In follicular lymphoma, the t(14;18) status of the peripheral blood and bone marrow analyzed by polymerase chain reaction (PCR) is assumed to correlate with disease activity in patients with relapsed disease. The clinical significance of quantitating circulating lymphoma cells by real-time PCR is reported in patients on first-line treatment. Thirty-four consecutive patients with previously untreated follicular lymphoma and detectable t(14;18)-positive cells in pretreatment peripheral blood samples were monitored. All patients were treated with standard chemotherapy in combination with interferon alfa-2b. Before and after induction therapy, blood samples were taken for quantitative analysis of t(14;18). At presentation, a median of 262 t(14;18)-positive cells per 75 000 normal cells was found (range, 1-75 000). Patients with lower numbers of circulating tumor cells more frequently had bulky disease (P = .02). Seventy-nine percent of the patients responded clinically to treatment. In 22 of 28 patients, including 4 patients in whom treatment had failed clinically, the number of circulating t(14;18)-positive cells decreased to undetectable or low levels after therapy. In the remaining responding patients, circulating tumor cells persisted after therapy. These quantitative data on circulating t(14;18)-positive cells call into question the usefulness of molecular monitoring of the blood in a group of patients with follicular lymphoma uniformly treated with a noncurative first-line regimen. T(14;18)-positive cells decreased in peripheral blood after treatment, irrespective of the clinical response. Therefore, the significance of so-called molecular remission should be reconsidered in follicular lymphoma.
Introduction
Follicular non-Hodgkin lymphoma (NHL) is characterized by the chromosomal translocation t(14;18)(q32;q21),1 leading to the overexpression ofbcl-2 with subsequent inhibition of apoptosis.2The presence of t(14;18)-positive cells, analyzed by polymerase chain reaction (PCR), is assumed to correlate with lymphoma activity. However, until now the clinical significance of the presence or absence of t(14;18)-positive cells in bone marrow and peripheral blood has not been settled.3-7 Quantitation of circulating t(14;18)-positive cells, showing variation at diagnosis and over time, may lead to a better understanding of the natural course of the disease and the effects of treatment.
Previously we observed an excellent correlation between numbers of t(14;18)-positive cells in peripheral blood, analyzed by real-time PCR, and the graft-versus-lymphoma effect of donor leukocyte infusion in a patient with follicular NHL who had a relapse after allogeneic bone marrow transplantation (BMT).8 Here, we present the results of prospective monitoring of circulating t(14;18)-positive cells by real-time quantitative PCR before and during first-line treatment of patients with follicular lymphoma, consisting of standard chemotherapy in combination with interferon alfa-2b and followed by interferon alfa-2b maintenance therapy.9 10
Patients and methods
Patients
In December 1994, the South-East Netherlands Comprehensive Cancer Centers Cooperative Group (Interzol) started an open, nonrandomized t(14;18) monitoring study in patients with previously untreated follicular NHL of low-grade malignancy in Ann Arbor stages II bulky (larger than 5 cm), III, and IV. Histologic diagnoses of the original lymph node biopsy samples were reviewed centrally according to the REAL-WHO classification. Furthermore, t(14;18) in the biopsy samples was analyzed through the use of conventional PCR (KH and JvK). Informed consent was obtained from all patients to collect blood samples for t(14;18) monitoring. Before the start of treatment, circulating t(14;18)-positive cells with abcl-2 gene breakpoint in the major breakpoint region (MBR) were identified in 34 consecutive patients. Circulating t(14;18)-positive cells were then quantitated in these patients before the start of treatment, after induction therapy, and every 3 months thereafter. Peripheral blood leukocyte and lymphocyte counts were recorded at all time points of t(14;18) sampling. All patients were treated with 8 courses once every 4 weeks of CVP (cyclophosphamide 750 mg/m2 intravenously on day 1, vincristine 2 mg intravenously on day 1, and prednisone 60 mg/d orally on days 1-5), in combination with interferon alfa-2b (5 × 106 IU subcutaneously 3 times a week). Thereafter, responding patients continued treatment with interferon alfa-2b maintenance therapy (5 × 106 IU subcutaneously 3 times a week) until intolerable toxicity, progressive disease, or death. An initial wait-and-see period was allowed before induction therapy was started.
Molecular analysis
Unmanipulated whole blood samples were directly frozen at −20°C and stored in the participating center until transport on dry ice to our laboratory for DNA isolation and subsequent molecular analysis.
Cells carrying t(14;18) with a bcl-2 gene breakpoint in the MBR were quantified performing real-time PCR analysis usingTaq polymerase and an internal probe in the ABI/Prism 7700 sequence detector (Applied Biosystems, Foster City, CA).11For this, 500 ng DNA of each patient sample, measured by optical density at 260 nm, was amplified in duplicate in the presence of 300 nM MBR-2 (5′-TCC CTT TGA CCT TGT TTC TTG A-3′) and JH-con oligonucleotides,12 200 nM dual-labeled fluorogenic MBR internal probe (5′-(FAM)-CAC AGA CCC ACC CAG AGC CC-(TAMRA)-3′), 250 μM dNTPs, 1.25 U AmpliTaq gold DNA polymerase, and 4 mM MgCl2 in sample buffer A (Applied Biosystems) in a total volume of 50 μL. Samples were heated for 10 minutes at 95°C and amplified for 50 cycles of 15 minutes at 95°C and 60 minutes at 60°C. In parallel reactions, 500 ng patient DNA was amplified in duplicate in the presence of 300 nM albumin forward (5′-TGA AAC ATA CGT TCC CAA AGA GTT T-3′) and reverse primer (CTC TCC TTC TCA GAA AGT GTG CAT AT-3′), 200 nM dual-labeled fluorogenic albumin probe (5′-(JOE)-TGC TGA AAC ATT CAC CTT CCA TGC AGA-(TAMRA)-3′), 250 μM dNTPs, 1.25 U AmpliTaq gold DNA polymerase, and 4 mM MgCl2 in sample buffer A in a total volume of 50 μL. Amplification conditions for the albumin reaction were as mentioned above. Given that the t(14;18) and albumin reactions had equivalent amplification efficiencies (E = 0.91 ± 0.05 andE = 0.93 ± 0.04, respectively),13 the t(14;18) PCR signal, reflecting the total number of lymphoma cells, was directly normalized to the albumin PCR signal, reflecting the total number of cells used in the assay. For quantitation, normalized patient PCR signals were compared to a single calibrator sample using the comparative Ct-method. Briefly, for each patient sample, the t(14;18) PCR Ct-value—that is, the cycle number at which emitted fluorescence exceeds the 10× SD of base-line emissions as measured from cycles 3 to 15—is normalized to the albumin PCR Ct-value by subtracting the albumin Ct-value from the t(14;18) Ct-value (ΔCtS). All patient samples are quantified against normalized Ct values of a calibrator sample (ΔCtC) using the following equation: ⇒ XSample = XCal × 2−(ΔCts−ΔCtc) = 750 × 2−ΔΔCt whereby XSampleis the number of t(14;18) lymphoma cells per 500 ng patient sample DNA. The calibrator sample was a 100-fold dilution of t(14;18)-positive cell line SU-DHL-6 into a DNA pool obtained from 4 Epstein-Barr virus–induced lymphoblastoid B-cell lines, with 750 t(14;18) carrying lymphoma cells per 500 ng DNA (equivalent to 75 000 cells). The sensitivity limit of our real-time PCR assay equals a single lymphoma cell in a background of 75 000 normal cells (data not shown). Earlier we had already shown that even after independent sampling, DNA isolation, and quantitation, the variation in results is within 1 log difference.8 All patient t(14;18) PCR products had been resolved on agarose gel to exclude PCR positivity due to contamination, revealing a unique PCR product compatible with a unique chromosomal rearrangement for each patient. In 25 of 30 analyzed lymph nodes, we were able to confirm lymph node t(14;18) MBR positivity performing conventional PCR. Presence of t(14;18) has been reported in benign lymphoid tissue14 and in the blood of healthy persons.15-17 To establish the lower noise level of our PCR, we analyzed t(14;18) in the blood of healthy persons. In 15 of 102 (15%), we found circulating t(14;18)-positive cells. Eight persons had one positive cell per 75 000 cells, and 7 had 2 to 7 positive cells per 75 000 cells. For 9 PCR products, we were able to determine the 14q+ breakpoint sequence that was unique for each person. These sequences did not differ from that of the chromosomal breakpoint regions observed in patients with follicular NHL, but none of the sequences was identical to that obtained previously in our laboratory, thereby excluding contamination. Recently, in a larger group of healthy persons, the presence of circulating t(14;18)-positive cells in approximately the same numbers, analyzed with real-time PCR, was reported.17
Statistical analysis
Patient characteristics between groups were compared with the χ2 test (level of significance: P < .05). Progression-free survival was measured from the start of induction therapy with CVP and interferon alfa until the time of disease progression or until the end of the observation period in patients without progressive disease. Ending the protocol treatment because of other causes than progressive disease—for example, intolerable toxicity or death from diseases other than malignant lymphoma—was censored. If a patient had had an initial wait-and-see policy, progression-free survival was still measured from the date induction therapy was started. Survival curves were calculated according to the Kaplan-Meier method; the log-rank test was used for analyzing differences between curves.
Results
Clinical characteristics
Table 1 summarizes the clinical characteristics of the 34 patients with circulating t(14;18)-positive cells at presentation. Seventy-nine percent of the patients responded to induction therapy with 8 CVP courses combined with interferon alfa-2b. After a median follow-up of 44 months (range, 8-69 months), the median progression-free survival time of patients was 23 months. Median overall survival has not yet been reached.
Clinical data of 34 patients with follicular lymphoma and pretreatment circulating t(14;18)-positive cells
| . | n . | % . |
|---|---|---|
| Sex | ||
| Male | 21 | 62 |
| Female | 13 | 38 |
| Age | ||
| Median (y) | 52 | |
| Range (y) | 30-79 | |
| Ann Arbor stage | ||
| Stage II bulky or III | 11 | 32 |
| Stage IV | 23 | 68 |
| Systemic symptoms | 7 | 21 |
| Bulky disease | 17 | 52 |
| Bone marrow involvement* | 23 | 72 |
| Leukemic | 2 | 6 |
| Previous wait-and-see policy | ||
| n | 7 | 21 |
| Median period (mo) | 20 | |
| Range (mo) | 9-32 | |
| Response to 8 CVP + IFN | ||
| Complete remission | 16 | 44 |
| Partial remission | 11 | 34 |
| Stable disease | 2 | 6 |
| Progressive disease | 5 | 16 |
| End of protocol treatment† | ||
| Insufficient response to induction | 7 | 29 |
| Toxicity of induction | 3 | 13 |
| Progression during maintenance | 9 | 37 |
| Toxicity of maintenance | 5 | 21 |
| . | n . | % . |
|---|---|---|
| Sex | ||
| Male | 21 | 62 |
| Female | 13 | 38 |
| Age | ||
| Median (y) | 52 | |
| Range (y) | 30-79 | |
| Ann Arbor stage | ||
| Stage II bulky or III | 11 | 32 |
| Stage IV | 23 | 68 |
| Systemic symptoms | 7 | 21 |
| Bulky disease | 17 | 52 |
| Bone marrow involvement* | 23 | 72 |
| Leukemic | 2 | 6 |
| Previous wait-and-see policy | ||
| n | 7 | 21 |
| Median period (mo) | 20 | |
| Range (mo) | 9-32 | |
| Response to 8 CVP + IFN | ||
| Complete remission | 16 | 44 |
| Partial remission | 11 | 34 |
| Stable disease | 2 | 6 |
| Progressive disease | 5 | 16 |
| End of protocol treatment† | ||
| Insufficient response to induction | 7 | 29 |
| Toxicity of induction | 3 | 13 |
| Progression during maintenance | 9 | 37 |
| Toxicity of maintenance | 5 | 21 |
CVP indicates cyclophosphamide, vincristine, and prednisone courses; IFN, interferon alpha-2b.
Bone marrow examination performed in 32 of 34 patients.
24 of 34 patients ended treatment according to the protocol; others continued treatment.
Quantitative t(14;18) at diagnosis
In blood samples taken before the start of treatment, the median number of t(14;18)-positive cells was 262 (range, 1-75 000) per 75 000 cells. Table 2 shows the clinical characteristics of patients with high numbers (≥ 262 per 75 000 cells) and low numbers (up to 262 per 75 000 cells) of t(14;18)-positive cells. In patients with the higher numbers of t(14;18)-positive cells, stage IV disease (mostly due to bone marrow involvement) was more frequent than in those with the lower numbers (P = .01). On the other hand, bulky disease was more often present in patients with the lower numbers of circulating t(14;18)-positive cells (P = .02). The highest numbers of t(14;18)-positive cells at presentation were found in the 2 patients with overt leukemic NHL, with lymphocyte counts of 15 and 22 × 109/L respectively (patients 1 and 2).
Clinical characteristics related to the number of t(14;18)-positive cells per 75 000 cells in blood before the start of therapy
| . | Below the median of 262 (n = 17) (%) . | Above the median of 262 (n = 17) (%) . | P . |
|---|---|---|---|
| Stage IV | 47 | 88 | .01 |
| Involved bone marrow* | 50 | 94 | .01 |
| Bulky disease | 71 | 29 | .02 |
| Prior wait-and-see | 29 | 12 | NS |
| . | Below the median of 262 (n = 17) (%) . | Above the median of 262 (n = 17) (%) . | P . |
|---|---|---|---|
| Stage IV | 47 | 88 | .01 |
| Involved bone marrow* | 50 | 94 | .01 |
| Bulky disease | 71 | 29 | .02 |
| Prior wait-and-see | 29 | 12 | NS |
NS indicates not significant.
Analyzed in 32 of 34 patients.
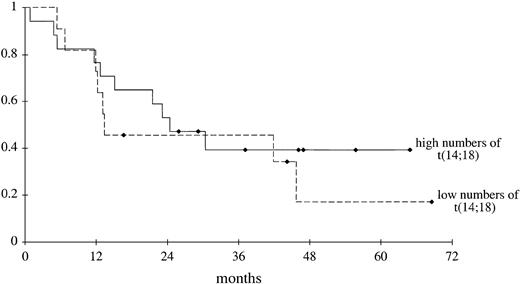
Progression-free survival curves of patients with higher and lower numbers of circulating t(14;18)-positive cells before therapy are shown in Figure 1. In the patients with lower numbers, we excluded those with up to 10 circulating t(14;18)-positive cells per 75 000 cells because this number approximates the maximum number of t(14;18)-positive cells we and others17 found in the blood of healthy persons through real-time PCR analysis. Progression-free survival did not differ between these groups (P = .26). Even if the patients with pretreatment 1 to 10 circulating t(14;18)-positive cells per 75 000 cells were included in the group of patients with lower numbers, progression-free survival of this group did not differ from that of the group with higher numbers (P = .23) (data not shown).
Progression-free survival of patients.
Progression-free survival was measured from the start of induction therapy until disease progression or, in patients without disease progression, until the end of the observation period. Seventeen patients had high (262 or more) and 11 patients had low (10-261) numbers of circulating t(14;18)-positive cells before treatment (P = .26, log-rank test). ♦ indicates end of observation period for individual patients.
Progression-free survival of patients.
Progression-free survival was measured from the start of induction therapy until disease progression or, in patients without disease progression, until the end of the observation period. Seventeen patients had high (262 or more) and 11 patients had low (10-261) numbers of circulating t(14;18)-positive cells before treatment (P = .26, log-rank test). ♦ indicates end of observation period for individual patients.
Sequential quantitative t(14;18) monitoring
In 28 patients, t(14;18)-positive cells could be quantitated in paired blood samples, taken before and after induction treatment with CVP and interferon alfa-2b (Table 3). In all but one patient, the number of circulating t(14;18)-positive cells dropped, irrespective of the clinical response. In 13 patients, t(14;18) could not be detected after treatment. Twelve of them had a clinical response, but one showed progressive disease. In 9 patients, numbers of t(14;18) dropped to low levels of 1 to 10 per 75 000 cells, yet treatment had failed in 3 of these. One of them (patient 5) was the only patient who, in retrospect, did not have low-grade lymphoma but had discordant NHL, with a small cell component in lymph node and bone marrow and a large cell component in the femur. After induction therapy, therapy-resistant progressive disease of the large cell type was found in lymph node and femur. Finally, in 4 responding patients, the numbers decreased but remained positive at levels of 12, 79, 177, and 361 per 75 000 cells, respectively. In one patient, a decrease to 11 per 75 000 cells was found though treatment failed. In most patients, circulating t(14;18)-positive cells decreased by one or more logs after induction therapy. Analysis of absolute leukocyte and lymphocyte counts in the blood before and after induction showed that the decrease in t(14;18) did not result from leukopenia or lymphopenia per se. As induction treatment led to a decrease in the median number of t(14;18)-positive cells from 262 to 1 per 75 000 cells, it led to a decrease in the median numbers of leukocytes from 7.5 to 4.0 × 109/L and to a decrease of lymphocytes from 1.3 to 0.7 × 109/L.
Clinical data and circulating t(14;18)-positive cells before and after induction treatment (patients numbered according to numbers of t(14;18)-positive cells before induction)
| Patient . | Stage . | Bone marrow . | Bulky disease . | Response to induction . | t(14;18) Lymph node . | t(14;18) Before induction3-150 . | t(14;18) After induction3-150 . |
|---|---|---|---|---|---|---|---|
| 1 | IV3-152 | + | − | PR | + | 76 145 | 1 |
| 23-151 | IV3-152 | + | − | PR | + | 31 890 | 3 |
| 3 | IV | + | + | PD | + | 8 573 | ND |
| 4 | IV | + | + | PD | + | 2 467 | ND |
| 5 | IV | + | + | PD | + | 2 217 | 10 |
| 6 | IV | + | − | PR | + | 1 779 | 1 |
| 7 | III | − | − | CR | + | 1 706 | 12 |
| 8 | IV | + | − | CR | ND | 1 684 | ND |
| 93-151 | IV | + | + | PR | + | 1 657 | 361 |
| 10 | IV | + | − | CR | + | 1 410 | ND |
| 11 | IV | + | − | CR | + | 1 234 | 0 |
| 12 | IV | + | + | PR | + | 991 | 79 |
| 13 | IV | + | − | CR | + | 436 | 1 |
| 14 | IV | + | − | CR | + | 351 | 1 |
| 15 | IV | + | − | PR | + | 319 | 0 |
| 16 | III | ND | − | CR | ND | 311 | 0 |
| 17 | IV | + | − | PR | + | 277 | 177 |
| 18 | IV | + | − | CR | + | 247 | 0 |
| 19 | II | − | + | SD | ND | 80 | 11 |
| 20 | IV | + | + | CR | ND | 71 | 0 |
| 21 | III | ND | + | PR | + | 70 | 0 |
| 22 | II | − | + | CR | + | 63 | 0 |
| 23 | III | − | + | CR | + | 34 | 0 |
| 243-151 | III | − | + | CR | + | 25 | 5 |
| 25 | II | − | + | PD | − | 24 | 1 |
| 26 | IV | + | − | CR | + | 16 | 0 |
| 27 | IV | + | + | SD | + | 16 | 1 |
| 283-151 | III | − | − | CR | − | 10 | ND |
| 29 | IV | + | − | CR | + | 7 | ND |
| 303-151 | IV | + | + | PR | − | 3 | 0 |
| 313-151 | III | − | + | PD | + | 2 | 0 |
| 32 | III | − | + | CR | − | 1 | 0 |
| 333-151 | IV | + | − | PR | + | 1 | 0 |
| 34 | IV | + | + | PR | − | 1 | 406 |
| Patient . | Stage . | Bone marrow . | Bulky disease . | Response to induction . | t(14;18) Lymph node . | t(14;18) Before induction3-150 . | t(14;18) After induction3-150 . |
|---|---|---|---|---|---|---|---|
| 1 | IV3-152 | + | − | PR | + | 76 145 | 1 |
| 23-151 | IV3-152 | + | − | PR | + | 31 890 | 3 |
| 3 | IV | + | + | PD | + | 8 573 | ND |
| 4 | IV | + | + | PD | + | 2 467 | ND |
| 5 | IV | + | + | PD | + | 2 217 | 10 |
| 6 | IV | + | − | PR | + | 1 779 | 1 |
| 7 | III | − | − | CR | + | 1 706 | 12 |
| 8 | IV | + | − | CR | ND | 1 684 | ND |
| 93-151 | IV | + | + | PR | + | 1 657 | 361 |
| 10 | IV | + | − | CR | + | 1 410 | ND |
| 11 | IV | + | − | CR | + | 1 234 | 0 |
| 12 | IV | + | + | PR | + | 991 | 79 |
| 13 | IV | + | − | CR | + | 436 | 1 |
| 14 | IV | + | − | CR | + | 351 | 1 |
| 15 | IV | + | − | PR | + | 319 | 0 |
| 16 | III | ND | − | CR | ND | 311 | 0 |
| 17 | IV | + | − | PR | + | 277 | 177 |
| 18 | IV | + | − | CR | + | 247 | 0 |
| 19 | II | − | + | SD | ND | 80 | 11 |
| 20 | IV | + | + | CR | ND | 71 | 0 |
| 21 | III | ND | + | PR | + | 70 | 0 |
| 22 | II | − | + | CR | + | 63 | 0 |
| 23 | III | − | + | CR | + | 34 | 0 |
| 243-151 | III | − | + | CR | + | 25 | 5 |
| 25 | II | − | + | PD | − | 24 | 1 |
| 26 | IV | + | − | CR | + | 16 | 0 |
| 27 | IV | + | + | SD | + | 16 | 1 |
| 283-151 | III | − | − | CR | − | 10 | ND |
| 29 | IV | + | − | CR | + | 7 | ND |
| 303-151 | IV | + | + | PR | − | 3 | 0 |
| 313-151 | III | − | + | PD | + | 2 | 0 |
| 32 | III | − | + | CR | − | 1 | 0 |
| 333-151 | IV | + | − | PR | + | 1 | 0 |
| 34 | IV | + | + | PR | − | 1 | 406 |
ND indicates not done; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; t(14;18) lymph node +, present (analyzed by conventional PCR in patients 1-31 and 33 and by karyotyping in patient 32); t(14;18) lymph node −, absent; t(14;18) lymph node ND, not done; t(14;18) blood, number of circulating t(14;18)-positive cells per 75 000 cells.
Number of circulating t(14;18)-positive cells per 75 000 cells.
Previous wait-and-see policy.
Leukemic.
Only one patient (patient 33) showed an increase after induction therapy from 1 to 406 circulating t(14;18)-positive cells per 75 000 cells. This patient had stage IVB bulky follicular NHL with bone marrow localization and achieved partial remission after induction therapy. In the original lymph node biopsy of this patient, t(14;18) could not be demonstrated. The course of t(14;18) in the peripheral blood in this patient remains unexplained.
Discussion
This is the first report on prospective quantitative monitoring of t(14;18)-positive cells in the blood of patients with follicular lymphoma receiving first-line treatment. In this multicenter study, we used peripheral blood as a source for t(14;18) analysis; serial bone marrow analyses are less attractive because of patient inconvenience and sampling error, and serial lymph node sampling is not feasible.
Thus far, only qualitative t(14;18) studies in follicular lymphoma have been reported, and the clinical significance of results has been conflicting. Several authors claimed that the risk for relapse after autologous stem cell transplantation and after standard chemotherapy correlated with the presence of t(14;18) in bone marrow or peripheral blood at the time of treatment or shortly thereafter.5-7 However, others found persistently t(14;18)-positive blood samples in patients with long-lasting remission.3 4 These patients have been followed up predominantly through clinical examination, making statements on real remission status impossible.
The median number of circulating t(14;18)-positive cells in this study was 262 per 75 000 cells (3.5 per 1000 cells) before the start of therapy, with only 6 patients having low numbers (1 to 10 per 75 000). Patients with stage IV disease and bone marrow involvement were more likely to have higher numbers of t(14;18)-positive cells than those with less advanced stages. This finding can be considered to reflect the systemic dissemination of stage IV disease. In contrast, patients with bulky disease were more likely to have lower numbers of circulating t(14;18)-positive cells than those without bulky disease. This finding may be explained by an increased expression of adhesion receptors, such as L-selectin, in bulky lymphoma, resulting not only in the formation of a large contiguous tumor mass but also in preferential homing of lymphoma cells in lymph nodes rather than in bone marrow and peripheral blood.18
Our finding that the progression-free survival rates of patients with higher numbers of circulating t(14;18)-positive cells before treatment did not differ from those of patients with lower numbers suggests that the lymphoma load in peripheral blood at presentation does not influence prognosis. First-line treatment appears to result in an easy clearing of lymphoma cells from the blood, irrespective of their numbers. In contrast, this therapy may not always affect other compartments of disease, such as lymph nodes or bone marrow, from which progressive disease may originate later in the course of the disease.
Earlier we observed a clear correlation between numbers of t(14;18)-positive cells in blood and bone marrow, and the post-BMT course of a patient with a follicular NHL.8 In this patient, t(14;18)-positive cells persisted in the blood after allogeneic BMT and increased before clinical relapse. After subsequent donor leukocyte infusion, t(14;18)-positive cells in blood and bone marrow decreased gradually while the patient responded clinically. A similar course was seen in another patient with follicular lymphoma treated with allogeneic BMT, in whom clinical findings correlated well with molecular analysis of t(14;18)-positive cells, quantitated by a competitive PCR method.19 These findings suggested a contribution of monitoring t(14;18)-positive cells in predicting response and relapse in patients with follicular lymphoma, as also assumed by Hirt and Dölken,20 quantitating circulating t(14;18)-positive cells in patients with follicular lymphoma treated with autologous BMT.
Real-time quantitative t(14;18) monitoring in serial blood samples in this previously untreated patient group showed a sharp decrease in t(14;18)-positive cells after conventional therapy with CVP and interferon alfa, regardless of the clinical response. This decrease in t(14;18) cannot be explained by a parallel lowering of circulating white blood cells. In most patients who responded to induction therapy, we found a considerable decrease or even disappearance of circulating t(14;18)-positive cells. When molecular remission in patients with follicular lymphoma may be indicative of cure or at least prolonged remission, the disappearance of t(14;18)-positive cells after noncurative treatment with CVP and interferon alfa cannot be expected. Remarkably, even in the 5 nonresponders, we observed a similar decrease in t(14;18). Increasing the sensitivity of this real-time PCR analysis of t(14;18) will not contribute to understanding the course of t(14;18) because this PCR is already capable of detecting t(14;18)-positive cells in healthy persons. A longer follow-up period with more consecutive samples for t(14;18) analysis will give information on variation in circulating t(14;18)-positive cells during maintenance therapy and off maintenance therapy.
It cannot be excluded that interferon alfa plays a role in the decrease of t(14;18)-positive cells after induction therapy. Therefore, we quantitated circulating t(14;18)-positive cells in another group of previously untreated patients with stages III and IV follicular lymphoma, treated now with 8 CVP courses without interferon alfa-2b according to a European protocol (EORTC 20963/HOVON 35). In this study 19 patients had pretreatment circulating t(14;18)-positive cells with a bcl-2 gene breakpoint in MBR (median, 23 per 75 000 cells; range, 1-5027 cells). After induction therapy, again irrespective of clinical response, a sharp decrease in circulating t(14;18)-positive cells to (nearly) undetectable levels was observed in all 12 patients whose blood was serially tested (data not shown). From these data, we conclude that it is unlikely that treatment with interferon alfa caused the decrease in circulating t(14;18)-positive cells in the patient group presented here.
Our observation that circulating t(14;18)-positive cells decreased or disappeared after induction therapy in most patients, regardless of clinical response, should prompt a reappraisal of so-called molecular remissions. At least after this conventional first-line treatment, monitoring t(14;18)-positive cells in the peripheral blood does not correlate with lymphoma activity. Whether correlations are more reliable in pretreated patients with more advanced disease, as suggested in studies of high-dose therapy and stem cell transplantation,5,7,8 19 must be analyzed in future prospective monitoring studies.
We thank the participating centers of the South-East Cancer Centers Cooperative Group for their collaboration. Furthermore, we thank Lian Roovers and Sandra Seeger (Data Center, Department of Hematology, University Medical Center Nijmegen) for excellent data management. We also thank Han van Krieken (Department of Pathology, University Medical Center Nijmegen) for assistance in reviewing original lymph node biopsy samples and for critically reading the manuscript. Finally, we thank Schering-Plough for technical support in collecting blood samples.
The following colleagues participated in this study by the Interzol (South-East Netherlands Cancer Centers Cooperative Group) (in order of number of patients included): Bron and Erdkamp, Maasland Ziekenhuis Sittard; Derleyn and Koch, Elkerliek Ziekenhuis Helmond; Raemaekers and Mandigers, Universitair Medisch Centrum St Radboud Nijmegen; Roelofs and Werter, St Elisabeth Ziekenhuis Venray, Dullemond, Twee Steden Ziekenhuis Tilburg; Liem and Wils, St Laurentius Ziekenhuis Roermond; Mol, Rijnstate Ziekenhuis Arnhem; Van Reisen, Ziekenhuis Zeeuws Vlaanderen Terneuzen; Smals and Smeets, St Anna Ziekenhuis Geldrop; Schade, St Anna Ziekenhuis Oss; Fickers, Atrium Heerlen; Burghouts and Croles, Bosch Medicentrum ‘s-Hertogenbosch; Oosten and Van Turnhout, Canisius Wilhelmina Ziekenhuis Nijmegen; Loosveld, De Baronie Breda; Vlasveld, Diaconessenhuis Eindhoven; Beudeker and Creemers, Ziekenhuis Walcheren Vlissingen; Wittebol, Eemland Ziekenhuis Amersfoort; Van der Heul, St Elisabeth Ziekenhuis Tilburg; Janssen, St Franciscus Ziekenhuis Roosendaal; Van Nierop, St Jansdal Harderwijk; Keuning, St Joseph Ziekenhuis Veldhoven; Stouthard, Merwede Ziekenhuis Dordrecht; Jonkers, Rijnland Ziekenhuis Leiderdorp.
A complete list of the members of the South-East Netherlands Comprehensive Cancer Centers Cooperative Group is given at the end of this article in the .
Submitted December 1, 2000; accepted March 30, 2001.
Supported by an unrestricted grant from Schering-Plough (J.R.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Caroline M. P. W. Mandigers, Department of Hematology, University Medical Center Nijmegen, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: c.mandigers@hemat.azn.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal