Erythropoiesis occurs in 2 distinct waves during embryogenesis: the primitive wave in the extra-embryonic yolk sac (YS) followed by the definitive wave in the fetal liver and spleen. Even though progenitors for both cell types are present in the YS blood islands, only primitive cells are formed in the YS during early embryogenesis. In this study, it is proposed that erythropoietin (Epo) expression and the resultant EpoR activation regulate the timing of the definitive wave. First, it was demonstrated that Epo and EpoR gene expressions are temporally and spatially segregated: though EpoR is expressed early (embryonic days 8.0-9.5) in the yolk sac blood islands, no Epo expression can be detected in this extra-embryonic tissue. Only at a later stage can Epo expression be detected intra-embryonically, and the onset of Epo expression correlates with the initiation of definitive erythropoiesis. It was further demonstrated that the activation of the EpoR signaling pathway by knocking-in a constitutively active form of EpoR (R129C EpoR) can lead to earlier onset of definitive erythropoiesis in the YS. Thus, these results provide the first in vivo mechanism as to how 2 erythroid progenitor populations can coexist concurrently in the YS yet always differentiate successively during embryogenesis.
Introduction
During embryogenesis, erythropoiesis consists of the sequential appearance of 2 distinct populations of erythrocytes: primitive erythroid cells (EryP) and definitive erythroid cells (EryD).1-3 In the mouse, EryPs appear first in the yolk sac (YS) blood islands at embryonic day 7.5 (E7.5). EryPs are large and nucleated, and they mainly express the embryonic globin genes (ε and βH1). EryDs predominate in the fetal liver after E12.5. EryD are smaller and enucleated, and they express the adult globin genes (β major).
Although primitive and definitive erythroid progenitors are present in the extra-embryonic yolk sac, no definitive erythrocytes are formed in this compartment before the commencement of fetal circulation. This raised the interesting question as to why primitive and definitive erythroid progenitor populations can coexist concurrently in the YS yet always differentiate successively during embryogenesis. No mechanism explains why primitive erythropoiesis peaks before definitive erythropoiesis when the YS is clearly competent to produce both populations of erythrocytes. We hypothesized that there may be an important regulatory factor crucial for triggering definitive erythroid progenitors to undergo terminal differentiation and produce mature EryDs. Furthermore, if the factor regulating this process were a positive or a stimulatory signal, it should be absent early during YS erythropoiesis and present later during intra-embryonic definitive erythropoiesis.
In this study, we show that this regulatory factor is erythropoietin (Epo). Before the onset of fetal circulation (E8.0), cells positive for EpoR expression are present in the YS blood islands and are undetectable in the embryo proper. However, no Epo expression can be detected in either YS or embryo proper at this stage. Right after the commencement of fetal circulation, both Epo and EpoR are expressed in the fetal liver rudiment and the aorta–gonado–mesonephras areas, regions known to be involved in definitive erythropoiesis. Because the initiation of primitive erythropoiesis is Epo independent whereas definitive erythropoiesis is Epo dependent, a cellular mechanism is provided for how the temporal and spatial expression of Epo and EpoR genes in early embryogenesis coordinate the appearance of the 2 waves of erythropoiesis. We also show that prematurely activating the EpoR by genetic knock-in of a constitutively active form of EpoR into the EpoR locus is sufficient to trigger earlier definitive erythropoiesis in the YS in vivo. Thus, our results suggest definitive erythroid progenitor cells can coexist with primitive erythroid progenitor cells and yet fail to contribute to YS erythropoiesis during earlier embryonic development because they lack Epo stimulation.
Materials and methods
Animals and organ culture
Mice with deletions in Epo and EpoR genes have been described,4 and staged embryos were obtained by mating between heterozygous animals. Embryos were staged according to criteria previously described.5 Yolk sacs were dissected and cultured separately on 0.1 μm Nucleopore membrane (Corning, Corning, NY) resting on a sterile wire mesh at the air–media interface. Dulbecco modified Eagle medium plus 15% fetal calf serum supplemented with or without 2 U/mL huEpo (Amgen, Thousand Oaks, CA) was used and changed every 1 to 2 days. Cultures were maintained for up to 7 days.
Cytology
Yolk sacs were individually washed in phosphate-buffered saline (PBS) twice and incubated with 1% collagenase (Sigma, St Louis, MO) in 10% fetal calf serum and Iscoves modified Dulbecco medium for 30 minutes at 37°C. After digestion, YS cells were drawn through a 23-gauge needle and pelleted at 400g for 10 minutes.6 Cells were resuspended in PBS, cytospun onto glass slides, and stained with a Wright-Giemsa stain as recommended by the manufacturer (Biochemical Sciences, Pittsburgh, PA).
In situ hybridization
RNA nonradioactive whole-mount in situ hybridization technique was performed as previously described.7Histologic sections were cut at 7-μm increments and visualized by Normaski optics. Mouse Epo cDNA used for the generation of the RNA probe is a BamH1/EcoR1 500-bp fragment corresponding to exons 4 and 5. Mouse EpoR probe is aXhoI/ HindIII 949-bp fragment corresponding to nt 270-1219 of the EpoR cDNA. Mouse ε and β major globin probes were derived by polymerase chain reaction amplified from primers specific to their cDNA sequences8 and subcloned in pBluescript (Stratagene, La Jolla, CA) for RNA probe synthesis.
X-Gal staining
Isolated embryos were fixed (2% formaldehyde and 0.2% glutaraldehyde in PBS) for 30 minutes at 4°C. After extensive washes with cold PBS, the embryos were stained in the X-Gal solution (5 mM K3Fe3[CN]6, 5 mM K4Fe[CN]6 · 3H2O, 2 mM MgCl2 and 1 mg/mL X-Gal [5-bromo-4chloro-3-indoxyl-b-D-galactopyranoside in 70% dimethyl formamide] in PBS) for 2 hours at 37°C.
Generation of knock-in vectors and chimeric mice
The targeting vector pEpoR-M2 was constructed by replacing the XhoI-ClaI genomic fragment with the hygro/tk selection cassette. pEpoR-R129C vector was generated by replacingXhoI-ClaI genomic fragment with the corresponding cDNA fragment carrying the R129C mutation. The double-replacement strategy has been described.9 Generation of chimeric mice was performed as previously described.4
Results
Developmental stage-specific functions of Epo and EpoR in erythropoiesis
Others and we have introduced null mutations into either Epo4 or EpoR4,10,11 genes. Studies of EpoR and Epo homozygous-null mice show impaired definitive erythropoiesis leading to anemia and embryonic lethality at E13.5, indicating that Epo and EpoR are crucial for definitive erythropoiesis in vivo in the fetal liver stage. Epo-dependent and Epo-responsive erythroid progenitors were present in homozygous fetal livers4,11 and YS (Lin et al11 and R.L. et al, unpublished results, August, 1998). Thus, neither Epo nor EpoR is required for erythroid lineage commitment or for the generation of definitive erythroid progenitors. Epo and EpoR are crucial in vivo for the proliferation and survival of the definitive progenitors and for their irreversible terminal differentiation.
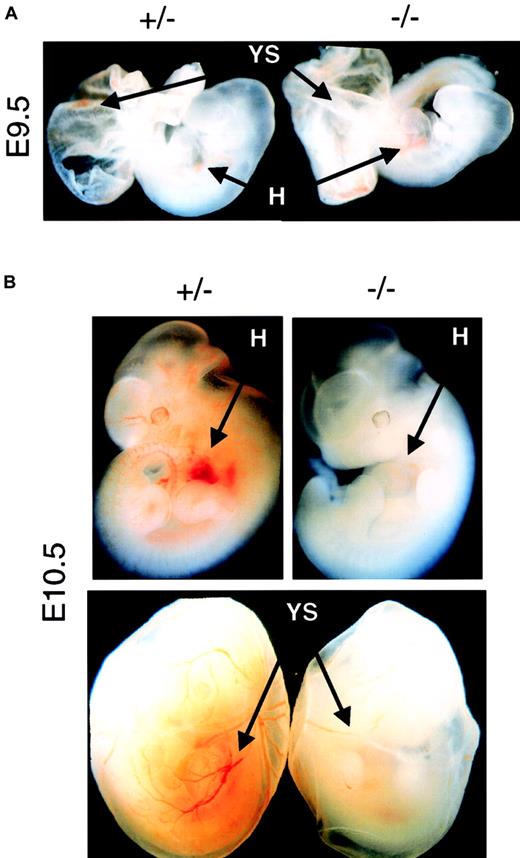
On the other hand, primitive erythropoiesis is not strictly dependent on Epo to initiate and to complete its differentiation program. No defect on the EryP erythropoiesis could be observed at E8.5 (data not shown), before the onset of fetal circulation, or at E9.5 (Figure1A), right after the commencement of fetal circulation, in Epo−/− or EpoR−/−embryos. However, 1 day later at E10.5, both Epo−/− and EpoR−/− began to show reduced levels of EryP in the embryo and YS (Figure 1B). Quantitative analysis indicated that there was a 5- to 10-fold reduction in the amount of EryP produced in E10.5 to E12.5 null embryos, which is consistent with results of previous studies.11 Because the onset of the EryP defect happens right after the commencement of blood circulation, it suggests that the function of Epo in EryP is developmental stage dependent and blood circulation related. Before fetal circulation, the initiation and differentiation of EryP is Epo-independent; after the commencement of fetal circulation, the expansion or survival12 of the EryP is Epo-dependent. Thus, Epo most likely begins to exert its physiological function between E9.5 and E10.5, a stage after the initiation of YS primitive erythropoiesis but before the onset of fetal liver definitive erythropoiesis.
Defective erythropoiesis in Epo−/− and EpoR−/− embryos.
(A) Comparing the amount of red blood cells in the heart (H) and yolk sac (YS) regions of E9.5 mutant embryos (−/−) with age-matched control embryos (+/− ). Normal levels of EryP can be detected in both types of mutant embryos. Original magnification × 10. (B) Epo−/− or EpoR−/− embryos isolated at E10.5 or later showed profound decreases in levels of erythropoiesis in both the embryo proper and the yolk sac. Epo and EpoR null mutants had identical erythropoietic phenotypes at all stages studied. Original magnification × 10.
Defective erythropoiesis in Epo−/− and EpoR−/− embryos.
(A) Comparing the amount of red blood cells in the heart (H) and yolk sac (YS) regions of E9.5 mutant embryos (−/−) with age-matched control embryos (+/− ). Normal levels of EryP can be detected in both types of mutant embryos. Original magnification × 10. (B) Epo−/− or EpoR−/− embryos isolated at E10.5 or later showed profound decreases in levels of erythropoiesis in both the embryo proper and the yolk sac. Epo and EpoR null mutants had identical erythropoietic phenotypes at all stages studied. Original magnification × 10.
Epo and EpoR are differentially expressed
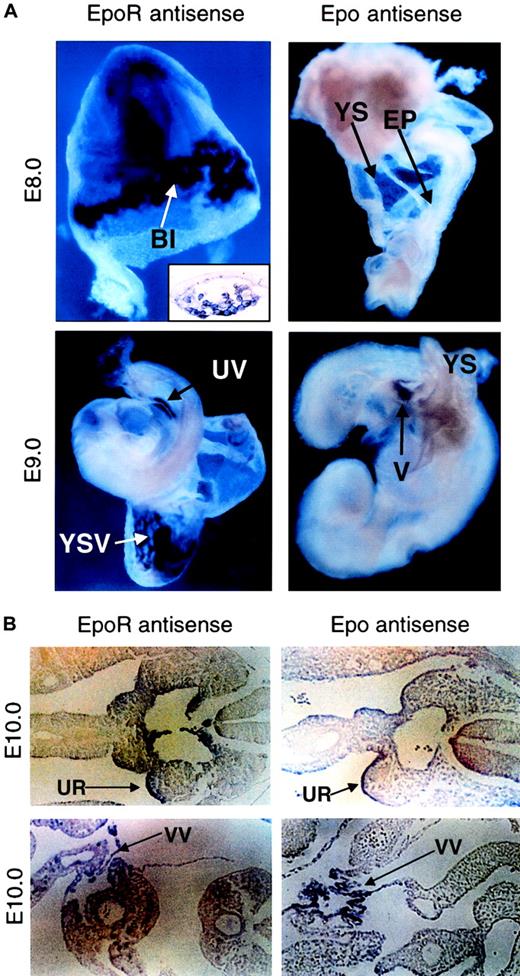
Temporally and spatially controlled gene expression plays a critical role in correct developmental. To answer why Epo and EpoR function in a developmental stage-specific manner, we surveyed the expression patterns of Epo and EpoR genes during early embryogenesis using nonradioactive whole-mount RNA in situ hybridization analysis. EpoR expression starts as early as E8.0 within YS blood islands (Figure2A, upper left and inset),13,14 but it remains undetectable in the embryo proper until E9.0, when it can be observed in the umbilical vein (Figure 2A, lower left). In contrast, Epo expression is never detected in the yolk sac and is absent in the embryo proper until E9.0, when it first appears in the vitelline (Figure 2A, right). Approximately 1 day later (E10), both EpoR and Epo expression can be detected in the urogenital ridge (Figure 2B, top) and vitelline vessels leading to the hepatic primordium (Figure 2B, bottom), regions known to be important for the initiation of definitive erythropoiesis. This temporal and spatial segregation of Epo and EpoR expression during early embryogenesis suggests that EpoR-positive, thus Epo-responsive, precursors are present in the extra-embryonic yolk sac before circulation (Cudennec et al15; Paul et al16; Wong et al17; and R.L. et al, unpublished data, August, 1998) but are quiescent because Epo is unavailable. This explains the observation that though mature EryDs cannot be found in vivo in the YS before blood circulation, EryD progenitors can be detected in the YS through in vitro analysis when supplemented with exogenous Epo.16-18 In contrast, the initiation and differentiation of primitive erythropoiesis is Epo independent and can proceed immediately to establish the first wave of erythropoiesis in the YS. Only when Epo is expressed later in the embryo proper can EryP cells be expanded and can definitive erythroid progenitors be stimulated to begin the second wave. Thus, these results provide evidence that the temporal and spatial expressions of the Epo and EpoR genes play a critical role in erythropoietic timing during embryogenesis. However, is premature EpoR activation sufficient to induce earlier definitive erythropoiesis in vivo?
Epo and EpoR gene expression are spatially and temporally segregated.
(A) Expression of EpoR and Epo at E8.0 (upper panel) and at E9.0 (lower panel), as detected by whole-mount in situ hybridization. EpoR expression starts in the blood islands of the yolk sac (top left). Histologic sections confirmed that EpoR staining is confined to the cells in the blood islands of the yolk sac (inset). At E9.0, EpoR expression can be observed in the vasculature of the yolk sac and in the embryo proper (lower left). No Epo expression can be detected in the yolk sac or the embryo proper at E8.0 or earlier (top right). Epo expression starts at approximately E9.0, when staining is present in the vitelline of the embryo proper but is not present in the yolk sac (lower right). Original magnifications, all main panels, × 10; original magnification, inset, × 40. (B) Histologic section showing EpoR and Epo expression present in the urogenital ridges in the AGM region of the embryo proper at E10.0 (upper panels). Positive staining of EpoR and Epo can also be observed in the vitelline vessels leading to the hepatic primordium (lower panels). Sense strand probes derived from either Epo or EpoR were used on littermate controls, and nonspecific binding was not observed (data not shown). Original magnifications, all panels, × 20. BI indicates yolk sac blood islands; YS, yolk sac; EP, embryo proper; UV, umbilical vein; YSV, vasculature of the yolk sac; V, vitelline; UR, urogenital ridge; VV, vitelline vessel.
Epo and EpoR gene expression are spatially and temporally segregated.
(A) Expression of EpoR and Epo at E8.0 (upper panel) and at E9.0 (lower panel), as detected by whole-mount in situ hybridization. EpoR expression starts in the blood islands of the yolk sac (top left). Histologic sections confirmed that EpoR staining is confined to the cells in the blood islands of the yolk sac (inset). At E9.0, EpoR expression can be observed in the vasculature of the yolk sac and in the embryo proper (lower left). No Epo expression can be detected in the yolk sac or the embryo proper at E8.0 or earlier (top right). Epo expression starts at approximately E9.0, when staining is present in the vitelline of the embryo proper but is not present in the yolk sac (lower right). Original magnifications, all main panels, × 10; original magnification, inset, × 40. (B) Histologic section showing EpoR and Epo expression present in the urogenital ridges in the AGM region of the embryo proper at E10.0 (upper panels). Positive staining of EpoR and Epo can also be observed in the vitelline vessels leading to the hepatic primordium (lower panels). Sense strand probes derived from either Epo or EpoR were used on littermate controls, and nonspecific binding was not observed (data not shown). Original magnifications, all panels, × 20. BI indicates yolk sac blood islands; YS, yolk sac; EP, embryo proper; UV, umbilical vein; YSV, vasculature of the yolk sac; V, vitelline; UR, urogenital ridge; VV, vitelline vessel.
Introducing a constitutively active form of EpoR (EpoRR129C) into the endogenous EpoR locus
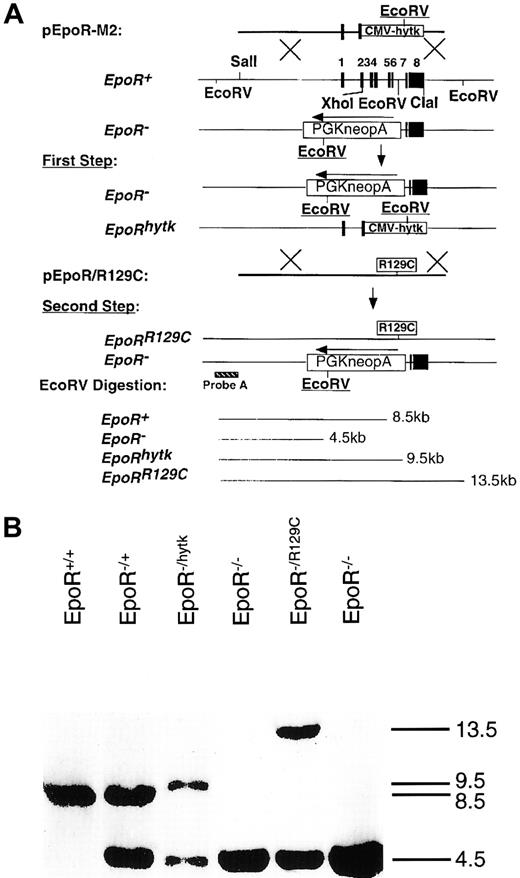
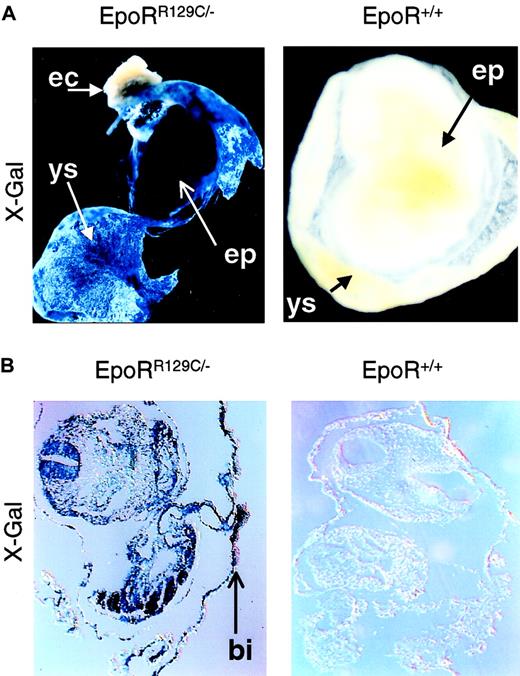
To test whether Epo is sufficient to induce definitive erythropoiesis in vivo, we had to express Epo or activate EpoR earlier in YS to demonstrate a concordant early appearance of EryD. For this, we knocked-in a constitutively active form of EpoR (EpoRR129C)19 into the endogenous EpoR locus using the double-replacement strategy9 (Figure3A) and confirmed it by Southern blot analysis (Figure 3B). This mutant form of EpoR consists of an arginine-to-cysteine substitution at amino acid position 129, resulting in intermolecular disulfide bond formation and ligand-independent EpoR activation.20 We have confirmed the function of the targeting construct pEpoR/R129C in Epo-dependent HCD57 cells before gene targeting (data not shown). Embryonic stem cells used for the knock-in procedure were EpoR+/− blue embryonic stem (ES) cells21 derived from EpoR+/− X ROSAβ-geo11+/+ mice, which allow β-galactosidase gene expression in every lineage derived from the ES cells, including mature erythrocytes.22 X-Gal staining of chimeric embryos derived from blastocysts injected with EpoRR129C/− ES cells showed high levels of chimerism in intra-embryonic (EP) and extra-embryonic tissues (YS), especially in the blood islands (Figure 4, left panels). No X-Gal–positive staining could be detected in noninjected, stage-matched embryos (Figure 4, right panels).
Introducing the constitutively active mutant of EpoR into the endogenous EpoR locus.
(A) Targeting of EpoRR129C mutation into the endogenous EpoR locus was performed using a double replacement. In the first step, pEpoR-M2 construct was used to replace the WT allele of the EpoR to generate EpoRhytk/− ES cells. For the second step, pEpoRR129C construct was electroporated into the EpoRhytk/− ES to replace the EpoRhytk allele. Predicted restriction fragments by EcoRV digestion were indicated. (B) Southern blot analysis for knock-in event. Genomic DNA was isolated and digested with EcoRV. Southern blot was probed with an external probe A.
Introducing the constitutively active mutant of EpoR into the endogenous EpoR locus.
(A) Targeting of EpoRR129C mutation into the endogenous EpoR locus was performed using a double replacement. In the first step, pEpoR-M2 construct was used to replace the WT allele of the EpoR to generate EpoRhytk/− ES cells. For the second step, pEpoRR129C construct was electroporated into the EpoRhytk/− ES to replace the EpoRhytk allele. Predicted restriction fragments by EcoRV digestion were indicated. (B) Southern blot analysis for knock-in event. Genomic DNA was isolated and digested with EcoRV. Southern blot was probed with an external probe A.
Generation of chimeric embryos with EpoRR129C/− ES cells.
EpoRR129C/− ES cells were injected into the early blastocysts. X-Gal staining of chimeric embryos shows high level of chimerism in both intra-embryonic (EP, embryo proper) and extra-embryonic tissue (YS, yolk sac) (top left). No expression is apparent in maternally derived tissues such as in the ectoplacental cone (EC). Histologic sections confirmed the presence of specific blue staining in the EpoRR129C/− embryos, especially in the YS blood islands (BI, bottom left). No X-Gal staining can be detected in stage-matched, noninjected chimeric embryos (right). Original magnifications, ×10.
Generation of chimeric embryos with EpoRR129C/− ES cells.
EpoRR129C/− ES cells were injected into the early blastocysts. X-Gal staining of chimeric embryos shows high level of chimerism in both intra-embryonic (EP, embryo proper) and extra-embryonic tissue (YS, yolk sac) (top left). No expression is apparent in maternally derived tissues such as in the ectoplacental cone (EC). Histologic sections confirmed the presence of specific blue staining in the EpoRR129C/− embryos, especially in the YS blood islands (BI, bottom left). No X-Gal staining can be detected in stage-matched, noninjected chimeric embryos (right). Original magnifications, ×10.
Early EpoR activation is sufficient to promote early definitive erythropoiesis
We then asked whether prematurely activating the EpoR signaling pathway alone would induce earlier expression of EryD-specific genes, such as adult β major globin. E8.25 chimeric embryos were isolated and tested for adult β major globin expression by in situ hybridization. High levels of β major globin expression could be detected in the YS blood islands of embryos injected with EpoRR129C/− ES cells (Figure5A, upper left and lower panels). In contrast, there was no β major expression in embryos injected with wild-type (WT) blue ES cells, EpoR−/− blue ES cells, or noninjected controls (Figure 5A, upper right). There was no positive staining in samples hybridized with sense probe (data not shown). This demonstrates that premature activation of EpoR in erythroid progenitors is sufficient to induce early β major expression in the YS.
Constitutively active form of EpoR induces premature expression of the adult β major globin gene and production of EryD.
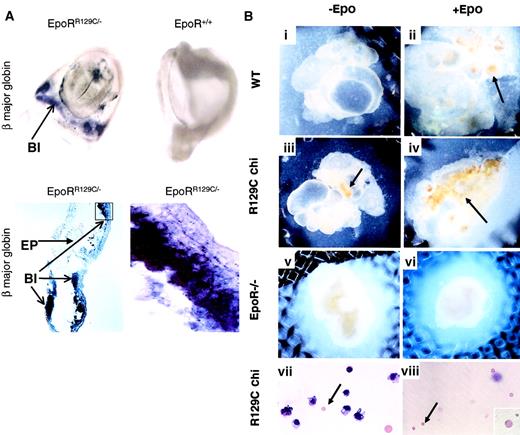
(A) E8.25 chimeric embryos injected with EpoRR129C/− ES cells show high level of adult β major globin expression in the YS blood islands (BI) by whole-mount in situ hybridization (top left). In contrast, β major expression is absent in embryos injected with WT or EpoR−/− blue ES cells or stage-matched noninjected controls (top right). Original magnifications, top panels, ×10. Histologic sections from EpoRR129C/− chimeric embryos show positive staining of β major expression in the blood islands (lower panels). No signal can be detected in the embryonic tissues (EP) or when probes derived from sense strand of β major globin cDNA fragment were used on matching samples (data not shown). Original magnification, bottom left, × 20; bottom right, × 100. (B) In vitro organ culture experiments (i-viii). E8 YS isolated from chimeric (chi) embryos injected with EpoRR129C/− ES cells were cultured with or without Epo (iii-iv). Epo-independent erythropoiesis (arrows) could be observed in cultures even without Epo (iii). When cultured YS in (iii) were dissociated and stained with Wright-Giemsa, the presence of enucleated erythrocytes (arrow), corresponding to mature definitive RBCs, was clearly evident. As controls, no mature erythrocytes were formed in the WT YS cultures without exogenous Epo (i), and no erythropoiesis could be detected when EpoR−/− YS were parallel cultured (v-vi). E8.5 YS from chimeric embryos without culturing also contained mature EryDs (viii). Original magnifications, panels i-vi, × 10; panels vii-viii, × 20; 2 insets, × 100.
Constitutively active form of EpoR induces premature expression of the adult β major globin gene and production of EryD.
(A) E8.25 chimeric embryos injected with EpoRR129C/− ES cells show high level of adult β major globin expression in the YS blood islands (BI) by whole-mount in situ hybridization (top left). In contrast, β major expression is absent in embryos injected with WT or EpoR−/− blue ES cells or stage-matched noninjected controls (top right). Original magnifications, top panels, ×10. Histologic sections from EpoRR129C/− chimeric embryos show positive staining of β major expression in the blood islands (lower panels). No signal can be detected in the embryonic tissues (EP) or when probes derived from sense strand of β major globin cDNA fragment were used on matching samples (data not shown). Original magnification, bottom left, × 20; bottom right, × 100. (B) In vitro organ culture experiments (i-viii). E8 YS isolated from chimeric (chi) embryos injected with EpoRR129C/− ES cells were cultured with or without Epo (iii-iv). Epo-independent erythropoiesis (arrows) could be observed in cultures even without Epo (iii). When cultured YS in (iii) were dissociated and stained with Wright-Giemsa, the presence of enucleated erythrocytes (arrow), corresponding to mature definitive RBCs, was clearly evident. As controls, no mature erythrocytes were formed in the WT YS cultures without exogenous Epo (i), and no erythropoiesis could be detected when EpoR−/− YS were parallel cultured (v-vi). E8.5 YS from chimeric embryos without culturing also contained mature EryDs (viii). Original magnifications, panels i-vi, × 10; panels vii-viii, × 20; 2 insets, × 100.
To confirm that β major expression detected in the chimeric yolk sac was caused by EryD production rather than activation of β major globin expression in EryP,23 we conducted 2 experiments. First, we used an in vitro organ culture system8,15 24 to test whether EryD progenitors derived from EpoRR129C/− ES can give rise to mature EryDs independent of Epo stimulation. E8.0 YS from chimeric or control embryos were carefully dissected and cultured separately, but intact, in the presence or absence of Epo. Using yolk sacs derived from E8.0 is particularly important because this time point precedes the onset of fetal circulation. Samples isolated after circulation begins could possibly receive Epo produced intra-embryonically. No significant erythropoiesis could be detected in wild-type samples without supplement of exogenous Epo (Figure 5Bi). In the presence of Epo, cultured WT yolk sac showed a clear burst of erythropoiesis, starting 4 to 5 days and peaking after 7 days in culture (Figure 5Bii). In contrast, erythropoiesis could be detected in EpoRR129C/− YS cultures without exogenous Epo (Figure 5Biii). Furthermore, when these cultured yolk sacs were dissociated and stained, small enucleated red blood cells characteristic of EryDs were found (Figure 5Bvii, arrow). This demonstrates the presence of EryD precursors in the EpoRR129C/− YS that are capable of proliferation and differentiation in the absence of Epo stimulation. As controls, we performed similar organ culture experiments using YS isolated from Epo−/− and EpoR−/− embryos. Cells in the Epo−/− YS responded identically to those of WT YS when supplemented with Epo (data not shown), whereas YS from EpoR−/− mice showed no response (Figure 5Bv-vi) without or with exogenous Epo supplementation, indicating an Epo/EpoR-specific response. We also studied the morphology of cells dissociated from E8.5 chimeric YS, but without in vitro culturing, by Wright-Giemsa staining. Mature enucleated EryDs were clearly present in the chimeric YS (Figure 5Bviii) but were absent in the control samples (data not shown). These results, along with Epo and EpoR expression patterns, argue strongly that the timing of Epo expression or EpoR activation regulates the initiation of the definitive erythropoiesis wave in vivo.
Discussion
The differential expression of a receptor and its ligand to control the timing of normal physiological function is a common regulatory theme. For example, in adult mammals, Epo is transcriptionally up-regulated in response to hypoxia and synthesized in the juxtaglomerular cells of the kidney, and then it serves as the endocrine signal to stimulate erythropoiesis.25 However, our study is the first direct genetic demonstration that the timing Epo expression can act as an important developmental signal coordinating the initiation of definitive erythropoiesis.
By describing the temporal and spatial expression patterns of Epo and EpoR and by genetically knocking-in a constitutively activated form of the EpoR, we demonstrated that the timing of EpoR activation on definitive erythroid progenitor cells in the YS was necessary and sufficient to control the timing of definitive erythrocyte production. Using reverse transcription–polymerase chain reaction, Epo expression has been detected in the mouse embryo as early as E6.5, before the onset of primitive or definitive erythropoiesis.26However, genetic studies of the Epo and EpoR knockout mice argue that the level of Epo expression becomes physiologically relevant for erythropoiesis between E9.5 and E10.5. This correlates with the embryonic stage when we can detect Epo expression in the embryo by in situ hybridization, and it explains how both EryP and EryD progenitors can coexist in the YS yet always initiate differentiation programs successively—Epo-dependent EryD progenitors in the YS fail to complete differentiation without Epo stimulation, whereas Epo-independent EryP progenitors mature normally. The function of EpoR in the YS before Epo expression is unclear. Because no second ligand has been found that can bind and activate EpoR, earlier expression of EpoR may be important to “prime” the progenitors to be “competent” when Epo becomes available.
Although primitive erythropoiesis is still poorly understood, the growing theme emerging from gene disruption experiments is that there seem to be 2 categories of regulatory genes controlling erythropoiesis, one critical for only definitive erythropoiesis but not primitive erythropoiesis, such as AML1/CBFα2,27CBFβ,28 EKLF,29 and c-myb,30 and the other important for both, such as GATA-2,31 SCL/tal-1,32 and Rbtn2/Lmo2.33 This dichotomy of genetic requirements between primitive and definitive erythropoiesis strongly suggests that these 2 erythrocyte populations have distinct developmental programs. In vitro ES differentiation studies showed that EryP and EryD progenitors share a common precursor,34 suggesting that other regulatory molecules exquisitely orchestrate the differentiation paths of this common progenitor. The distinct developmental programs of EryP and EryD progenitors may also reflect different cell lineages.35 Both interpretations are possible and consistent with the observation that the earliest definitive erythroid progenitor is present in the YS (Palis et al18 and this study). Our work extends these models by identifying Epo as a critical signal for coordinating when and which developmental program predominates in vivo. This happens by stimulating existing EryD progenitors toward terminal differentiation rather than inducing de novo formation of specific progenitors.
Whether these earliest EryD progenitors are derived from the pluripotent hematopoietic stem cell or an alternative source for erythroid progenitors is unclear.35 The fact that Epo expression begins in the vitelline vasculature and near the AGM region suggests that Epo could play a role in supporting and expanding these progenitor cells. This further suggests that the apparent different origins of the hematopoietic progenitor cells could be more reflective of its environmental milieu and less of the potency of the progenitor cell itself.36 37 What is clear is that the onset of Epo expression in the embryo marks a control point of the second wave of erythropoiesis in vivo.
We thank Dr E. Goldwasser for mouse Epo cDNA; Dr S. Watowich for EpoRR129C cDNA; Dr Jing Gao for technical support; and Drs Owen Witte, Ke Shuai, Judy Gasson, Xin Liu, and members of our laboratory for useful discussions and for critical reading of the manuscript.
Supported by the Medical Scientist Training Program training grant (R.L. and A.J.). S.B.J. is supported by a National Institutes of Health Predoctoral NRSA in Biotechnology. H.W. is a V Foundation Scholar and an Assistant Investigator of the Howard Hughes Medical Institute.
R.L. and N.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hong Wu, Molecular Biology Institute, Department of Molecular and Medical Pharmacology, and Howard Hughes Medical Institute, UCLA School of Medicine, Los Angeles, CA 90095-1735; e-mail:hwu@mednet.ucla.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal