown which of these collagen receptors is the most potent stimulator of platelets. However, a recent analysis of (GP)VI-deficient platelets under flow has suggested that (GP)VI plays a major role in inducing platelet activation and thrombus growth.31 Based on our observations of PECAM-1–deficient platelets under flow, we predict that PECAM-1 serves a functional role in the negative regulation of stationary adhesion and not in the initial tethering and rolling of platelets on the thrombogenic collagen surface. This hypothesis is supported by previous observations in which different anti-murine PECAM-1 monoclonal antibodies delayed platelet adhesion–aggregation at sites of endothelium injury in mouse cerebral arterioles.32-34 A recent study by Vollmer et al35 demonstrated no change in in vivo vascular thrombosis in PECAM-1–deficient mice compared to wild-type control mice. In this study, thrombus formation was initiated by a photochemical reaction. Under these conditions, it is difficult to know whether the damage to the subendothelial matrix of the blood vessel would expose types I and III collagen, which are deep in the wall of the blood vessel. Types VI and IV collagen are likely to be exposed by the photochemical injury, but because type VI collagen is a weak stimulus, its role in thrombus growth is unclear. We want to emphasize that in our study we examined platelet–platelet interactions with immobilized type I fibrillar collagen, a strong stimulus of thrombus growth under physiological conditions of flow, and we observed a distinct increase in thrombus growth in the absence of PECAM-1. Based on these observations, further studies will be required to more precisely define the mechanisms by which PECAM-1 regulates the growth of thrombi in vivo.
In conclusion, our studies demonstrate that PECAM-1 elicits a negative regulatory role in collagen-platelet interactions, particularly involving ITAM-associated collagen (GP)VI receptor-Fc γ-chain activation events that culminate in dense granule platelet secretion and aggregation. A recent report by Patil et al,36published during review of our manuscript, confirms an important role of PECAM-1 in mouse platelets. Few examples of naturally occurring inhibitors of platelet-collagen interactions have been described. Prostacyclin and nitric oxide represent natural inhibitors released from the endothelium, but until now there has been no evidence of a natural inhibitor that serves an autoregulatory role when platelets come into contact with each other after exposure to collagen. Our studies provide strong evidence that PECAM-1–PECAM-1 interactions participate in an autoregulatory mechanism to negatively regulate collagen-induced platelet activation. Given the importance of collagen in promoting platelet adhesion and thrombus formation in vivo, activators of PECAM-1 may provide a novel therapeutic approach to prevent arterial thrombotic diseases.
We thank Prof Peter Newman for supplying anti–PECAM-1 antibodies and recombinant human PECAM-1–immunoglobulin chimeras. We thank Dr Chris Buckley for supplying the mutant K89A human PECAM-1–immunoglobulin chimera. We also thank Dr Tak Mak and Dr Gordon Duncan (Amgen Institute, University of Toronto, Ontario, Canada) for kindly providing the PECAM-1–deficient mice.
Supported by National Heart Foundation grant G00A 0517 (D.E.J.) and by National Health and Medical Research Council of Australia grant 129700 (D.E.J.). D.E.J. is the recipient of an NHMRC RD Wright Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Denise E. Jackson, Division of Haematology, Hanson Centre for Cancer Research, IMVS, Frome Road, Adelaide; Australia 5000; e-mail: denise.jackson@imvs.sa.gov.au.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal