Rituximab is a chimeric monoclonal antibody that targets B-cell–specific antigen CD20 and an effective treatment for B-cell non-Hodgkin lymphoma. Although it is readily used in clinical practice, the exact mechanism of its antitumor effect is unclear. One potential mechanism involves complement-mediated cytotoxicity. It has been shown that rituximab induces complement-mediated cytotoxicity in follicular lymphoma cells in vitro, and complement inhibitors CD55 and CD59 may regulate this process. To determine whether complement inhibitors play a role in regulating the antitumor effect of rituximab, the expression of complement inhibitors CD46, CD55, and CD59 was analyzed in pretreatment tumor cells from 29 rituximab-treated follicular lymphoma patients. Among them, 8 patients achieved complete responses, 11 patients achieved partial responses, and 10 patients showed no or minimal responses to rituximab treatment. Expression of surface CD20, CD46, CD55, and CD59 was determined by 2-color flow cytometry. Although the CD59 level was slightly lower in the complete response group, there was no statistically significant difference in the expression of individual complement inhibitor CD46 (mean channel fluorescence [MCF]: NR, 26.4; PR, 21.9; CR, 29.9), CD55 (MCF: NR, 16.4; PR, 14.9; CR, 23.2), or CD59 (MCF: NR, 41.6; PR, 40.6; CR, 30.6), the combination of any 2 inhibitors, or all 3 on tumor cells from 3 response groups. In addition, there was no difference in the rituximab-induced complement-mediated cytotoxicity in an in vitro assay using tumor cells from 3 response groups. Thus, CD46, CD55, and CD59 expression on pretreatment tumor cells, or their susceptibility to in vitro complement-mediated killing, does not predict clinical outcome after rituximab treatment.
Introduction
The B-cell–specific antigen CD20 is first expressed on the surfaces of B-cell precursors shortly after the appearance of CD19 and throughout the mature B-cell stage1and on more than 90% of B-cell non-Hodgkin lymphoma (NHL).2 Although the structure of CD20 suggests a function as a membrane transporter or an ion channel,3 the function of CD20 in B cells is still not clearly understood. CD20 has been the target for monoclonal antibody (mAb) immunotherapy of B-cell NHL.4 A recently developed chimeric anti-CD20 mAb, rituximab, is an effective treatment for low-grade or follicular B-cell NHL; its response rate is approximately 50%. In phase 1 studies, rituximab induced a rapid depletion of CD20+ normal and lymphoma cells.5,6 Phase 2 trials with low-grade or follicular lymphoma showed a 50% response rate,7 whereas intermediate- to high-grade lymphomas showed a lower response rate.8 The reason for the heterogeneity of the response of different histologies and different patients is unclear.
The exact mechanism of CD20 mAb-induced antitumor or anti–B-cell effect remains unknown. It is generally accepted that immune-effector mechanisms, which include antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated cytotoxicity, are involved.9,10 This notion has been supported by several observations. First, though rituximab (IgG1) effectively depleted CD20+ B cells, an equivalent IgG4γ version of rituximab was unable to deplete B cells in vivo in primates.11 This is consistent with a role by complement or ADCC effector cells because they mediate cytotoxic effects best through the IgG1 constant region. Second, rituximab showed significant decrease in its antitumor effect in Fc receptor-deficient mice.12 This argues that an Fc receptor-dependent mechanism (ie, ADCC) contributes substantially to rituximab's antitumor effect. Third, in vitro study showed that lymphoma cell lines and freshly isolated follicular lymphoma cells are targets for ADCC and complement-mediated cytotoxicity in the presence of rituximab.9,10 Recently, rituximab-induced, complement-mediated cytotoxicity has been demonstrated in cell lines derived from patients with follicular lymphoma and in follicular lymphoma cells from patient samples in vitro.10Additionally, complement inhibitors CD55 and CD59 were suggested to regulate rituximab's antitumor effect because blocking antibodies specific to CD55 or CD59 increased tumor susceptibility to rituximab-induced, complement-mediated cytotoxicity.10However, though the serum complement levels decreased significantly after thrice weekly rituximab infusion in patients with chronic lymphocytic leukemia,13 no such change was observed after weekly rituximab infusion in patients with indolent lymphomas.7
We analyzed the expression of complement inhibitors CD46, CD55, and CD59, and performed in vitro complement-mediated cytotoxicity assay on pretreatment tumor cells from 29 rituximab-treated patients, whose outcome of therapy was known, to determine whether the relative levels of complement inhibitors or their susceptibility to in vitro complement-mediated cytotoxicity correlated with clinical outcome. This work was aimed at testing whether complement inhibitor expression pattern or in vitro complement-mediated cytotoxicity can predict the clinical outcome in rituximab-treated patients.
Materials and methods
Tumor cells
Tumor cells in this study were from patients treated with the chimeric mAb rituximab at Stanford Medical Center between 1993 and 2000. Suspensions of pretreatment tumor cells were isolated from lymph node biopsy specimens and cryopreserved in liquid nitrogen, affording the opportunity to study pretreatment tumor cells from patients whose responses to therapy were known.
For analysis, tumor cells were thawed, washed twice with culture medium (RPMI 1640 supplemented with 2% fetal calf serum, L-glutamine, penicillin, and streptomycin), and subjected to Ficoll-Paque Plus (Amersham Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation to remove dead cells. Viability of tumor cells as determined by trypan blue dye exclusion at the time of analysis exceeded 90% in all cases. Percentages of tumor cells in all samples were estimated using the light-chain restriction expressed in tumor cells. Coexisting normal B cells included cells expressing the other light chain and some of the cells expressing the same light chain as tumor cells. The number of normal B cells expressing the same light chain can be calculated by doubling the number of the other light chain (in λ light chain) or halving that number (in κ light chain), given the assumption that the normal distribution of κ to λ in the normal B-cell population is roughly 2:1. Based on this calculation, all but 3 samples were estimated to have more than 80% of tumor cells using right light-chain restriction (Table 1).
Patient characteristics according to response to rituximab treatment
| Characteristics . | NR (N = 10) . | PR (N = 11) . | CR (N = 8) . | All (N = 29) . |
|---|---|---|---|---|
| Sex (M/F) | 8/2 | 6/5 | 5/3 | 19/10 |
| Age (y) | 50 ± 9.4 | 59 ± 14.3 | 53 ± 5.7 | 54 ± 11.1 |
| Pathology | ||||
| FSC | 6 | 4 | 5 | 15 |
| FM | 3 | 6 | 2 | 11 |
| FLC | 1 | 1 | 1 | 3 |
| Average no. of prior chemotherapy courses | 2.40 | 2.55 | 2.13 | 2.38 |
| Prior transplantation | 1 | 3 | 1 | 5 |
| Bulky disease | 5 | 5 | 2 | 12 |
| Time between diagnosis and treatment (mo) | 63 ± 35 | 73 ± 39 | 69 ± 51 | 68 ± 40 |
| Estimated tumor cells* in biopsied samples (%) | 99, 97, 98, 96, 83, 98, 98, 93, 95, 97 | 87, 97, 94, 99, 99, 95, 95, 99, 89, 99, 74 | 76, 98, 94, 96, 91, 82, 75, 90 |
| Characteristics . | NR (N = 10) . | PR (N = 11) . | CR (N = 8) . | All (N = 29) . |
|---|---|---|---|---|
| Sex (M/F) | 8/2 | 6/5 | 5/3 | 19/10 |
| Age (y) | 50 ± 9.4 | 59 ± 14.3 | 53 ± 5.7 | 54 ± 11.1 |
| Pathology | ||||
| FSC | 6 | 4 | 5 | 15 |
| FM | 3 | 6 | 2 | 11 |
| FLC | 1 | 1 | 1 | 3 |
| Average no. of prior chemotherapy courses | 2.40 | 2.55 | 2.13 | 2.38 |
| Prior transplantation | 1 | 3 | 1 | 5 |
| Bulky disease | 5 | 5 | 2 | 12 |
| Time between diagnosis and treatment (mo) | 63 ± 35 | 73 ± 39 | 69 ± 51 | 68 ± 40 |
| Estimated tumor cells* in biopsied samples (%) | 99, 97, 98, 96, 83, 98, 98, 93, 95, 97 | 87, 97, 94, 99, 99, 95, 95, 99, 89, 99, 74 | 76, 98, 94, 96, 91, 82, 75, 90 |
FSC indicates follicular small cleaved; FM, follicular mixed; FLC, follicular large cell.
Plus-minus values are means ± SD.
Calculation described in “Materials and methods.”
Antibodies and flow cytometry
Reagents and methods used to analyze expression of cell surface antigens by flow cytometry have been previously described.14 Monoclonal antibodies specific to CD3, CD20, CD46, and CD55 were labeled with fluorescein isothiocyanate (FITC), and mAbs specific to CD59 and CD20 were labeled with phycoerythrin (PE). All were purchased from BD Pharmingen (San Diego, CA). Antibodies specific to κ and λ light chains were labeled with FITC or PE and were purchased from Biosource (Camarillo, CA). All analyses were conducted using 2-color staining with FITC- and PE-labeled mAb. Briefly, 5 × 105 cells were stained with FITC-labeled anti-CD20, anti-CD46, anti-CD55, or PE-labeled anti-CD59 and subsequently stained with a second fluorescence-labeled anti–κ or anti–λ light chain. In an analysis of T-cell percentages in the tumor samples, cells were stained with FITC-labeled anti-CD3 and PE-labeled anti-CD20. Cells were then fixed in 1% paraformaldehyde and analyzed on a FACScan (Becton Dickinson, San Jose, CA). Negative controls consisted of cells stained with equal concentrations of isotype-matched FITC- or PE-labeled myeloma proteins. Expression of CD20, CD46, CD55, and CD59 level was determined on right light chain-positive cells based on the light-chain restriction of each patient sample. Mean channel fluorescence (MCF) of CD20, CD46, CD55, and CD59 staining was determined after subtracting the signal obtained from the isotype-matched antibody from the specific mAb staining. All 29 samples were stained and were analyzed by flow cytometry on the same day.
Complement-mediated cytotoxicity assay
Lymphoma cells (5 × 105) were washed with phosphate-buffered saline (PBS) once and resuspended in 500 μL PBS. Cells were incubated with 5 μg/mL rituximab or control human IgG1 for 30 minutes at 4°C. Twenty percent of human serum from healthy donors was added, and incubation was carried out for 1 hour at 37°C. Human serum was not heat-inactivated to preserve the complement activities. The extent of complement-mediated lysis was measured by flow cytometric analysis of propidium iodide (PI)-stained cells on a FACScan (Becton Dickinson, San Jose, CA). All experiments were performed in triplicate. Specific complement-mediated cytotoxicity was determined by subtracting the percentage of PI-positive cells in IgG1-treated samples from that in the rituximab-treated sample, then dividing by the percentage of CD20+ cells in individual patient samples. The formula used was:
Statistical analysis
Differences in the means of continuous measurement (MCF signal, T-cell percentage, and complement-mediated lysis) were tested by single-factor analysis of variance (ANOVA) and checked by Kruskal-Wallis (nonparametric) test. Multivariate ANOVA was used to test the difference in combinations of 2 or all complement inhibitor levels. All statistical analyses were performed using SPSS 6.1.1 for Macintosh (SPSS, Chicago, IL).
Results
Patient characteristics
Tumor samples from 29 patients treated with rituximab at Stanford Medical Center between October 1993 and December 2000 were analyzed in this study. These patients were selected because of the availability of their pretreatment tumor cells and their known responses to rituximab. Pathology review of biopsy specimens was performed at Stanford Medical Center for all patients—15 with follicular small-cleaved lymphoma, 11 with follicular mixed lymphoma, and 3 with follicular large-cell lymphoma. All except 2 patients received 2 to 3 courses of chemotherapy before rituximab. However, none underwent chemotherapy within 2 months before rituximab infusion. Twenty patients underwent chemotherapy between the time of biopsy and rituximab infusion. Twenty-seven patients were treated with 4 weeks of weekly rituximab infusion at 375 mg/m2, one patient with 8 weeks of 375 mg/m2infusion, and one with 4 weeks of 250 mg/m2 infusion. Clinical responses were evaluated by physical examination and computed tomography studies. Eight patients achieved complete response (CR), 11 patients achieved partial response (PR), and 10 patients showed no or minimal response (NR) after rituximab therapy. There were no significant differences in average age at the time of treatment, in time between diagnosis and treatment, or in average number of prior chemotherapy course among the 3 groups (Table 1). Five patients had prior autologous bone marrow transplantation before rituximab treatment. Overall response rate (CR+PR = 65%) was slightly higher but similar to that in the pivotal trial (CR+PR = 60%, follicular lymphoma group).7 Forty percent of the patients had bulky disease (5 cm or greater) at the time of rituximab treatment. Overall response rate was slightly higher in patients with nonbulky disease (CR+PR = 71%) than in patients with bulky disease (CR+PR = 58%).
CD20 expression in pretreatment follicular lymphoma cells
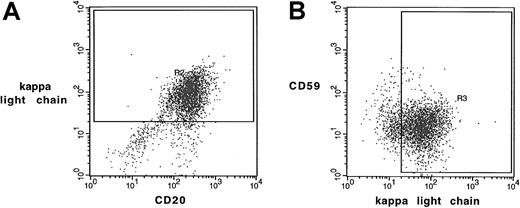
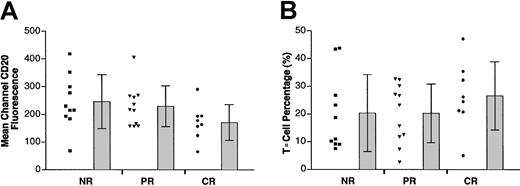
Using 2-color flow cytometry, we determined CD20 surface expression in the pretreatment tumor cells from these 3 groups of patients. Cell suspensions from biopsy specimens were stained with FITC-labeled anti-CD20 mAb, followed by PE-labeled anti–light chain antibody corresponding to individual patients based on light-chain restriction. An example of this 2-color flow cytometric analysis is shown in Figure 1A. Using this approach, the FITC signal (representing surface CD20 expression) from specific light-chain–positive cells (gated cells shown in Figure 1A) was evaluated. MCF levels from the CD20 staining of the tumor cells from the 29 patients varied between 66 and 418. Cell surface CD20 levels expressed by the patients' tumor cells according to their clinical outcomes are shown in Figure 2A. Values were MCF = 245.8 for tumors from patients showing no responses, MCF = 229.0 for tumors from partial responders, and MCF = 171.2 for complete responders (Table 2). However, statistical analysis reveals no significant differences among the 3 groups.
Two-color flow cytometry analysis of follicular lymphoma cells.
Scatterplots by 2-color staining with (A) anti-CD20–FITC and anti–κ light chain–PE or with (B) anti–κ light chain–FITC and anti-CD59–PE from a typical tumor sample were shown. Mean channel fluorescence of CD20 and CD59 (similar in CD46 and CD55) were determined in κ light chain- (or λ light chain- in other cases) positive cells as gated in panels A and B.
Two-color flow cytometry analysis of follicular lymphoma cells.
Scatterplots by 2-color staining with (A) anti-CD20–FITC and anti–κ light chain–PE or with (B) anti–κ light chain–FITC and anti-CD59–PE from a typical tumor sample were shown. Mean channel fluorescence of CD20 and CD59 (similar in CD46 and CD55) were determined in κ light chain- (or λ light chain- in other cases) positive cells as gated in panels A and B.
Expression of surface CD20 and percentage of coexistent T cells in tumor specimens.
(A) Scatterplot in the left column of each group represents the mean channel fluorescence of CD20 of individual tumors in that group. Bars at the right represent the mean and SD in each group. (B) Percentages of T cells detected in the tumor specimens were plotted.
Expression of surface CD20 and percentage of coexistent T cells in tumor specimens.
(A) Scatterplot in the left column of each group represents the mean channel fluorescence of CD20 of individual tumors in that group. Bars at the right represent the mean and SD in each group. (B) Percentages of T cells detected in the tumor specimens were plotted.
Expression of CD20, CD46, CD55, and CD59 according to response to rituximab treatment
| Antigen . | MCF . | P . | ||
|---|---|---|---|---|
| NR (N = 10) . | PR (N = 11) . | CR (N = 8) . | ||
| CD20 | 245.8 ± 97 | 229.0 ± 74 | 171.2 ± 64 | .150 |
| (223) | (218) | (171) | ||
| CD46 | 26.4 ± 7.1 | 21.9 ± 6.5 | 29.9 ± 14.1 | .197 |
| (25.9) | (22.9) | (27.5) | ||
| CD55 | 16.4 ± 10.2 | 14.9 ± 7.6 | 23.2 ± 7.3 | .115 |
| (12.2) | (14.7) | (23.6) | ||
| CD59 | 41.6 ± 24.7 | 40.6 ± 31.1 | 30.6 ± 22.8 | .648 |
| (32.2) | (26.9) | (22.7) | ||
| Antigen . | MCF . | P . | ||
|---|---|---|---|---|
| NR (N = 10) . | PR (N = 11) . | CR (N = 8) . | ||
| CD20 | 245.8 ± 97 | 229.0 ± 74 | 171.2 ± 64 | .150 |
| (223) | (218) | (171) | ||
| CD46 | 26.4 ± 7.1 | 21.9 ± 6.5 | 29.9 ± 14.1 | .197 |
| (25.9) | (22.9) | (27.5) | ||
| CD55 | 16.4 ± 10.2 | 14.9 ± 7.6 | 23.2 ± 7.3 | .115 |
| (12.2) | (14.7) | (23.6) | ||
| CD59 | 41.6 ± 24.7 | 40.6 ± 31.1 | 30.6 ± 22.8 | .648 |
| (32.2) | (26.9) | (22.7) | ||
All probability values are 2-sided and are considered statistically significant for P < .05 (single-factor ANOVA). Plus-minus values are means ± SD. Values in parentheses are medians.
The number of T cells in the biopsy specimens also varied from patient to patient (range, 2.7%-47.1%). However, again, there was no statistically significant difference between the percentage of T cells in the biopsy specimens in the 3 different groups (P = .476, ANOVA). Means of T-cell percentages were 20.3%, 20.2%, and 26.6% for nonresponder, partial responder, and complete responder, respectively (Figure 2B).
Expression of complement inhibitors CD46, CD55, and CD59 in pretreatment follicular lymphoma cells
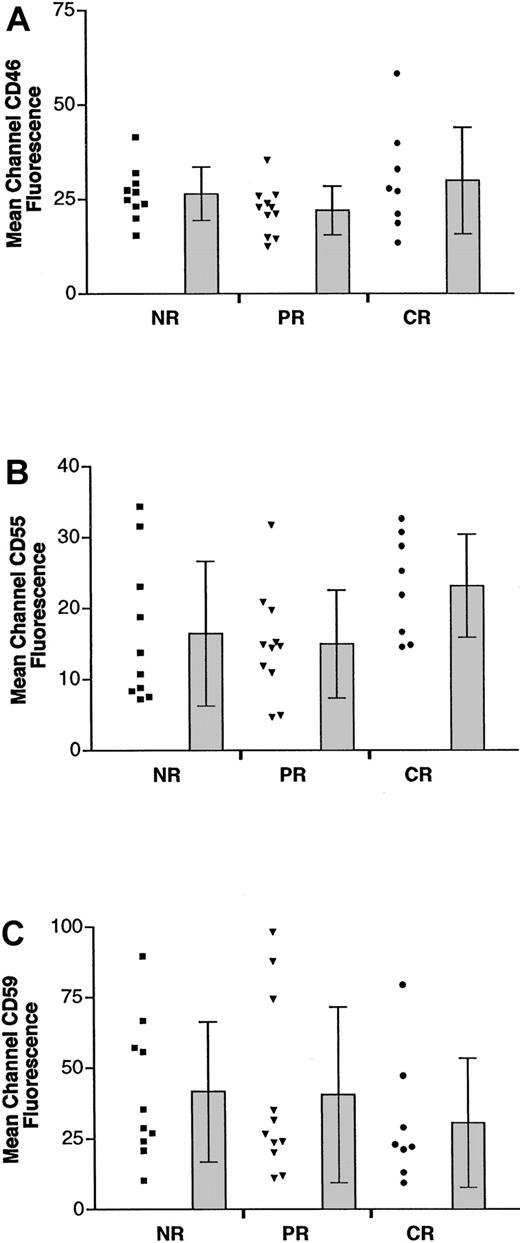
To determine the possible role of complement inhibitors in the antitumor effect of rituximab, we then analyzed the surface expression of CD46, CD55, and CD59. Two-color flow cytometric staining using corresponding anti–light chain antibody and mAbs against CD46, CD55, and CD59 was performed on all tumors. CD46, CD55, and CD59 levels were determined only on corresponding light-chain–positive cells (shown as gated cells in Figure 1B). MCF was determined by subtracting the signal of isotype-matched antibody staining from the specific mAb staining. Although CD46 and CD55 expression varied from patient to patient (MCF ranges, 12.5-58.2 for CD46, 4.9-34.4 for CD55; Figure3A-B), there were no statistically significant differences between the levels of CD46 and CD55 expression in the 3 response groups. As shown in Table 2, the means of MCF were 26.4 (NR), 21.9 (PR), and 29.9 (CR) for CD46, and they were 16.4 (NR), 14.9 (PR), and 23.2 (CR) for CD55, respectively. CD59 expression was slightly lower in patients who achieved CR (MCF = 30.6) than in patients who achieved PR or NR (MCF = 40.6 and 41.6, respectively; Figure 3C, Table 2). However, there was no statistically significant difference among the 3 groups regarding surface CD59 level. In addition, there was no statistically significant difference in the percentage of cells expressing individual complement inhibitor among the 3 groups (Table 3).
Expression of complement inhibitors CD46, CD55, and CD59.
Scatterplot in the left column of each group represents the mean channel fluorescence of CD46 (A), CD55 (B), or CD59 (C) of individual tumors in that group. Bars at the right represent the mean and SD in each group.
Expression of complement inhibitors CD46, CD55, and CD59.
Scatterplot in the left column of each group represents the mean channel fluorescence of CD46 (A), CD55 (B), or CD59 (C) of individual tumors in that group. Bars at the right represent the mean and SD in each group.
Percentage of CD20, CD46, CD55, and CD59 expression
| Antigen . | Percentage of positive cells . | P . | ||
|---|---|---|---|---|
| NR (N = 10) . | PR (N = 11) . | CR (N = 8) . | ||
| CD20 | 96.8 ± 7.9 | 97.7 ± 3.6 | 97.5 ± 3.8 | .916 |
| (99.4) | (99.5) | (99.2) | ||
| CD46 | 85.0 ± 12.2 | 69.7 ± 22.3 | 82.2 ± 14.8 | .119 |
| (88.8) | (83.7) | (85.5) | ||
| CD55 | 47.6 ± 21.6 | 47.7 ± 27.1 | 66.6 ± 19.6 | .171 |
| (47.5) | (47.9) | (72.5) | ||
| CD59 | 71.1 ± 20.2 | 67.5 ± 22.5 | 56.4 ± 20.2 | .336 |
| (76.7) | (66.9) | (56.1) | ||
| Antigen . | Percentage of positive cells . | P . | ||
|---|---|---|---|---|
| NR (N = 10) . | PR (N = 11) . | CR (N = 8) . | ||
| CD20 | 96.8 ± 7.9 | 97.7 ± 3.6 | 97.5 ± 3.8 | .916 |
| (99.4) | (99.5) | (99.2) | ||
| CD46 | 85.0 ± 12.2 | 69.7 ± 22.3 | 82.2 ± 14.8 | .119 |
| (88.8) | (83.7) | (85.5) | ||
| CD55 | 47.6 ± 21.6 | 47.7 ± 27.1 | 66.6 ± 19.6 | .171 |
| (47.5) | (47.9) | (72.5) | ||
| CD59 | 71.1 ± 20.2 | 67.5 ± 22.5 | 56.4 ± 20.2 | .336 |
| (76.7) | (66.9) | (56.1) | ||
All probability values are 2-sided and are considered statistically significant for P < .05 (single-factor ANOVA). Plus-minus values are means ± SD. Values in parentheses are medians.
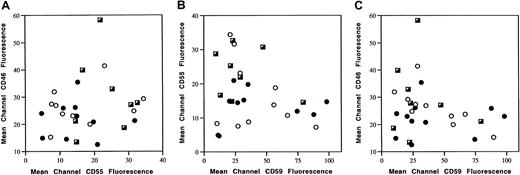
To test the possibility that the combined biologic effect of complement inhibitors might determine rituximab's antitumor effect, scatterplots were generated using the expression level of 2 complement inhibitors at a time. As shown in Figure 4, each point represents one patient plotted according to the MCF of either CD46/CD55 (Figure 4A), CD55/CD59 (Figure 4B), or CD46/CD59 (Figure 4C). There was no identifiable pattern or segregation of the 3 response groups in each case. In addition, there were no differences in the sums of levels among the 3 response groups, as follows: CD46 + CD55 (NR = 42.8 ± 13.9; PR = 36.9 ± 9.8; CR = 53.0 ± 16.0); CD55 + CD59 (NR = 58.1 ± 22.8; PR = 55.5 ± 31.6; CR = 53.8 ± 21.9); CD46 + CD59 (NR = 68.0 ± 20.1; PR = 62.5 ± 32.1; CR = 60.5 ± 25.0); CD46 + CD55 + CD59 (NR = 84.5 ± 18.8; PR = 77.4 ± 32.6; CR = 83.7 ± 24.3). Because CD46, CD55, and CD59 potentially inhibit complement-mediated cytotoxicity at different steps of the complement activation cascade in a sequential way, it is possible that their inhibitory effects are synergistic instead of additive. If this were the case, analysis of the sum of individual complement inhibitors may not be able to reveal its significance. To test this possibility, we then performed multivariate ANOVA on levels of 2 complement inhibitors and on all 3 at a time. Again, we did not find any relationship between expression status of any combination of 2 inhibitors or all 3 inhibitors and clinical outcome.
Expression status of complement inhibitor combination.
Expression of 2 complement inhibitors at a time were compared using scatterplot generated by corresponding MCF of CD46 and CD55 (A), CD55 and CD59 (B), or CD46 and CD59 (C) of individual tumor samples. ○ indicates NR; ●, PR; ┌, CR.
Expression status of complement inhibitor combination.
Expression of 2 complement inhibitors at a time were compared using scatterplot generated by corresponding MCF of CD46 and CD55 (A), CD55 and CD59 (B), or CD46 and CD59 (C) of individual tumor samples. ○ indicates NR; ●, PR; ┌, CR.
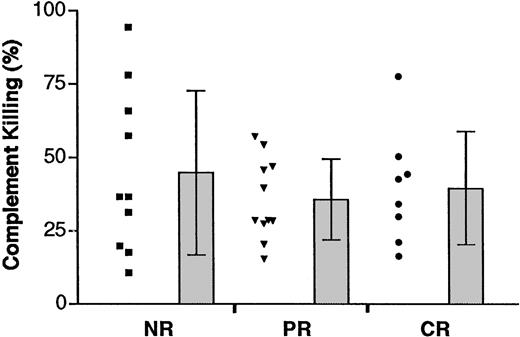
Susceptibility of pretreatment follicular lymphoma cells to in vitro complement-mediated cytotoxicity
In vitro complement-mediated cytotoxicity assay was then performed to determine whether the susceptibility of complement-mediated killing correlated with clinical outcome. The range of rituximab-induced complement-mediated killing varied widely in all 3 response groups (NR: range, 10.7%-94.3%; PR: range, 15.5%-57.1%; CR: range, 16.4%-50.3%) (Figure 5). However, there was no statistically significant difference of rituximab-induced complement-mediated killing in the 3 response groups (means: NR, 44.8% ± 28%; PR, 35.8% ± 14%; CR, 39.6% ± 19%) (Table4). Further statistical analysis did not show clear correlation between the expression of CD20 or individual complement inhibitors and complement-mediated killing (data not shown).
In vitro complement-mediated cytotoxicity.
Scatterplot in the left column of each group represents the mean percentage of complement-mediated killing of individual tumors in that group. Bars at the right represent the mean and SD in each group.
In vitro complement-mediated cytotoxicity.
Scatterplot in the left column of each group represents the mean percentage of complement-mediated killing of individual tumors in that group. Bars at the right represent the mean and SD in each group.
Susceptibility to in vitro complement-mediated cytotoxicity assay
| Specific complement-mediated killing (%) . | P . | ||
|---|---|---|---|
| NR (N = 10) . | PR (N = 11) . | CR (N = 8) . | |
| 44.8 ± 28 | 35.8 ± 14 | 39.6 ± 19 | .619 |
| (36.6) | (28.7) | (38.5) | |
| Specific complement-mediated killing (%) . | P . | ||
|---|---|---|---|
| NR (N = 10) . | PR (N = 11) . | CR (N = 8) . | |
| 44.8 ± 28 | 35.8 ± 14 | 39.6 ± 19 | .619 |
| (36.6) | (28.7) | (38.5) | |
All probability values are 2-sided and are considered statistically significant for P < .05 (single-factor ANOVA). Plus-minus values are means ± SD. Values in parentheses are medians.
Discussion
Rituximab, a chimeric mAb that targets the B-cell–specific antigen CD20, is the first therapeutic mAb available for the treatment of non-Hodgkin lymphoma (NHL). The advantages of targeting CD20 are 2-fold: (1) CD20 is a B-cell–specific antigen and is highly expressed on more than 90% of B-cell NHL; (2) on binding to mAb, CD20 does not down-modulate significantly from the cell surface, which makes it a continuous target for treatment. Although rituximab has been integrated into clinical practice in the treatment of B-cell lymphoma, the response is not uniform among different histologies or among patients with the same histologic diagnosis. The lower level of CD20 on the cell surface may explain the lower response rate in patients with chronic lymphocytic leukemia15 but not in patients with follicular lymphoma because CD20 is highly expressed in most patients. Consistent with this notion, we showed no difference in CD20 level on tumors from patients with follicular lymphoma who did or did not respond after rituximab treatment (Table 2, Figure 2A). Interestingly, the average level of CD20 in complete responders was slightly lower than in partial responders and in nonresponders. This cannot be explained by the down-modulation of surface CD20 or by a selective process after antibody treatment because all analyses were performed on pretreatment tumor cells. It is possible that there is a threshold of CD20 expression for cells to become susceptible to rituximab treatment. Given that the second lowest CD20 expression was found in one of the complete responders, we can potentially assume all but one tumor in this study exceeded that threshold.
One novel idea in the design of rituximab was to replace the constant region of the original mouse antibody with the human IgG1 constant region. This chimeric human constant region is probably very important for rituximab's antitumor effect through immune-mediated cytotoxicity.16,17 One potential model of rituximab's antitumor mechanism is complement-mediated cytotoxicity. In this model, rituximab binds to cell surface CD20 and then initiates activation of complement cascade, which eventually lyses the cell.18,19 Several membrane-bound complement inhibitors were recently identified to play regulatory roles in the above-described complement-mediated cytotoxicity.20 Among these complement inhibitors, CD46, CD55, and CD59 are readily expressed in human B cells. These 3 complement inhibitors interfere with the different steps of complement activation.21-23 This is an attractive model because the heterogeneity of rituximab response in different patients may be explained by the difference in the expression status of complement inhibitors on their tumor cells.
In this study, we examined the expression of complement inhibitors CD46, CD55, and CD59 on tumor cells from patients treated with rituximab. The hypothesis tested is that tumors from patients responding to treatment express less membrane-bound complement inhibitors than the ones from nonresponders. Using flow cytometry, we did not detect a significant difference in the level of any of the 3 complement inhibitors in patients with or without clinical response (Table 2). Additional analysis revealed no significant difference in the combined expression level of 2 or all 3 complement inhibitors in the 3 response groups (“Results” and Figure 4). In contrast to our expectation, tumors from patients who achieved CR expressed slightly higher levels of CD46 and CD55 than did those in the other 2 response groups. On the other hand, the complete responders had slightly lower levels of surface CD59 and fewer cells expressing CD59 (Tables 2, 3). Additional study using in vitro complement-mediated cytotoxicity assay showed a wide range of complement-mediated killing in the presence of rituximab in all 3 response groups (Table 4). However, it failed to show a relationship between susceptibility to complement-mediated killing and clinical outcome. In summary, there was no correlation between the expression of complement inhibitors on pretreatment tumors or their susceptibility to in vitro complement-mediated cytotoxicity and their clinical response to rituximab treatment.
In contrast to previous studies that suggested a regulatory role of complement inhibitors in rituximab-induced cytotoxicity,11,17 our data do not show a relationship between the expression of complement inhibitors CD46, CD55, or CD59 and complement-mediated killing in follicular lymphoma cells. It is possible that other unidentified complement inhibitors or additional regulatory factors expressed by lymphoma cells are involved in this complement-mediated cytotoxicity. In addition, the expression status of these 3 complement inhibitors does not predict the clinical outcome after rituximab treatment in patients with follicular lymphoma. However, this study does not address the possibility that complement inhibitors may play a role in the development of resistance to rituximab after treatment. A recent study13 in chronic lymphocytic leukemia showed that residual tumor cells have higher levels of surface CD55 and CD59 after rituximab treatment. Further study of tumor cells after rituximab treatment should be conducted to see whether the follicular lymphoma cells resistant to treatment express higher levels of complement inhibitors or whether they are less sensitive to complement-mediated killing. Twenty patients in our study underwent chemotherapy between the time of lymph node biopsy and rituximab treatment. Although one study reports a decrease in CD59 level on adenocarcinoma cell lines after in vitro treatment with levamisole, the effect of various types of chemotherapy on expression of complement inhibitors on lymphoma cells in vivo is unknown.24
Although the susceptibility to complement-mediated cytotoxicity does not predict clinical outcome in this study, our data do not exclude the potential involvement of complement-mediated cytotoxicity in rituximab's antitumor effect or a regulatory role of complement inhibitors in the treatment of other B-cell malignancies or in rituximab-induced normal B-cell depletion. It is conceivable that rituximab's anti–normal B-cell effect uses a different mechanism from its antitumor effect in patients with follicular lymphoma—there are significant differences between the micro-environment surrounding the circulating B cells and the one surrounding follicular lymphoma cells in the lymph node.
Another potential mechanism proposed to play a role in rituximab's antitumor effect is ADCC. In vitro studies11 support the involvement of ADCC by showing rituximab-induced lymphoma cell lines lysis in the presence of peripheral blood mononuclear effector cells. More supporting evidence is from the study12 in Fc receptor-deficient mice, in which rituximab shows significantly decreased antitumor effect. One possible explanation is that rituximab failed to recruit effector cells through Fc receptor to complete ADCC in these animals. In addition to immune-mediated mechanisms by ADCC and complement-mediated cytotoxicity, a recent study25 shows that rituximab can induce apoptosis of some lymphoma cell lines through the caspase pathway. It suggests a possible role of CD20-mediated signal transduction in rituximab's antitumor effect. Additional experiments to explore these potential mechanisms, including ADCC, apoptosis, and CD20-mediated signal transduction, should be conducted to determine their roles in rituximab's antitumor effect. This information may facilitate the development of new immunotherapy reagents.
We thank Debra Czerwinski for her technical assistance with flow cytometry analysis. We also thank Dr Robert Tibshirani for his assistance with statistical analysis.
Supported by grant CA34233 from the US Public Health Service–National Institutes of Health. W.K.W. is the recipient of a postdoctoral fellowship from the National Institutes of Health (training grant CA09287). R.L. is an American Cancer Society Clinical Research Professor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ronald Levy, Division of Oncology, CCSR 1126, Stanford University School of Medicine, Stanford, CA 94305-5306; e-mail: levy@leland.stanford.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal