The myelodysplastic syndromes (MDSs) are characterized by bilineage or trilineage dysplasia. Although diagnostic criteria are well established for MDS, a significant number of patients have blood and bone marrow findings that make diagnosis and classification difficult. Flow cytometric immunophenotyping is an accurate and highly sensitive method for detection of quantitative and qualitative abnormalities in hematopoietic cells. Flow cytometry was used to study hematopoietic cell populations in the bone marrow of 45 patients with straightforward MDS. The results were compared with those obtained in a series of patients with aplastic anemia, healthy donors, and patients with a history of nonmyeloid neoplasia in complete remission. The immunophenotypic abnormalities associated with MDS were defined, and the diagnostic utility of flow cytometry was compared, with morphologic and cytogenetic evaluations in 20 difficult cases. Although morphology and cytogenetics were adequate for diagnosis in most cases, flow cytometry could detect immunophenotypic abnormalities in cases when combined morphology and cytogenetics were nondiagnostic. It is concluded that flow cytometric immunophenotyping may help establish the diagnosis of MDS, especially when morphology and cytogenetics are indeterminate.
Introduction
The myelodysplastic syndromes (MDSs) are a heterogeneous group of bone marrow disorders characterized by dysplasia in 2 or more lineages and increased risk of acute leukemia. Patients usually present with peripheral blood cytopenias and hypercellular bone marrow, although the bone marrow is hypocellular in one quarter of the cases.1-3 A combination of morphology, to detect multilineage dysplasia in the bone marrow and peripheral blood, and cytogenetics, to detect characteristic clonal abnormalities, are used in establishing a diagnosis of MDS. The French-American-British cooperative group proposed 5 subgroups of MDS in 19824—namely, refractory anemia (RA), RA with ringed sideroblasts (RARS), chronic myelomonocytic leukemia (CMML), RA with excess blasts (RAEB), and RAEB in transformation (RAEB-T). Subclassification of MDS is based primarily upon the presence of ringed sideroblasts and the number of blast cells in the bone marrow. Although diagnostic criteria are well established, a significant number of patients have blood and bone marrow findings that make diagnosis and classification difficult.5-7 Morphology may be difficult to evaluate in some patients, either due to hypocellularity or fibrosis of the marrow. Because hypocellular marrows in the setting of pancytopenia may be observed in MDS or aplastic anemia, the differentiation of MDS from aplastic anemia based upon morphology alone may be impossible if bone marrow aspirates do not yield a sufficient number of cells for accurate assessment. In addition, bone marrow fibrosis, a nonspecific finding in a number of acute and chronic disorders, may obscure specific morphologic features and make a diagnosis of MDS challenging.5,6 Although cytogenetic evaluation is helpful in the diagnosis of MDS and provides prognostic information as well, clonal karyotypic abnormalities are present in only 20% to 60% of MDS cases.8-10 Molecular studies have been investigated as a diagnostic modality in MDS, but the relatively low incidence of defined translocations makes this technique not generally applicable.
Flow cytometric immunophenotyping is an accurate method for quantitative and qualitative evaluation of hematopoietic cells, and several groups have used flow cytometry in the study of MDS.11-14 Cell cycle analysis of myeloid lineage cells in normal versus MDS bone marrows has revealed differences in proliferative activity.14 In addition, increased numbers of CD34-expressing bone marrow blasts in MDS is associated with a poorer prognosis.13 Kuiper-Kramer et al observed decreased transferrin expression by erythroblasts in MDS, although this was also observed in anemia of chronic disease.11 Bowen and Davis studied the pattern of CD16 and CD11b expression by maturing granulocytes in the bone marrow of patients with MDS and healthy controls. There was a highly consistent normal pattern of CD11b and CD16 expression in the granulocytic series in healthy subjects, but in MDS patients there was an increased percentage of granulocytic cells with low CD16 or both low CD16 and low CD11b.12 Although these studies defined abnormalities in MDS, they did not address the potential contribution of flow cytometric evaluation to the diagnosis of MDS. In this study, we examined the diagnostic utility of flow cytometric immunophenotyping in 45 patients with typical MDS and compared the results to those obtained in patients with aplastic anemia, patients in remission after treatment for nonmyeloid neoplasia, and healthy donors. We have defined immunophenotypic abnormalities in MDS and compare our results with the diagnostic utility of morphologic and cytogenetic evaluations in 20 patients ultimately diagnosed with MDS but with equivocal initial morphology.
Patients, materials, and methods
Patient information
Bone marrow aspirates from 65 consecutive patients with a diagnosis of MDS (confirmed at our institution) who were referred to the National Institutes of Health for treatment (Tables1 and 2) were subjected to flow cytometric immunophenotyping. These patients gave written consent for analysis and treatment in an experimental immunosuppressive phase II study reviewed by the National Heart, Lung, and Blood Institute Internal Review Board (protocol No. 95-H-0189 and 98-H-0122). All 65 patients had a diagnosis of de novo MDS prior to referral and eventually received a confirmation of this diagnosis based upon clinical history, morphology, cytogenetics, and other clinical data. In addition, bone marrow aspirates from the following patients were studied: 15 patients with a long-term diagnosis of aplastic anemia and no morphologic, cytogenetic, or other indications of dysplasia; 10 patients in remission after treatment for nonmyeloid neoplasms (6 non-Hodgkin lymphoma, 1 chronic lymphocytic leukemia, 1 acute lymphoblastic leukemia, 2 sarcoma); and 4 healthy volunteers (Table 3).
Myelodysplastic syndrome patients
| Number . | Sex . | Race . | Age . | Initial BM diagnosis MDS . | Review BM diagnosis MDS . | Flow bilineage or trilineage dysplasia . | Cytogenetics clonal abnormality . | Final diagnosis . | Erythroid . | Megakaryocytic . | Myeloid . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphology dysplasia . | Flow dysplasia . | Morphology dysplasia . | Flow dysplasia . | Morphology dysplasia . | Flow dysplasia . | |||||||||
| 1 | F | H | 34 | − | + | + | NE | RA | − (NE) | + | + | − | NE | + |
| 2 | F | W | 70 | + | + | + | + | RA | + | − | + | + | + | + |
| 3 | M | W | 60 | + | + | + | − | RA | + | + | + | − | + | + |
| 4 | M | B | 32 | − | + | + | − | RA | + | + | + | + | + | + |
| 5 | M | W | 65 | + | + | + | − | RA | + | + | + | − | − | + |
| 6 | F | W | 72 | − | + | + | + | RA | − | − | + | + | + | + |
| 7 | F | W | 46 | − | + | − | − | RA | + | − | + | − | + | + |
| 8 | M | W | 27 | + | + | + | NE | RA | + | + | + | + | + | + |
| 9 | F | A | 58 | + | + | NE | − | RA | + | + | + | − | + | NE |
| 10 | F | W | 62 | + | + | + | − | RA | + | + | − | − | + | + |
| 11 | M | A | 77 | − | + | + | + | RA | + | + | + | + | + | NE |
| 12 | M | W | 76 | + | + | + | − | RA | + | + | + | − | + | + |
| 13 | M | B | 61 | + | + | + | + | RA | + | + | + | − | + | + |
| 14 | F | W | 67 | + | + | + | + | RA | + | + | + | + | − | + |
| 15 | F | W | 49 | + | + | + | + | RA | − | + | + | + | + | + |
| 16 | M | W | 60 | − | + | + | + | RA | NE | + | + | + | − | + |
| 17 | M | W | 69 | + | + | + | − | RA | + | + | + | + | + | + |
| 18 | M | W | 47 | − | + | − | NE | RA | + | − | + | − | − | + |
| 19 | F | W | 37 | − | + | + | − | RA | + | + | + | NE | + | + |
| 20 | F | W | 43 | + | + | + | − | RA | − | + | + | + | + | + |
| 21 | M | W | 45 | + | + | − | − | RA | + | − | + | − | + | + |
| 22 | M | W | 56 | + | + | + | − | RA | − | + | + | + | + | + |
| 23 | M | W | 57 | + | + | + | − | RARS | + | + | + | + | + | + |
| 24 | M | W | 68 | + | + | + | NE | RARS | + | + | + | + | + | + |
| 25 | M | W | 72 | + | + | + | + | RARS | + | + | + | + | + | + |
| 26 | M | W | 60 | + | + | + | + | RARS | + | + | − | + | − | + |
| 27 | F | W | 66 | + | + | + | + | RARS | + | + | + | − | + | + |
| 28 | F | W | 44 | − | + | + | − | RARS | + | + | + | + | + | − |
| 29 | F | W | 67 | + | + | + | + | RARS | + | + | − | + | + | + |
| 30 | M | W | 61 | − | + | − | NE | RARS | + | − | + | − | NE | + |
| 31 | M | W | 67 | + | + | + | − | RARS | + | − | + | + | − | + |
| 32 | M | W | 69 | + | + | + | + | RARS | + | − | + | + | − | + |
| 33 | M | W | 63 | + | + | + | + | RAEB | − | + | + | + | + | + |
| 34 | M | W | 64 | − | + | NE | NE | RAEB | − | NE | − | − | + | + |
| 35 | F | W | 69 | + | + | + | + | RAEB | + | + | + | + | + | + |
| 36 | F | W | 61 | + | + | + | − | RAEB | + | + | + | − | + | + |
| 37 | M | W | 65 | + | + | + | + | RAEB | + | + | + | + | + | + |
| 38 | M | W | 58 | + | + | + | + | RAEB | + | + | + | − | + | + |
| 39 | M | W | 51 | − | + | − | − | RAEB | + | − | + | − | − | + |
| 40 | M | W | 81 | + | + | + | + | RAEB | + | + | + | + | + | NE |
| 41 | M | W | 71 | + | + | + | − | RAEB | − | − | + | + | + | + |
| 42 | F | W | 61 | + | + | + | + | RAEB | + | + | + | + | + | + |
| 43 | M | W | 72 | + | + | + | + | RAEB | + | + | + | + | + | + |
| 44 | M | W | 64 | + | + | + | − | RAEB | + | + | + | − | + | + |
| 45 | F | W | 63 | + | + | + | + | RAEB-T | + | + | + | − | + | + |
| Number . | Sex . | Race . | Age . | Initial BM diagnosis MDS . | Review BM diagnosis MDS . | Flow bilineage or trilineage dysplasia . | Cytogenetics clonal abnormality . | Final diagnosis . | Erythroid . | Megakaryocytic . | Myeloid . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphology dysplasia . | Flow dysplasia . | Morphology dysplasia . | Flow dysplasia . | Morphology dysplasia . | Flow dysplasia . | |||||||||
| 1 | F | H | 34 | − | + | + | NE | RA | − (NE) | + | + | − | NE | + |
| 2 | F | W | 70 | + | + | + | + | RA | + | − | + | + | + | + |
| 3 | M | W | 60 | + | + | + | − | RA | + | + | + | − | + | + |
| 4 | M | B | 32 | − | + | + | − | RA | + | + | + | + | + | + |
| 5 | M | W | 65 | + | + | + | − | RA | + | + | + | − | − | + |
| 6 | F | W | 72 | − | + | + | + | RA | − | − | + | + | + | + |
| 7 | F | W | 46 | − | + | − | − | RA | + | − | + | − | + | + |
| 8 | M | W | 27 | + | + | + | NE | RA | + | + | + | + | + | + |
| 9 | F | A | 58 | + | + | NE | − | RA | + | + | + | − | + | NE |
| 10 | F | W | 62 | + | + | + | − | RA | + | + | − | − | + | + |
| 11 | M | A | 77 | − | + | + | + | RA | + | + | + | + | + | NE |
| 12 | M | W | 76 | + | + | + | − | RA | + | + | + | − | + | + |
| 13 | M | B | 61 | + | + | + | + | RA | + | + | + | − | + | + |
| 14 | F | W | 67 | + | + | + | + | RA | + | + | + | + | − | + |
| 15 | F | W | 49 | + | + | + | + | RA | − | + | + | + | + | + |
| 16 | M | W | 60 | − | + | + | + | RA | NE | + | + | + | − | + |
| 17 | M | W | 69 | + | + | + | − | RA | + | + | + | + | + | + |
| 18 | M | W | 47 | − | + | − | NE | RA | + | − | + | − | − | + |
| 19 | F | W | 37 | − | + | + | − | RA | + | + | + | NE | + | + |
| 20 | F | W | 43 | + | + | + | − | RA | − | + | + | + | + | + |
| 21 | M | W | 45 | + | + | − | − | RA | + | − | + | − | + | + |
| 22 | M | W | 56 | + | + | + | − | RA | − | + | + | + | + | + |
| 23 | M | W | 57 | + | + | + | − | RARS | + | + | + | + | + | + |
| 24 | M | W | 68 | + | + | + | NE | RARS | + | + | + | + | + | + |
| 25 | M | W | 72 | + | + | + | + | RARS | + | + | + | + | + | + |
| 26 | M | W | 60 | + | + | + | + | RARS | + | + | − | + | − | + |
| 27 | F | W | 66 | + | + | + | + | RARS | + | + | + | − | + | + |
| 28 | F | W | 44 | − | + | + | − | RARS | + | + | + | + | + | − |
| 29 | F | W | 67 | + | + | + | + | RARS | + | + | − | + | + | + |
| 30 | M | W | 61 | − | + | − | NE | RARS | + | − | + | − | NE | + |
| 31 | M | W | 67 | + | + | + | − | RARS | + | − | + | + | − | + |
| 32 | M | W | 69 | + | + | + | + | RARS | + | − | + | + | − | + |
| 33 | M | W | 63 | + | + | + | + | RAEB | − | + | + | + | + | + |
| 34 | M | W | 64 | − | + | NE | NE | RAEB | − | NE | − | − | + | + |
| 35 | F | W | 69 | + | + | + | + | RAEB | + | + | + | + | + | + |
| 36 | F | W | 61 | + | + | + | − | RAEB | + | + | + | − | + | + |
| 37 | M | W | 65 | + | + | + | + | RAEB | + | + | + | + | + | + |
| 38 | M | W | 58 | + | + | + | + | RAEB | + | + | + | − | + | + |
| 39 | M | W | 51 | − | + | − | − | RAEB | + | − | + | − | − | + |
| 40 | M | W | 81 | + | + | + | + | RAEB | + | + | + | + | + | NE |
| 41 | M | W | 71 | + | + | + | − | RAEB | − | − | + | + | + | + |
| 42 | F | W | 61 | + | + | + | + | RAEB | + | + | + | + | + | + |
| 43 | M | W | 72 | + | + | + | + | RAEB | + | + | + | + | + | + |
| 44 | M | W | 64 | + | + | + | − | RAEB | + | + | + | − | + | + |
| 45 | F | W | 63 | + | + | + | + | RAEB-T | + | + | + | − | + | + |
BM indicates bone marrow; MDS, myelodysplastic syndrome; H, Hispanic; + or −, whether diagnostic criteria for MDS or dysplasia were met as described in “Patients, materials, and methods” and “Results”; NE, not evaluable; RA, refractory anemia; W, white; B, black; RARS, RA with ringed sideroblasts; RAEB, RA with excess blasts; RAEB-T, RAEB in transformation.
Difficult cases of myelodysplastic syndrome
| Number . | Sex . | Race . | Age . | Initial BM diagnosis MDS . | Review BM diagnosis MDS . | Morphology indeterminate . | Cytogenetics clonal abnormality . | Final diagnosis . | Erythroid . | Megakaryocytic . | Myeloid . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphology dysplasia . | Flow dysplasia . | Morphology dysplasia . | Flow dysplasia . | Morphology dysplasia . | Flow dysplasia . | |||||||||
| 1 | M | W | 80 | − | +* | + | RA | + | + | + | − | + | + | |
| 2 | M | W | 45 | − | +* | − | RA | + | − | − | − | − | + | |
| 3 | F | W | 28 | − | +* | − | RA | + | − | − | + | − | + | |
| 4 | F | W | 69 | + | +* | + | RA | + | + | + | + | + | − (NE) | |
| 5 | M | W | 70 | − | +* | + | RA | + | + | + | − | + | + | |
| 6 | F | W | 61 | − | +* | + | RA | − | + | + | + | − | + | |
| 7 | M | W | 27 | − | +* | − | RA | − (NE) | − | − (NE) | − | − (NE) | + | |
| 8 | F | B | 41 | − | +* | − | RA | − (NE) | − | − (NE) | + | − (NE) | − | |
| 9 | M | W | 64 | − | +* | NE | RA | + | + | + | − | + | + | |
| 10 | M | A | 72 | − | +* | + | RAEB | + | + | − | − | + | + | |
| 11 | M | W | 72 | − | +* | − | RAEB | + | + | + | + | + | + | |
| 12 | M | W | 68 | + | +* | − | RAEB | + | + | − (NE) | − | + | + | |
| 13 | M | W | 55 | − | − | x | − | RA | + | − | − | − | − | − |
| 14 | M | W | 54 | − | − | x | − | RA | − | − | + | + | − | + |
| 15 | M | W | 59 | − | − | x | − | RA | − (NE) | + | − (NE) | − | − (NE) | + |
| 16 | F | B | 55 | − | − | x | − | RA | + | + | − | − | − | + |
| 17 | F | W | 57 | − | − | x | NE | RA | − | + | − | − | − | + |
| 18 | F | H | 37 | − | − | x | − | RA | + | + | + | + | + | + |
| 19 | M | W | 70 | − | − | x | − | RA | − | − | + | + | + | + |
| 20 | F | W | 42 | + | − | x | − | RA | − | − | − | − | − | + |
| Number . | Sex . | Race . | Age . | Initial BM diagnosis MDS . | Review BM diagnosis MDS . | Morphology indeterminate . | Cytogenetics clonal abnormality . | Final diagnosis . | Erythroid . | Megakaryocytic . | Myeloid . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphology dysplasia . | Flow dysplasia . | Morphology dysplasia . | Flow dysplasia . | Morphology dysplasia . | Flow dysplasia . | |||||||||
| 1 | M | W | 80 | − | +* | + | RA | + | + | + | − | + | + | |
| 2 | M | W | 45 | − | +* | − | RA | + | − | − | − | − | + | |
| 3 | F | W | 28 | − | +* | − | RA | + | − | − | + | − | + | |
| 4 | F | W | 69 | + | +* | + | RA | + | + | + | + | + | − (NE) | |
| 5 | M | W | 70 | − | +* | + | RA | + | + | + | − | + | + | |
| 6 | F | W | 61 | − | +* | + | RA | − | + | + | + | − | + | |
| 7 | M | W | 27 | − | +* | − | RA | − (NE) | − | − (NE) | − | − (NE) | + | |
| 8 | F | B | 41 | − | +* | − | RA | − (NE) | − | − (NE) | + | − (NE) | − | |
| 9 | M | W | 64 | − | +* | NE | RA | + | + | + | − | + | + | |
| 10 | M | A | 72 | − | +* | + | RAEB | + | + | − | − | + | + | |
| 11 | M | W | 72 | − | +* | − | RAEB | + | + | + | + | + | + | |
| 12 | M | W | 68 | + | +* | − | RAEB | + | + | − (NE) | − | + | + | |
| 13 | M | W | 55 | − | − | x | − | RA | + | − | − | − | − | − |
| 14 | M | W | 54 | − | − | x | − | RA | − | − | + | + | − | + |
| 15 | M | W | 59 | − | − | x | − | RA | − (NE) | + | − (NE) | − | − (NE) | + |
| 16 | F | B | 55 | − | − | x | − | RA | + | + | − | − | − | + |
| 17 | F | W | 57 | − | − | x | NE | RA | − | + | − | − | − | + |
| 18 | F | H | 37 | − | − | x | − | RA | + | + | + | + | + | + |
| 19 | M | W | 70 | − | − | x | − | RA | − | − | + | + | + | + |
| 20 | F | W | 42 | + | − | x | − | RA | − | − | − | − | − | + |
For abbreviations, see Table 1.
Diagnosis could not be made based upon a single specimen.
Control data
| Clinical diagnosis . | Morphology dysplasia . | Myeloid flow dysplasia . | Erythroid flow dysplasia . | Megakaryocyte flow increased . | Discrete blasts on CD45/SSC . | CD64 granulocytes . | CD56 monocytes . | Hypogranular granulocytes . | CD11B CD16 pattern . | CD13 CD16 pattern . | CD10 granulocytes . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aplastic anemia | |||||||||||
| 1 | − | − | NE | − | No | + | − | + | nl | nl | + |
| 2 | − | − | + CD71/GlyA | − | No | + | − | + | nl | nl | + |
| 3 | − | + | − | − | No | − | + | + | nl | nl | + |
| 4 | − | − | − | − | No | + | − | − | nl | nl | + |
| 5 | − | − | + CD71/GlyA | − | No | + | − | − | nl | nl | + |
| 6 | − | − | − | − | No | + | − | + | nl | nl | + |
| 7 | − | − | + CD71/GlyA | − | No | + | − | − | nl | nl | + |
| 8 | − | − | − | − | No | + | − | − | nl | nl | + |
| 9 | − | − | − | − | No | + | − | − | nl | nl | NE |
| 10 | − | − | − | − | No | + | − | + | nl | nl | + |
| 11 | − | − | − | − | No | + | − | + | nl | nl | + |
| 12 | − | − | − | − | No | + | − | − | nl | nl | + |
| 13 | − | − | − | − | No | − | − | − | nl | nl | + |
| 14 | − | − | − | − | No | + | − | − | nl | nl | + |
| 15 | − | − | − | − | No | + | − | − | nl | nl | + |
| Nonmyeloid in remission | |||||||||||
| 1 LCL | − | − | − | NE | No | + | − | − | nl | nl | + |
| 2 FL | − | − | − | NE | No | + | − | − | nl | NE | NE |
| 3 FL | − | − | − | − | No | + | − | − | nl | nl | + |
| 4 ALCL | − | − | − | − | No | + | − | − | nl | nl | NE |
| 5 CLL | − | − | − | − | No | + | − | − | nl | nl | NE |
| 6 ALL | − | − | − | − | No | + | − | − | NE | NE | + |
| 7 Rhabdo | − | − | − | + | No | + | − | − | nl | nl | + |
| 8 Ewing | − | − | − | − | No | + | − | − | nl | nl | + |
| 9 LCL (HIV) | − | − | − | NE | No | + | − | − | nl | nl | NE |
| 10 LCL | − | − | − | + | No | + | − | − | nl | NE | + |
| Normal | |||||||||||
| 1 | NE | − | − | + | No | + | − | − | nl | nl | + |
| 2 | NE | − | − | − | No | + | − | − | nl | nl | + |
| 3 | NE | − | − | + | No | + | − | − | nl | nl | + |
| 4 | NE | − | − | + | No | + | − | − | nl | nl | + |
| Clinical diagnosis . | Morphology dysplasia . | Myeloid flow dysplasia . | Erythroid flow dysplasia . | Megakaryocyte flow increased . | Discrete blasts on CD45/SSC . | CD64 granulocytes . | CD56 monocytes . | Hypogranular granulocytes . | CD11B CD16 pattern . | CD13 CD16 pattern . | CD10 granulocytes . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aplastic anemia | |||||||||||
| 1 | − | − | NE | − | No | + | − | + | nl | nl | + |
| 2 | − | − | + CD71/GlyA | − | No | + | − | + | nl | nl | + |
| 3 | − | + | − | − | No | − | + | + | nl | nl | + |
| 4 | − | − | − | − | No | + | − | − | nl | nl | + |
| 5 | − | − | + CD71/GlyA | − | No | + | − | − | nl | nl | + |
| 6 | − | − | − | − | No | + | − | + | nl | nl | + |
| 7 | − | − | + CD71/GlyA | − | No | + | − | − | nl | nl | + |
| 8 | − | − | − | − | No | + | − | − | nl | nl | + |
| 9 | − | − | − | − | No | + | − | − | nl | nl | NE |
| 10 | − | − | − | − | No | + | − | + | nl | nl | + |
| 11 | − | − | − | − | No | + | − | + | nl | nl | + |
| 12 | − | − | − | − | No | + | − | − | nl | nl | + |
| 13 | − | − | − | − | No | − | − | − | nl | nl | + |
| 14 | − | − | − | − | No | + | − | − | nl | nl | + |
| 15 | − | − | − | − | No | + | − | − | nl | nl | + |
| Nonmyeloid in remission | |||||||||||
| 1 LCL | − | − | − | NE | No | + | − | − | nl | nl | + |
| 2 FL | − | − | − | NE | No | + | − | − | nl | NE | NE |
| 3 FL | − | − | − | − | No | + | − | − | nl | nl | + |
| 4 ALCL | − | − | − | − | No | + | − | − | nl | nl | NE |
| 5 CLL | − | − | − | − | No | + | − | − | nl | nl | NE |
| 6 ALL | − | − | − | − | No | + | − | − | NE | NE | + |
| 7 Rhabdo | − | − | − | + | No | + | − | − | nl | nl | + |
| 8 Ewing | − | − | − | − | No | + | − | − | nl | nl | + |
| 9 LCL (HIV) | − | − | − | NE | No | + | − | − | nl | nl | NE |
| 10 LCL | − | − | − | + | No | + | − | − | nl | NE | + |
| Normal | |||||||||||
| 1 | NE | − | − | + | No | + | − | − | nl | nl | + |
| 2 | NE | − | − | − | No | + | − | − | nl | nl | + |
| 3 | NE | − | − | + | No | + | − | − | nl | nl | + |
| 4 | NE | − | − | + | No | + | − | − | nl | nl | + |
SSC indicates side light scatter; nl, normal; LCL, large cell lymphoma; FL, follicular lymphoma; ALCL, anaplastic large cell lymphoma; CLL, chronic lymphocytic leukemia; ALL, acute lymphoblastic leukemia; Rhabdo, rhabdomyosarcoma; Ewing, Ewing sarcoma; HIV, human immunodeficiency virus; for other abbreviations, see Table 1.
Flow cytometry studies of bone marrow aspirate specimens
Heparinized bone marrow aspirate specimens were stained within 12 hours of collection using a whole blood lysis technique as described15 and a panel of directly conjugated antibodies (Table 4). Because this panel evolved during the study and additional antibodies were used on later cases, data are not available with all antibodies on all 65 MDS cases. Five-parameter, 3-color flow cytometry was performed with a FACScan flow cytometer equipped with a 15-mW argon laser (excitation at 488 mm) (Becton Dickinson, San Jose, CA). The sensitivity of fluorescence detectors was set and monitored using Calibrite Beads (Becton Dickinson) according to manufacturer's recommendations. In addition, Quantum fluorescence calibration beads (FCSC, San Juan, PR) were run to allow comparison in fluorescence intensity measured on different dates. Compensation was adjusted using FITC-CD4/PE-CD8/PerCP-CD3–stained cells. Biological negative control cells within each tube (eg, B cells in a tube stained for T cells) and cells stained with isotypic controls for IgG1-FITC, IgG2-PE, and IgG2a-PerCP were used as negative controls. Data (collected in list mode) were analyzed with Lysis II and CellQuest software (Becton Dickinson). CD45 versus side scatter was used to select the populations of granulocytes, monocytes, lymphocytes, nucleated red cell precursors (erythroid), and primitive hematopoietic cells consistent with blasts. CD19, CD20, CD3, CD14, and CD34 populations were back-gated to determine if analysis gates were appropriate. Orthogonal light scatter (SSC) was compared with that obtained for mature granulocytes in healthy control peripheral blood specimens processed on the same day. Multiparameter data analysis of antibody staining patterns and granulation was used to access the various populations for different trends in antigen expression.
Antibody panel used
| CD/FITC, PE, PerCP . | Cell population defined . |
|---|---|
| CD45/PerCP | All hematopoietic cells |
| CD14/PE | Monocytes |
| CD61/FITC | Megakaryocytes |
| CD41/FITC | Magakaryocytes |
| CD3/FITC, PE | Mature T cells |
| CD8/FITC, PE | T cells, NK cells |
| CD4/FITC, PE | T cells |
| CD2/FITC | T cells |
| CD5/FITC | T cells |
| Anti–glycophorin A/PE | Erythroid lineage |
| CD71/FITC | Transferrin receptor—different expression in multiple lineages |
| CD11b/PE | Mature and intermediate differentiated myeloid series |
| CD19/FITC | B cells |
| CD20/PE | B cells |
| CD22/PE | B cells |
| CD16/PE | T cells, NK cells, and myeloid lineage |
| CD57/FITC | T cells, NK cells |
| CD56/PE | T cells, NK cells |
| CD64/PE | Myeloid cells, mature granulocytes, and monocytes |
| Anti-κ/FITC | B cells |
| Anti-λ/PE | B cells |
| CD36/FITC | Monocytic, erythroid, and megakaryocytic lineages |
| CD7/PE | T cells |
| CD10/PE | Pre-B cells, mature granulocytes |
| CD/FITC, PE, PerCP . | Cell population defined . |
|---|---|
| CD45/PerCP | All hematopoietic cells |
| CD14/PE | Monocytes |
| CD61/FITC | Megakaryocytes |
| CD41/FITC | Magakaryocytes |
| CD3/FITC, PE | Mature T cells |
| CD8/FITC, PE | T cells, NK cells |
| CD4/FITC, PE | T cells |
| CD2/FITC | T cells |
| CD5/FITC | T cells |
| Anti–glycophorin A/PE | Erythroid lineage |
| CD71/FITC | Transferrin receptor—different expression in multiple lineages |
| CD11b/PE | Mature and intermediate differentiated myeloid series |
| CD19/FITC | B cells |
| CD20/PE | B cells |
| CD22/PE | B cells |
| CD16/PE | T cells, NK cells, and myeloid lineage |
| CD57/FITC | T cells, NK cells |
| CD56/PE | T cells, NK cells |
| CD64/PE | Myeloid cells, mature granulocytes, and monocytes |
| Anti-κ/FITC | B cells |
| Anti-λ/PE | B cells |
| CD36/FITC | Monocytic, erythroid, and megakaryocytic lineages |
| CD7/PE | T cells |
| CD10/PE | Pre-B cells, mature granulocytes |
All antibodies were obtained from Becton Dickinson, with the exception of CD7, which was obtained from Coulter Immunology, Hialeah, FL.
FITC indicates fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin chlorophyll protein; NK, natural killer.
Review of bone marrow aspirate morphology and cytogenetics
Bone marrow biopsies and nonanticoagulated aspirates were done on all patients at the time that flow cytometric immunophenotyping was performed. Multiple readers provided the initial diagnosis on the bone marrow aspirates. All bone marrow specimens were reviewed by a single hematologist (M.E.R.) for consistency. Specimens were considered adequate when aspirates and cores allowed evaluation of myeloid, erythroid, and megakaryocytic lineages. The features used to define dysplastic changes are outlined by Bennett et al.4Peripheral blood findings included the following: cytopenias; granulocyte abnormalities such as Pelger-Huet anomaly, hypersegmentation, bizarre nuclear shape (eg, rings or large lobes), increased chromatin clumping, hypogranular or agranular cells, persistence of basophilia in mature cells, and blasts; platelet abnormalities, including giant platelets and hypogranulation; and erythroid abnormalities, eg, nucleated red cells that show dyserythropoietic changes or megaloblastic features. Bone marrows were reviewed for dysgranulopoiesis, dysmegakaryocytopoiesis, and dyserythropoiesis. Examples of dysgranulopoiesis include abnormalities in primary granules such as decreased staining or large granules, decreased or absent secondary granules, nuclear abnormalities similar to those described in the peripheral blood granulocytes above, and left shift or increased blasts. Dysmegakaryocytopoiesis was considered present when the following was observed: micromegakaryocytes, large mononuclear or binuclear forms, multiple small nuclei, reduced numbers. More than 15% ringed sideroblasts, nuclear fragments, multiple nuclei, nuclear lobation, internuclear bridges, megaloblastic erythropoiesis, macronormoblastic erythropoiesis, irregular cytoplasmic staining, and fewer than 5% erythroid cells were considered indicative of dyserythropoiesis. Mild megaloblastic changes without dyspoiesis in other cell lines were not considered sufficient for a diagnosis of MDS.
Adequate cytogenetic data were obtained for bone marrow aspirates from 57 (87.7%) of 65 cases. Adequacy of data was determined based upon technical aspects and whether the specimen submitted for cytogenetic analysis was concurrent with the specimen submitted for flow cytometric evaluation or when identical results were obtained from specimens collected prior to and after the flow cytometric analysis. All cytogenetic reports were reviewed by a single cytogeneticist (D.C.A.).
Results
Patient data
All 65 patients studied received an ultimate diagnosis of MDS based upon clinical data as well as morphologic and cytogenetic data on the initial and repeat bone marrow studies. Of the 65 MDS patients studied, there were 40 males and 25 females (male:female ratio 1.6:1). The mean age was 58.7 years with an SD of 13.3. The demographics of the patients in our study differ somewhat from those cited at primary care institutions. The National Institutes of Health Clinical Center is a tertiary care facility where all patients are enrolled in experimental protocols and must meet eligibility criteria. The patients studied were seeking enrollment on a research protocol focusing on treating the marrow failure component of MDS—not the cellular preleukemic end of the spectrum. Therefore, the population had a younger mean age and a slightly increased proportion of RA. Bone marrow biopsies and aspirates performed at the time of sampling for flow cytometric analysis were available on all 65 patients, although some aspirates were inadequate. All bone marrow specimens were reviewed by a single experienced hematologist (M.E.R.). A firm morphologic diagnosis of MDS was rendered based solely upon the marrow concurrent with the flow cytometry specimen in 45 (69%) of 65 cases (Table 1). The initial diagnosis that had been made by multiple other readers on these marrows was revised in 12 (27%) of 45 cases (Table 1) upon review and reflects the difficulty and potential individual variation in morphologic diagnosis by different readers. In 20 (31%) of 65 cases, a morphologic diagnosis of MDS could not be made on the initial bone marrow evaluation, and additional studies were necessary (Table 2). Adequate cytogenetic data were available for 57 (87.7%) of 65 patients. Clonal cytogenetic abnormalities consistent with MDS were detected in bone marrow aspirates from 25 (44%) of 57 patients. Thirty-nine of the patients with morphologic evidence of MDS in the initial bone marrow evaluation had adequate cytogenetics; 20 (51%) of 39 had clonal cytogenetic abnormalities consistent with MDS (Table 1).
Additional studies were required to render a diagnosis of MDS in 20 (31%) of 65 cases (Table 2). In 12 (60%) of 20 of these cases, a diagnosis could be made within the context of previous and subsequent biopsy material, allowing a final morphologic diagnosis of MDS (Table2). In 8 (12%) of 65 patients, initial and repeat morphology was not diagnostic of MDS (Table 2). Of the 20 patients requiring additional tests, clonal cytogenetic abnormalities consistent with MDS were detected in 5 (25%).
In the parallel studies performed on 15 patients with a diagnosis of aplastic anemia, bone marrow morphology was consistent with aplastic anemia and cytogenetics were normal in all 15 cases (Table 3). In addition, flow cytometric studies were performed on bone marrow aspirates from 4 healthy volunteers and 10 patients with a history of nonmyeloid neoplasia (6 non-Hodgkin lymphomas, 1 chronic lymphocytic leukemia, 2 sarcoma, 1 acute lymphoblastic leukemia) with morphology indicating complete remission (cytogenetics not performed).
Immunophenotypic evaluation of myeloid dysplasia
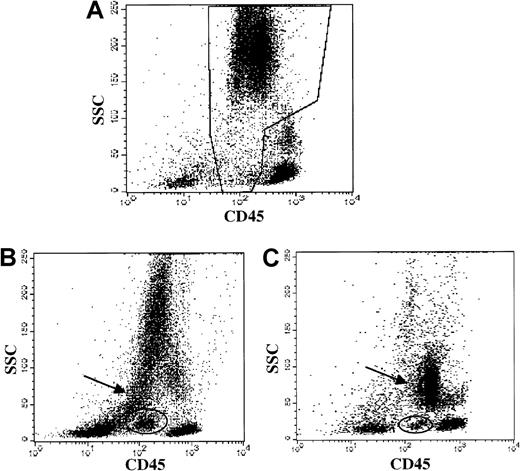
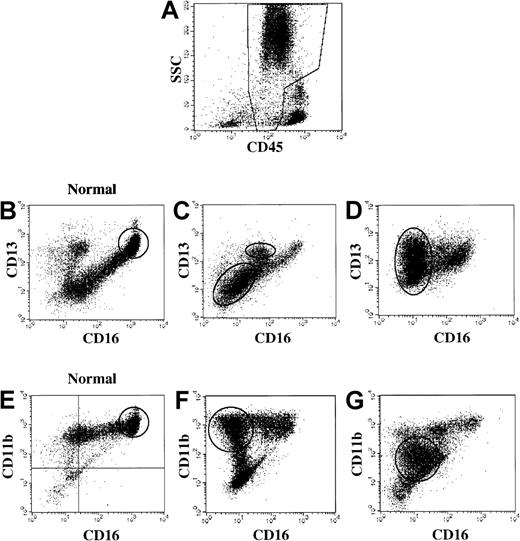
Forty-two cases with a morphologic diagnosis of MDS on the concurrent specimen were evaluated for granulocytic and monocytic abnormalities by flow methods, and multiple granulocytic and monocytic abnormalities were detected (Table 5). Identification of abnormal antigen expression depended upon extensive antibody panels and knowledge of normal patterns. Frequently observed abnormalities included the following: hypogranulation of neutrophils (based upon orthogonal light scatter, Figure1) in 38 (84%) of 45; CD64−granulocytes in 27 (66%) of 41; discrete blast population by CD45 versus orthogonal light scatter (Figure 1) in 24 (57%) of 42; and myeloid cells expressing nonmyeloid antigens in 17 (38%) of 45. Although the patterns of CD11b versus CD16 and of CD13 versus CD16 (Figure 2) were only evaluated in a smaller subpopulation, abnormalities were noted with high frequency in this subgroup (19 [70%] of 27 abnormal CD11b versus CD16; and 21 [78%] of 27 abnormal CD13 versus CD16).
Granulocytic and monocytic lineage abnormalities detected by flow cytometry
| . | MDS patients (%) . |
|---|---|
| Hypogranulation of neutrophils by orthogonal light scatter | 38/45 (84) |
| CD64−granulocytes | 27/41 (66) |
| Discrete blasts by CD45 vs orthogonal light scatter | 24/42 (53) |
| CD56+granulocytes | 97/33 (21) |
| CD56+ monocytes | 11/33 (33) |
| CD11b/CD16 pattern abnormal | 19/27 (70) |
| CD13/CD16 pattern abnormal | 21/27 (78) |
| CD10−granulocytes | 5/45 (11) |
| Bright CD2+ myeloid blasts | 12/45 (27) |
| Myeloid lineage expressing nonmyeloid antigens (eg, B-cell antigen CD22 or T-cell antigen CD7) | 17/45 (38) |
| . | MDS patients (%) . |
|---|---|
| Hypogranulation of neutrophils by orthogonal light scatter | 38/45 (84) |
| CD64−granulocytes | 27/41 (66) |
| Discrete blasts by CD45 vs orthogonal light scatter | 24/42 (53) |
| CD56+granulocytes | 97/33 (21) |
| CD56+ monocytes | 11/33 (33) |
| CD11b/CD16 pattern abnormal | 19/27 (70) |
| CD13/CD16 pattern abnormal | 21/27 (78) |
| CD10−granulocytes | 5/45 (11) |
| Bright CD2+ myeloid blasts | 12/45 (27) |
| Myeloid lineage expressing nonmyeloid antigens (eg, B-cell antigen CD22 or T-cell antigen CD7) | 17/45 (38) |
MDS indicates myelodysplatic syndrome.
Myeloid abnormalities in MDS demonstrated by CD45 versus side light scatter.
Data from patients with straightforward myelodysplastic syndrome, diagnosed by morphology. (A) Healthy donor bone marrow: normal granulocytes and precursors in the boxed region. (B) MDS patient bone marrow (ungated): hypogranular neutrophils with low side scatter (arrow) and a discrete blast population (oval) are demonstrated. (C) MDS patient bone marrow (ungated): hypogranular neutrophils with low side scatter (arrow) and a discrete blast population (oval) are demonstrated.
Myeloid abnormalities in MDS demonstrated by CD45 versus side light scatter.
Data from patients with straightforward myelodysplastic syndrome, diagnosed by morphology. (A) Healthy donor bone marrow: normal granulocytes and precursors in the boxed region. (B) MDS patient bone marrow (ungated): hypogranular neutrophils with low side scatter (arrow) and a discrete blast population (oval) are demonstrated. (C) MDS patient bone marrow (ungated): hypogranular neutrophils with low side scatter (arrow) and a discrete blast population (oval) are demonstrated.
Immunophenotypic myeloid abnormalities in MDS.
(A) Analysis gate used: the analysis gate used is based on CD45 (x-axis) versus side light scatter (SSC, y-axis) and includes granulocytes and precursors (CD45 dim and spectrum of SSC) and is demonstrated in the boxed region of this healthy donor bone marrow. (B) Healthy donor bone marrow. FITC-CD16 (x-axis) versus PE CD13 (y-axis). Oval indicates the majority of cells in this gate in normal bone marrow. (C-D) MDS patient bone marrow: FITC-CD16 (x-axis) versus PE CD13 (y-axis). Ovals highlight abnormal populations. (E) Healthy donor bone marrow. FITC-CD16 (x-axis) versus PE CD11b (y-axis). Oval indicates the majority of cells in this gate in normal bone marrow. (F-G) MDS patient bone marrow: FITC-CD16 (x-axis) versus PE CD11b (y-axis). Ovals highlight abnormal populations.
Immunophenotypic myeloid abnormalities in MDS.
(A) Analysis gate used: the analysis gate used is based on CD45 (x-axis) versus side light scatter (SSC, y-axis) and includes granulocytes and precursors (CD45 dim and spectrum of SSC) and is demonstrated in the boxed region of this healthy donor bone marrow. (B) Healthy donor bone marrow. FITC-CD16 (x-axis) versus PE CD13 (y-axis). Oval indicates the majority of cells in this gate in normal bone marrow. (C-D) MDS patient bone marrow: FITC-CD16 (x-axis) versus PE CD13 (y-axis). Ovals highlight abnormal populations. (E) Healthy donor bone marrow. FITC-CD16 (x-axis) versus PE CD11b (y-axis). Oval indicates the majority of cells in this gate in normal bone marrow. (F-G) MDS patient bone marrow: FITC-CD16 (x-axis) versus PE CD11b (y-axis). Ovals highlight abnormal populations.
Immunophenotypic myeloid abnormalities were considered to be present only when 2 or more abnormalities were detected. The range of myeloid abnormalities was 1 to 7, with a mean of 4.21 (SD = 1.44). Using these criteria, myeloid dyspoiesis was detected by immunophenotype in 41 (98%) of 42 evaluable cases of MDS (Table 1). The bone marrow aspirates were adequate for morphologic evaluation of the myeloid lineage in 43 of 45 cases, and dysplasia was observed in 35 (81%) of 43. A single myeloid immunophenotypic abnormality (Table 3) was noted in 6 of 15 aplastic anemia patients (5 hypogranular granulocytes, 1 equivocal CD64 staining). We conclude that hypogranular granulocytes alone are not a definitive finding for MDS and that other myeloid abnormalities should be detected to conclude myeloid dysplasia is present. Three immunophenotypic myeloid abnormalities were observed in one aplastic anemia patient (CD56+ monocytes, equivocal CD64 staining, and hypogranular granulocytes), although erythroid and megakaryocytic analyses were normal. The bone marrow in this aplastic anemia patient is hypocellular, with no morphologic evidence of dysplasia, and cytogenetics were normal. The patient is being monitored for possible development of MDS. No immunophenotypic myeloid abnormalities were detected in the healthy donor bone marrow aspirates or in the 10 remission bone marrow aspirates from patients with a history of nonmyeloid neoplasia (Table 3).
As mentioned, the immunophenotypic panels used evolved during the study, and not all antigens were studied in the entire MDS patient group. In the earlier series (4 to 7 specific myeloid patterns studied per case) an average of 3.2 ± 1.5 (mean ± SD) abnormalities were observed per case. In the later series (8 to 10 specific myeloid patterns studied per case) an average of 5.0 ± 1.33 (mean ± SD) abnormalities were observed per case. We conclude that the larger myeloid panel yielded a greater probability of detection of myeloid abnormalities in MDS. In contrast, the larger panels did not yield more abnormalities in the controls; in the aplastic anemia cases there were 0.53 ± 0.83 (mean ± SD) myeloid abnormalities per case, in the healthy controls no abnormalities were detected, and in the nonmyeloid neoplastic controls no abnormalities were detected. All of the healthy controls and aplastic anemia cases had 9 or more myeloid patterns assessed, and 9 of the 10 nonmyeloid neoplastic controls had 8 or more myeloid antigens studied. Despite the larger panels in these control specimens, the number of immunophenotypic abnormalities remained low.
Immunophenotypic evaluation of erythroid dysplasia
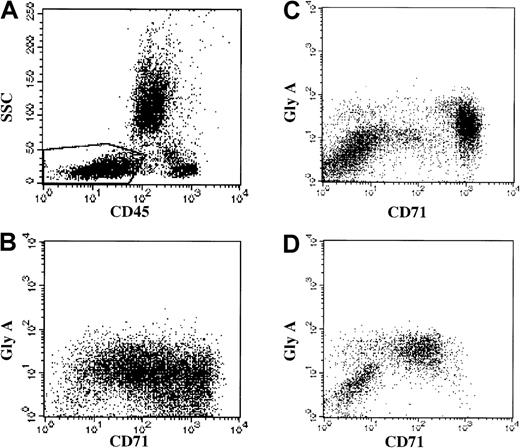
Forty-four cases with a morphologic diagnosis of MDS on the concurrent specimen were evaluated for erythroid abnormalities by flow cytometric immunophenotyping. Immunophenotypic evaluation of erythroid maturation was more challenging than in the myeloid series because fewer antibodies are available for study of the erythroid lineage. Nucleated erythroid cells normally have very high levels of transferrin receptor (CD71) expression. During development from the basophilic erythroblast to the erythrocyte there is decreasing CD45. An increase in glycophorin A is observed early upon differentiation from the basophilic erythroblast to the orthochromic erythroblast. The following were considered evidence of erythroid abnormalities and possible dysplasia (Figure 3): abnormal levels of CD71 (transferrin receptor) expression; dis-synchronous expression of CD45 versus CD71; dis-synchronous expression of CD45 versus glycophorin A; and dis-synchronous expression of CD71 versus glycophorin A on nucleated red cell precursors. Abnormal erythroid precursors were detected based upon immunophenotype in 34 (77%) of 44 typical MDS cases studied (Table 1). The most frequent immunophenotypic abnormality noted was very low CD71 expression on glycophorin A–positive erythroid precursors. This abnormality was noted in 3 of 15 aplastic anemia patients and correlates with morphologic evidence of megaloblastic erythropoiesis in these patients (Table 3). No erythroid lineage abnormalities were noted in the 10 bone marrow aspirates of patients in complete remission from nonmyeloid neoplasia or in healthy controls (Table 3). Morphologic evaluation determined the presence of dysplastic (megaloblastic morphology most common) erythroid precursors in 36 (84%) of 43 cases evaluated.
Immunophenotypic erythroid abnormalities in MDS.
(A) Analysis gate used: the analysis gate used includes nucleated red cell precursors (CD45 dim to negative and low SSC) and is demonstrated in the boxed region of this bone marrow. (B) Healthy donor bone marrow: there is high-level staining for the transferrin receptor (FITC-CD71, x-axis) in the glycophorin A–positive (GLY A, y-axis) nucleated erythroid cells. (C) MDS patient bone marrow: FITC-CD71 (x-axis) versus PE anti–glycophorin A (y-axis). There is a broad spectrum of CD71 expression—from normal to very low. (D) MDS patient bone marrow: FITC-CD71 (x-axis) versus PE anti–glycophorin A (y-axis). There is decreased CD71 expression in the glycophorin A–positive cells.
Immunophenotypic erythroid abnormalities in MDS.
(A) Analysis gate used: the analysis gate used includes nucleated red cell precursors (CD45 dim to negative and low SSC) and is demonstrated in the boxed region of this bone marrow. (B) Healthy donor bone marrow: there is high-level staining for the transferrin receptor (FITC-CD71, x-axis) in the glycophorin A–positive (GLY A, y-axis) nucleated erythroid cells. (C) MDS patient bone marrow: FITC-CD71 (x-axis) versus PE anti–glycophorin A (y-axis). There is a broad spectrum of CD71 expression—from normal to very low. (D) MDS patient bone marrow: FITC-CD71 (x-axis) versus PE anti–glycophorin A (y-axis). There is decreased CD71 expression in the glycophorin A–positive cells.
Immunophenotypic evaluation of megakaryocytes
Detection of megakaryocytic abnormalities was also hampered by a lack of sufficient antibodies to evaluate megakaryocytic maturation and by much smaller numbers in bone marrow aspirates. In addition, giant platelets express megakaryocytic antigens and, because of size, are difficult to differentiate from other cell populations based upon light scatter characteristics. Megakaryocytes express CD45 at approximately the same level as granulocytes but have a different pattern of light scatter (increased forward and decreased orthogonal light scatter). Therefore, an analysis gate for megakaroyctes can be drawn based upon CD45 versus orthogonal light scatter. Megakaryocytes are also positive for CD41a and CD61, and measuring expression of these antigens allows quantitation of megakaryocytes within this gate.16 Flow cytometry detected increased numbers of megakaryocytes (mean 24.6%, range 1%-76%) in MDS specimens compared with aplastic anemia specimens (mean 5.3%) but not compared with normals (mean 23%) or the remission bone marrows from patients with nonmyeloid neoplasia (mean 14%). Increased numbers of megakaryocytes were defined as more than 15% of the gate, which is 4 SDs above that observed in aplastic anemia patients. Increased numbers of megakaryocytes were detected by flow cytometry in 26 (59%) of 44 of the MDS cases (Table 1). Flow cytometric immunophenotyping detected low numbers of megakaryocytes in all of the aplastic anemia cases (Table3). However, 2 of 10 remission bone marrow aspirates from patients with a history of nonmyeloid neoplasia had a high percentage of megakaryocytes (25% and 42% of gate). Normal donor bone marrow aspirates contained a greater number of normal blasts and higher average percentage of megakaryocytes compared with MDS patients (36%, 8%, 28%, and 20% of the gate in the 4 studied). Because the mean percentage of megakaryocytic cells is not significantly higher in MDS compared with normal or nonmyeloid neoplasia controls but is significantly higher compared with aplastic anemia, the percentage of megakaryocytes may only be helpful in distinguishing MDS from aplastic anemia. Morphologic evaluation revealed abnormal megakaryocytes in 40 (91%) of 44 cases and thus had a greater sensitivity than flow cytometry for detection of megakaryocytic abnormalities. Because only quantitative flow cytometric findings were seen in the megakaryocytic lineage and morphologic evaluation was more sensitive, flow cytometric evaluation of megakaryocytic lineage abnormalities is of limited use in MDS. As more antibodies to study megakaryocytic maturation become available, flow cytometric immunophenotyping may be of greater value in detecting dysplasia in this lineage.
Flow cytometric detection of dysplasia in MDS
Because MDS is typified by dyspoiesis in 2 or more lineages, we examined the granulocytic/monocytic, erythroid, and megakaryocytic lineages for abnormalities in the 45 cases with a morphologic diagnosis of MDS on the concurrent bone marrow aspirate. Immunophenotypic data were available on all 3 lineages in 40 (89%) of the 45 MDS patients (Table 1). All specimens were adequate for flow cytometric analysis, and indeterminate flow cytometric results were due to inadequate antibody panels, not inadequate specimens. The rate of detection of abnormalities in 2 or more lineages was 35 (88%) of 40 by flow cytometric immunophenotyping and 37 (93%) of 40 by morphology. Trilineage abnormalities were detected by immunophenotype in 18 (45%) of 40 versus 22 (55%) of 40 cases by morphology (Table 1). Bilineage abnormalities were detected by immunophenotype in 17 (43%) of 40 of the cases versus 15 (38%) of 40 cases by morphology. A single-lineage abnormality was determined in 5 (13%) of 40 cases by immunophenotype and in 3 (8%) of 40 cases by morphology. In all 5 cases the single-lineage immunophenotypic abnormalities were in the myeloid series, where we have greatest sensitivity of detection of abnormalities because of the extensive number of antibodies available to study this lineage. In 2 cases dysplastic megakaryocytes and peripheral blood changes allowed a morphologic diagnosis of MDS despite inadequate aspirate material for assessment of erythroid and granulocytic precursors. In 1 case ringed sideroblasts were detected without morphologic evidence of megakaryocytic or granulocytic dysplasia. Immunophenotypic data were available on 2 lineages in the remaining 5 of 45 cases. In 3 of these cases there were abnormalities in both lineages, bringing the total detection of abnormalities in 2 or more lineages to 38 (84%) of 45 by flow cytometric immunophenotyping and 41 (91%) of 45 by morphology.
Adequate cytogenetic data were available on 39 patients with a morphologic diagnosis of MDS on the initial specimen. Clonal cytogenetic abnormalities consistent with MDS were detected in bone marrow aspirates from 20 (51%) of 39 (Table 1). Flow cytometric analysis detected bilineage or trilineage abnormalities in all 20 (100%) cases with clonal cytogenetic abnormalities consistent with MDS. However, in the 18 cases with normal cytogenetics results that were adequately evaluated by flow cytometry, flow cytometric analysis detected a lower incidence of bilineage or trilineage abnormalities (15 [83%] of 18 cases). Thus, there was a correlation between the detection of bilineage or trilineage abnormalities by flow cytometry and clonal karyotypic abnormalities by cytogenetics.
Flow cytometric immunophenotypic abnormalities in a single lineage were detected in bone marrow aspirates from 4 of 15 aplastic anemia patients, 2 of 10 non-Hodgkin lymphoma patients in complete remission, and 2 of 4 normal bone marrow aspirate specimens (Table 3). Dual-lineage or trilineage abnormalities were not detected. In view of the control specimen results, we would consider immunophenotypic abnormalities in 2 or more lineages to be diagnostic of MDS in the appropriate clinical setting.
Correlation of flow cytometric with morphologic determination of dyspoiesis
Detection of abnormalities by flow cytometric and morphologic methods were compared in the 45 cases of straightforward MDS. The Fisher exact test was used for correlation of the 2 and the McNemar test to determine sensitivity (Table 6). There is no apparent association between flow cytometric detection of lineage abnormalities and French-American-British classification as determined by the Fisher-Freeman-Halton test (P values .85, .31, and .26). The correlation P value is large for the myeloid series because of the high sensitivity of flow cytometric detection. Forty cases had adequate samples to compare flow cytometric and morphologic data in the myeloid series. Flow cytometric immunophenotyping for myeloid dyspoiesis was more sensitive than morphology, detecting abnormal maturation of the myeloid series in 39 (98%) of the 40 cases that had both adequate antibody panels and morphology for the assessment of myeloid series. In comparison, morphology detected 32 (80%) of these 40 cases. There was concordance between the 2 methods in 31 (78%) of the 40 cases with adequate morphology and immunophenotypic data. In 1 of 40 cases dysplastic changes in the myeloid series were detected by morphology but the myeloid immunophenotype was normal. In 8 of 40 cases there was immunophenotypic evidence of myeloid abnormalities but not definitive myeloid dysplasia detected by morphology. In addition, flow cytometric immunophenotyping detected myeloid abnormalities in 2 cases in which the aspirates collected for morphology were inadequate. This was observed despite the first, and arguably best, aspirate being obtained for morphology, indicating that specimen requirements are not as demanding for flow cytometric immunophenotyping.
Flow cytometric and morphologic detection of dysplasia: correlation between the two methods and comparison of sensitivity
| Dysplastic lineage . | No. of pairs . | Correlation,P . | Sensitivity, P . | Flow positive . | Morphology positive . |
|---|---|---|---|---|---|
| Erythroid | 42 | .62 | .39 | 32/42 | 36/42 |
| Megakaryocytic | 44 | 1.0 | .00013 | 26/44 | 40/44 |
| Myeloid | 40 | 1.0 | .039 | 39/40 | 32/40 |
| Dysplastic lineage . | No. of pairs . | Correlation,P . | Sensitivity, P . | Flow positive . | Morphology positive . |
|---|---|---|---|---|---|
| Erythroid | 42 | .62 | .39 | 32/42 | 36/42 |
| Megakaryocytic | 44 | 1.0 | .00013 | 26/44 | 40/44 |
| Myeloid | 40 | 1.0 | .039 | 39/40 | 32/40 |
Although morphology appeared slightly more sensitive than immunophenotype in detecting erythroid abnormalities, this was not statistically significant (Table 6). There was concordance between morphology and immunophenotype in 30 (71%) of the 42 cases with adequate morphology and immunophenotypic data on the erythroid lineage (Table 6). In 6 cases there was immunophenotypic but no definitive morphologic evidence of erythroid abnormalities—due to inadequate aspirate in 2 cases and no definitive morphologic evidence of dysplasia in 4 cases with good cellularity. In 8 cases erythroid abnormalities were detected by morphology but not immunophenotype.
Forty-four cases had adequate flow cytometric data and morphology for assessment of megakaryocytes. Morphology was more sensitive than immunophenotype in detecting megakaryocytic abnormalities (Table 6). Dysplastic megakaryocytes, including micromegakaryocytes and hypolobulated forms, were observed by morphology in 40 (91%) of the 44 cases with adequate morphology and flow cytometric analysis. There was an increased percentage of megakaryocytic lineage cells detected by flow cytometric immunophenotype in 26 of these 44 cases. The higher percentage of megakaryocytes observed may reflect an increased aspirability of dysplastic megakaryocytes or simply increased numbers of megakaryocytes. There was concordance between dysplastic megakaryocytes by morphology and increased numbers by immunophenotype in 26 (59%) of 44 cases in which both tests were adequate. In 16 cases abnormal megakaryocytes were noted by morphology but not immunophenotype. In 2 cases there were increased megakaryocytes detected by flow, but morphology was normal.
Diagnostic utility of flow cytometric immunophenotyping in cases of MDS with indeterminate morphology
Morphologic and cytogenetic evaluations are the gold standards in the diagnosis of MDS. In our series of 65 patients with MDS, a diagnosis of MDS could not be made in 20 patients based upon morphology alone in a single specimen (the specimen that was concurrent with the flow cytometric sample). Clonal cytogenetic abnormalities consistent with MDS were detected in 5 of the original bone marrow aspirates (5 [25%] of 20 cases). In 12 (60%) of 20 patients a diagnosis of MDS could be made based upon review of the biopsy in conjunction with previous bone marrows and repeat specimens (Table 2). These 12 patients included the 5 patients in which clonal cytogenetic abnormalities consistent with MDS were demonstrated. Therefore, a diagnosis of MDS could be made based upon morphology and cytogenetics in a single bone marrow specimen in 25% of difficult MDS cases (50 [77%] of 65 total cases). Repeat bone marrows increased the diagnostic accuracy to 12 (60%) of 20 in difficult-to-diagnose cases of MDS and to 57 (88%) of 65 cases studied. The remaining 8 patients with indeterminate morphology on initial and repeat bone marrow studies and no evidence of abnormal cytogenetics studies ultimately received a diagnosis of MDS on later follow-up examination. Flow cytometric immunophenotyping was informative in 15 (75%) of 20 patients with indeterminate morphology on concurrent bone marrow (Table 2) by demonstrating dual-lineage or trilineage immunophenotypic abnormalities, a finding absent in all aplastic anemia, healthy donor, and remission nonmyeloid neoplasia marrows studied. Multiple myeloid abnormalities were detected in all but 1 (95%) of the 19 cases with adequate myeloid immunophenotypic data. However, multiple myeloid abnormalities were only detected in 1 aplastic anemia patient (erythroid and megakaryocytic data normal in this patient) and none of the healthy donor or nonmyeloid neoplasia in remission marrows studied. Flow cytometric immunophenotyping detected abnormal erythroid precursors in 12 of 20 cases and increased megakaryocytes in 8 of 20 cases. This is in comparison to abnormal erythroid precursors in 3 (20%) of 15 and no cases of increased megakaryocytes encountered by immunophenotype in the aplastic anemia specimens. In 8 of 20 patients, initial morphology as well as cytogenetics and a repeat morphologic evaluation did not lead to a diagnosis of MDS. Flow cytometric immunophenotyping demonstrated dual-lineage or trilineage immunophenotypic abnormalities in 6 (75%) of 8 patients with normal cytogenetic studies and indeterminate morphology on initial and repeat bone marrow (Table7). Thus, flow cytometry could detect immunophenotypic abnormalities in cases when combined morphology and cytogenetics were nondiagnostic.
Contribution of flow cytometric immunophenotyping in myelodysplatic syndrome cases negative by morphology and cytogenetics
| Patient . | Morphology . | Cytogenetics . | Flow cytometric immunophenotyping . | ||
|---|---|---|---|---|---|
| Erythroid . | Megakaryocytic . | Myeloid . | |||
| 1 | − | − | − | − | − |
| 2 | − | − | − | + | + |
| 3 | − | − | + | − | + |
| 4 | − | − | + | − | + |
| 5 | − | NA | + | − | + |
| 6 | − | − | + | + | + |
| 7 | − | − | − | + | + |
| 8 | − | − | − | − | + |
| Patient . | Morphology . | Cytogenetics . | Flow cytometric immunophenotyping . | ||
|---|---|---|---|---|---|
| Erythroid . | Megakaryocytic . | Myeloid . | |||
| 1 | − | − | − | − | − |
| 2 | − | − | − | + | + |
| 3 | − | − | + | − | + |
| 4 | − | − | + | − | + |
| 5 | − | NA | + | − | + |
| 6 | − | − | + | + | + |
| 7 | − | − | − | + | + |
| 8 | − | − | − | − | + |
NA indicates not available.
Discussion
The MDSs are a group of heterogeneous hematologic disorders characterized by bilineage or trilineage dysplastic morphology, abnormal clonal populations, progressive bone marrow failure, and a high rate of transformation to acute myeloid leukemia. The incidence of MDS has increased significantly over the last 20 years, with possible causes including an aging population and increased environmental exposure to toxins.17 In most cases the diagnosis is straightforward according to the French-American-British classification scheme.1,2,4 However, morphologic evaluation of blood and bone marrow is not adequate for diagnosis in a significant number of MDS patients. This is often due to failure to obtain suitable bone marrow aspirates, either because of hypocellularity or fibrosis of the marrow.3,5,6 The distinction between hypoplastic MDS and aplastic anemia can be especially difficult because both can present with hypocellular bone marrows and pancytopenia. Furthermore, patients with aplastic anemia can develop MDS and even acute leukemia years after successful treatment by antilymphocyte globulin. This has led investigators to propose that some forms of aplastic anemia and MDS may have a common pathophysiology and etiology.7 The distinction between these 2 diseases is, however, important because the risk of progression to acute leukemia is much greater in MDS.6 7
The standard criteria for the diagnosis of MDS are based upon morphology and demonstration of clonal cytogenetic abnormalities.1,2,4 5 In this study we evaluated the utility of flow cytometric immunophenotyping in the diagnosis of MDS. We studied 45 patients with straightforward MDS (as diagnosed by morphologic evaluation of the initial bone marrow specimen) and compared results with those obtained in healthy controls, patients with aplastic anemia, and patients in complete remission after treatment for nonmyeloid neoplasia. Flow cytometric immunophenotyping of bone marrow aspirates resulted in detection of myeloid, erythroid, and megakaryocytic abnormalities in the 45 patients with MDS. Detection of abnormalities in 2 or more lineages was specific for MDS and was not observed in aplastic anemia, healthy controls, or remission bone marrow aspirates from patients with nonmyeloid neoplasia. The rate of detection of abnormalities in 2 or more lineages was 88% for flow cytometric immunophenotyping and 93% by morphology. A diagnosis of MDS by morphology was rendered in 7% of the 45 cases without bilineage dysplasia. In 2 cases dysplastic megakaryocytes and peripheral blood changes allowed a morphologic diagnosis of MDS despite inadequate aspirate material for the assessment of erythroid and granulocytic precursors. In 1 case ringed sideroblasts were detected without morphologic evidence of megakaryocytic or granulocytic dysplasia. Flow cytometric immunophenotyping was more sensitive than morphology in detecting myeloid abnormalities but less sensitive in megakaryocytic dysplasia. Although flow cytometric immunophenotyping was slightly less sensitive than morphology in detecting erythroid dysplasia, this difference was not statistically significant. In addition, flow cytometric analysis was informative in cases in which the aspirates collected for morphology were inadequate despite the first (and arguably best) aspirate being obtained for morphology. This indicates that specimen requirements are not as rigorous for flow cytometric immunophenotyping and diagnostic interpretation is possible with highly hypocellular bone marrow aspirates. Therefore, flow cytometric evaluation of bone marrow aspirates may be very helpful in differentiating hypocellular MDS from aplastic anemia. Both flow cytometric immunophenotyping and morphologic evaluation were more sensitive than cytogenetics in detecting MDS. As more antibodies useful in studying erythroid and megakaryocytic maturation are developed, the sensitivity of flow cytometric testing may increase.
The difficulty of accurate diagnosis of MDS is well illustrated in the current study. Review of biopsies and aspirates by an experienced morphologist resulted in a change in diagnosis in 12 of 45 cases, indicating the pitfalls inherent in morphologic evaluation of this disease. A diagnosis of MDS could be made based upon morphologic evaluation of a single bone marrow specimen in only 45 of 65 cases. In 20 cases additional studies were required to render a diagnosis of MDS. A diagnosis of MDS could be made based upon cytogenetics in the initial bone marrow specimen in 5 (25%) of the 20 difficult cases. Repeat biopsies with morphologic evaluation resulted in a diagnosis of MDS in 12 of 20 difficult cases (clonal cytogenetic abnormalities were demonstrated in 5 of these cases). With cytogenetic analysis and repeat bone marrow sampling, 12 of 20 difficult cases received a diagnosis of MDS. Thus, patients with pancytopenia and indeterminate bone marrow morphology benefit from cytogenetic assessment, consultation with a morphologist with extensive experience in this disease entity, and repeat bone marrow examination. Eight patients ultimately received a diagnosis of MDS despite an inability to render this diagnosis based upon morphology and cytogenetics at presentation. Flow cytometric immunophenotyping was informative in 6 of these 8 patients due to demonstration of dual-lineage or trilineage immunophenotypic abnormalities, a finding absent in all aplastic anemia, healthy donor, and nonmyeloid neoplasia in remission marrows studied. We conclude that flow cytometric immunophenotypic evaluation may be of benefit in difficult cases in which morphology and cytogenetics are nondiagnostic but clinical suspicion of MDS is high.
In conclusion, because morphology and cytogenetics combined are sufficient to render a diagnosis in most MDS cases and because the panels needed for complete immunophenotypic analysis of all 3 lineages are extensive as well as costly, we do not recommend flow cytometric evaluation as a screening procedure for MDS. Morphologic and cytogenetic evaluation, with repeat analysis where indicated, are optimal screening tools in this disease. However, in cases in which morphology and cytogenetics are indeterminate and repeat analyses are noninformative, flow cytometric immunophenotyping can help establish the diagnosis of MDS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maryalice Stetler-Stevenson, Flow Cytometry Unit, Laboratory of Pathology, DCS, NCI, NIH, Bldg 10, Rm 2N-108, Bethesda, MD, 20892; e-mail: stetler@box-s.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal