Chemokines play a pivotal role in regulating leukocyte migration as well as other biological functions. CC chemokine receptor 9 (CCR9) is a specific receptor for thymus-expressed CC chemokine (TECK). It is shown here that engagement of CCR9 with TECK leads to phosphorylation of Akt (protein kinase B), mitogen-activated protein kinases (MAPKs), glycogen synthase kinase–3β (GSK-3β), and a forkhead transcription factor, FKHR, in a human T-cell line, MOLT4, that naturally expresses CCR9. By means of chemical inhibitors, it is shown that phosphoinositide-3 kinase (PI-3 kinase), but not MAPK, is required for CCR9-mediated chemotaxis. Akt, GSK-3β, FKHR, and MAPK have been previously implicated in cell survival signals in response to an array of death stimuli. When MOLT4 cells, which expressed Fas as well as CXCR4, were stimulated with cycloheximide (CHX), an agonistic anti-Fas antibody, or a combination of these, the cells rapidly underwent apoptosis. However, costimulation of MOLT4 cells with TECK or stromal derived factor–1 significantly blocked CHX-mediated apoptosis, whereas stimulation only with TECK partially blocked Fas-mediated apoptosis. Concomitant with this blocking, cleavage of poly (adenosine 5′-diphosphate–ribose) polymerase and activation of caspase 3 were significantly attenuated, but the expression level of FLICE inhibitory protein c-FLIPL, which had been shown to be regulated by CHX, was unchanged. This demonstrates that activation of CCR9 leads to phosphorylation of GSK-3β and FKHR and provides a cell survival signal to the receptor expressing cells against CHX. It also suggests the existence of a novel pathway leading to CHX-induced apoptosis independently of c-FLIPL.
Introduction
Chemokines refer to a group of cytokines whose function was originally confined to regulation of leukocyte trafficking.1 Chemokines have since been implicated in other biological functions.2 These involve regulation of hematopoiesis, T helper cell development, inflammation, angiogenesis/angiostasis, regulation of cytokine production from certain dendritic cells, and cell survival. These diverse functions of chemokines result primarily from the interaction of chemokines with their cognate receptors, collectively termed chemokine receptors. Chemokine receptors are a subfamily of the G protein–coupled receptor supergene family that uses heterotrimeric G proteins (Gαβγ) for activity.
Upon binding of certain chemokines to chemokine receptors, signaling events are noted; these include F-actin formation; transient calcium mobilization; inhibition of intracellular cyclic adenosine monophosphate (cAMP) levels; activation of mitogen-activated protein kinases (MAPKs); protein kinase C (PKC); c-Jun-NH2 terminal kinase; Pyk2; focal adhesion kinase; phosphoinositide-3 kinase (PI-3 kinase); Akt (protein kinase B); the Jak/signal transducer and activator of transcription pathway; and nuclear factor κ-B (NF-κB).3-5 Although some examples, which use specific inhibitors, show that PI-3 kinase, MAPK, or PKC appear to be involved in chemokine-mediated chemotaxis in certain cell types,6-10 the underlying mechanisms of chemokine actions, especially for chemotaxis, have not been fully elucidated. Although release of Gα and Gβγ subunits from a heterotrimeric complex is believed to be a priming step for activation of these downstream targets, the mechanisms through which activated Gα or Gβγ subunits activate downstream targets and through which involvement of activated downstream targets in the aforementioned biologic functions differ from that of chemotaxis remain largely unclear.
We and others identified CC chemokine receptor 9 (CCR9), previously designated GPR-9-6, as a specific receptor for thymus-expressed chemokine (TECK).12,13 One interesting feature of CCR9 is that it is expressed mainly in immature T cells such as double-positive (DP) T cells or gut-associated T cells.14 This makes CCR9 a valuable model system for understanding T-cell development and tissue-specific homing. Additionally, DP T cells undergo extensive apoptosis, thereby becoming single-positive T cells.14Therefore, it may be possible that CCR9-mediated signaling is associated with this apoptosis. Because several studies have shown that chemokine receptor–mediated signaling activates a battery of protein or lipid kinases that are generally thought to be involved in cell survival or cell proliferation,3-5 it is possible that CCR9/TECK interaction provides antiapoptotic signaling to DP T cells. Thus, we decided to evaluate signaling components activated following CCR9/TECK interaction. This led to the finding that this receptor/ligand interaction induces activation of PI-3 kinase as well as its downstream target Akt, MAPK, glycogen synthase kinase–3β (GSK-3β), and FKHR, and resistance to death stimuli from cycloheximide and Fas.
Materials and methods
Chemokines and reagents
Human TECK was purchased from Peprotech (Rocky Hill, NJ). Anti-Fas antibody, CH-11, anti–caspase 8, and anti–inhibitor of caspase-activated DNase (ICAD) were purchased from MBL (Watertown, MA). The annexin-V staining kit, anti–Bcl-2, and anti-BclX were obtained from Pharmingen (San Diego, CA). The PI-3 kinase inhibitor wortmannin, the MAPK inhibitor PD98059, and the translation inhibitor rapamycin were obtained from Sigma (St Louis, MO). These were reconstituted in dimethyl sulfoxide (DMSO) and used at active concentrations. Anti–rabbit immunoglobulin-G horseradish peroxidase–conjugated antibody was purchased from Roche (Indianapolis, IN). Phospho-specific Akt (S473 [single-letter amino acid code]), extracellular signaling-regulated kinase 1 (pErk1/Erk2), Bad (S112), p70S6 kinase (T389 [single-letter amino acid code]), GSK-3β (S9), and FKHR (S256) antibodies were purchased from Cell Signaling Technologies (Beverly, MA). Pan–GSK-3β and FKHR antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). A chemiluminescent detection kit was purchased from Pierce Chemicals (Rockford, IL).
Cell culture, fluorescence-activated cell-sorting analysis, and transfection
MOLT4 cells were kindly provided by Dr Z Brahmi (Indiana University, School of Medicine) and cultured in RPMI complete medium. Cos7 cells were cultured in Dulbecco modified Eagle medium (complete medium). Immunofluorescent staining was performed, essentially as described in Pharmingen's technical protocols. For intracellular active caspase-3 staining, cytofix/cytoperm buffer (Pharmingen) was used according to the manufacturer's instructions to permeabilize cells, and cells were subsequently stained with anti–active-capsase-3 monoclonal antibody (Pharmingen). After washing with cytoperm buffer, active caspase-3 fluorescence intensity was measured by flow cytometry. Data were analyzed by means of the the WinMDI program (The Scripps Research Institute, La Jolla, CA) (program available at http://facs.scripps.edu/software.html#winapps).
Construction of expression plasmids and transfection
The CCR9 expression vector has been previously described.12 Akt complementary DNA (cDNA) was obtained from MOLT4 cDNA library. Akt cDNA was cloned into pCEP4 (Invitrogen, Carlsbad, CA) that had been digested with XhoI andBamHI. The transcriptional unit encompassing the cytomegalovirus early promoter, Akt, and 3′ polyadenylation site was released by SalI digestion and cloned into the CCR9 expression vector. This expression vector was then transfected into Cos7 cells by means of Fugene 6 (Roche). After 48 hours, the transfected cells were serum deprived for 2 hours and stimulated with TECK, and cell lysates were prepared. Activated Akt was detected by the phospho-specific Akt antibody.
Calcium flux assay
Receptor activation was assessed in chemokine receptor-expressing cell lines by real-time measurement of (Ca++)i changes by means of an MSIII fluorometer (Photon Technology International, S Brunswick, NJ). The cell preparation was made as described.11 Excitation scans between 300 and 400 nm were performed to determine whether fura-2AM was appropriately loaded. Excitation ratios were measured at 340 and 380 nm.
Detection of phosphorylated kinases and downstream targets of Akt
MOLT4 cells were serum starved overnight or for 2 hours and stimulated with TECK (1 μg/mL) for varying time points. Cell lysates were prepared as described elsewhere,15 and protein concentrations were determined by means of a Pierce chemical kit. Equal amounts of protein lysates were employed for Western blot analysis. Subsequently, protein blots were incubated with proper concentrations of phospho-specific antibodies or respective pan-antibodies. Target protein bands were detected by chemiluminescence.
Chemotaxis assay
A bare-filter transwell assay was performed as previously described.13 MOLT4 cells were washed once with chemotaxis assay buffer (RPMI, 20 mM Hepes, pH 7.4, 1% bovine serum albumin) and resuspended in the same buffer. We placed 5 × 105 cells in 100 μL buffer in the upper inset, and optimal concentrations of TECK in 600 μL buffer were placed in the lower chamber. Cell migration was allowed to take place at 37°C for 4 hours. Migrated cells were harvested and counted with a hemocytometer. For chemotaxis inhibition assay, MOLT4 cells were pretreated with varying concentrations of wortmannin, PD98059 (50 μM), and pertussis toxin (1 μg/mL) or vehicle for 1 hour, washed once with phosphate-buffered saline (PBS), and used for chemotaxis.
Apoptosis assay
We deprived 1 × 106 MOLT cells of serum overnight. These cells were preincubated with cycloheximide (CHX) at a final concentration of 2.5 μg/mL for 1 hour. Afterwards, cells were stimulated with CH-11 (0.5 μg/mL) and 1 μg/mL TECK for 6 hours. For inhibitor studies, CHX-treated cells were incubated with wortmannin (25 or 50 nM), PD98059 (50 μM), and rapamycin (10 μM) for 30 minutes and then stimulated with CH-11 and TECK for 6 hours. The treated cells were washed twice with cold PBS and stained with annexin V for 15 minutes. The apoptotic cell population that was annexin-V–positive and propidium iodide–negative was measured by means of flow cytometry.
Results
PI-3 kinase–dependent migration of MOLT4 cells in response to TECK
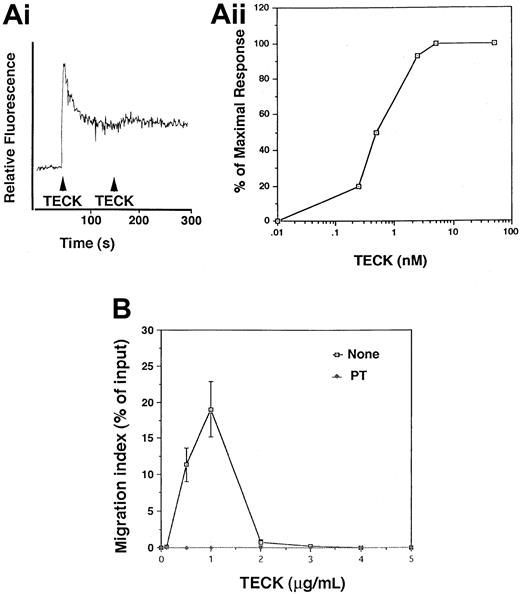
MOLT4 cells have been shown to express high levels of CCR9.13,14 CCR9 is a specific receptor for TECK.12 13 Binding of TECK to CCR9 induced a rapid and transient calcium flux (Figure 1A). In order to evaluate the chemotactic stimulating ability of TECK, bare-filter transwell migration assays were performed with MOLT4 cells. The maximal chemotactic responsiveness of MOLT4 cells to TECK was seen at 1 μg/mL, with migration desensitized at higher concentrations (Figure 1B). TECK-mediated migration of MOLT4 cells was completely abrogated by pertussis toxin (PT) (Figure 1B), suggesting that CCR9 is coupled to Gαi proteins in the cells.
Transient calcium mobilization and chemotaxis of MOLT4 cells in response to TECK.
(A) MOLT4 cells were loaded with Fura-2AM and stimulated with 100 nM TECK. Calcium flux was monitored by measuring relative fluorescence of Fura-2AM (i). Fura-2AM–loaded MOLT4 cells were stimulated with the indicated concentrations of TECK (1 to 100 nM), and fluorescence was monitored. The peak amplitude of the calcium response was plotted (ii). Representative data from 3 different experiments are shown. (B) Chemotactic responsiveness of MOLT4 cells to TECK. A half-million MOLT4 cells were placed into a transwell chamber and allowed to migrate in the presence of varying concentrations of TECK for 4 hours. The number of cells were counted by a hemocytometer. MOLT4 cells were also pretreated with pertussis toxin (PT) (1 μg/mL) for 1 hour and used for chemotaxis in response to TECK. SD was calculated from 3 independent experiments.
Transient calcium mobilization and chemotaxis of MOLT4 cells in response to TECK.
(A) MOLT4 cells were loaded with Fura-2AM and stimulated with 100 nM TECK. Calcium flux was monitored by measuring relative fluorescence of Fura-2AM (i). Fura-2AM–loaded MOLT4 cells were stimulated with the indicated concentrations of TECK (1 to 100 nM), and fluorescence was monitored. The peak amplitude of the calcium response was plotted (ii). Representative data from 3 different experiments are shown. (B) Chemotactic responsiveness of MOLT4 cells to TECK. A half-million MOLT4 cells were placed into a transwell chamber and allowed to migrate in the presence of varying concentrations of TECK for 4 hours. The number of cells were counted by a hemocytometer. MOLT4 cells were also pretreated with pertussis toxin (PT) (1 μg/mL) for 1 hour and used for chemotaxis in response to TECK. SD was calculated from 3 independent experiments.
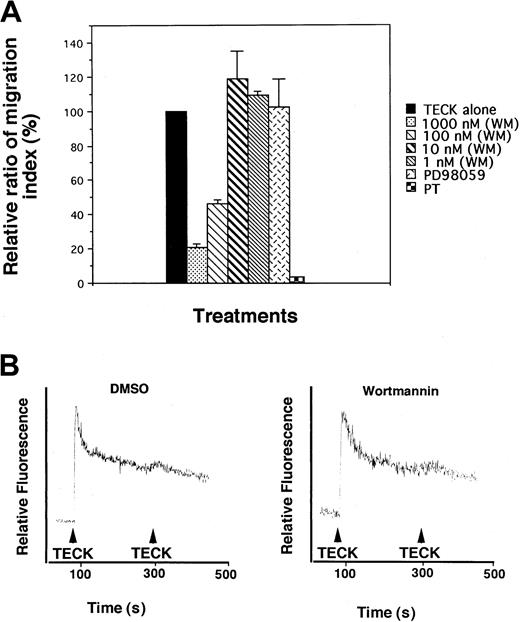
PI-3 kinases and MAPK have been implicated in cell movement or cell shape change.16,17 However, information on involvement of PI-3 kinase or MAPK in chemokine-mediated chemotaxis is fairly limited. It has been shown that CXCR4 or RANTES-receptor–mediated chemotaxis is inhibited by PI-3 kinase inhibitors.6,18 The MAPK inhibitor PD99059 has been shown to block monocyte and eosinophil chemotaxis in response to monocyte chemoattractant protein (MCP)–1 and eotaxin-1, respectively.8 19 To determine whether these kinases were responsible for TECK-mediated chemotaxis, MOLT4 cells were pretreated with varying concentrations of wortmannin, a potent PI-3–kinase inhibitor; PD98059, a MAPK inhibitor; or vehicle, DMSO. Transwell migration assays were performed. As seen in Figure2A, only wortmannin significantly inhibited cell migration, an effect that was maximally 5- to 6-fold at 1 μM. PD98059 did not inhibit cell migration. Wortmannin at the same concentrations did not affect the ability of TECK to induce transient calcium mobilization (Figure 2B). These data suggests that PI-3 kinase, but not MAPK, plays an important role in TECK-mediated chemotaxis.
Inhibition of chemotactic responsiveness of MOLT4 cells to TECK by wortmannin.
(A) MOLT4 cells were pretreated with varying concentrations of wortmannin (WM), PD98059, or pertussis toxin (PT) for 1 hour, washed twice with serum-free medium, and employed for transwell migration assay. (B) MOLT4 cells treated with DMSO or wortmannin (1 μM) were loaded with Fura-2AM and stimulated with 100 nM TECK. Calcium flux was monitored by measuring relative fluorescence of Fura-2AM. SDs were obtained from at least 3 independent experiments.
Inhibition of chemotactic responsiveness of MOLT4 cells to TECK by wortmannin.
(A) MOLT4 cells were pretreated with varying concentrations of wortmannin (WM), PD98059, or pertussis toxin (PT) for 1 hour, washed twice with serum-free medium, and employed for transwell migration assay. (B) MOLT4 cells treated with DMSO or wortmannin (1 μM) were loaded with Fura-2AM and stimulated with 100 nM TECK. Calcium flux was monitored by measuring relative fluorescence of Fura-2AM. SDs were obtained from at least 3 independent experiments.
PI-3-kinase– and G protein–dependent activation of Akt by TECK
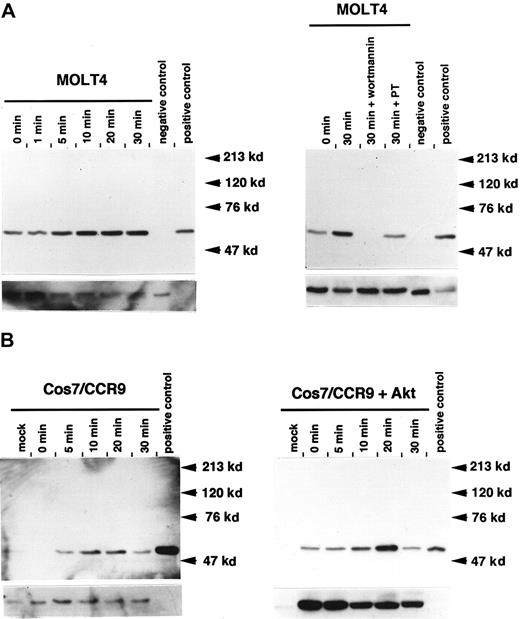
A growing body of evidence has demonstrated that Akt is a downstream target of PI-3 kinase.20 Upon activation of growth factor receptors, Akt is phosphorylated and activated in the presence of PI–(3,4,5)P3 (PIP3).21 This lipid product appears to recruit the cytosolic Akt to the membrane compartment by interaction with the pleckstrin homology (PH) domain of Akt. To investigate whether TECK activates Akt, MOLT4 cells were stimulated with TECK, and activation of Akt was assessed at different times by means of phopho-specific Akt antibody. As shown in Figure3A, an increase in Akt phosphorylation was observed within 10 minutes, and maximal phosphorylation was attained at 30 minutes. This was sustained for 1 hour (data not shown). Wortmannin or pertussis toxin completely abrogated this TECK-enhanced effect, suggesting that activation of Akt is both PI-3-kinase– and Gαi-protein–dependent. In terms of loading controls, we found that complete stripping of the phospho-specific Akt antibody at stringent conditions rarely occurred, and this interfered with use of the pan-Akt antibody. However, separate loading of equal amounts of cell lysates gave uniform intensity of phosphorylation. See an example of this in Figure 4. It has been recently suggested that CCR11 is also a receptor for TECK.22Therefore, we evaluated whether the CCR9/TECK interaction leads to activation of Akt in heterologous cells. When CCR9 was ectopically expressed in TECK-stimulated Cos7 cells, mainly marginal activation of endogenous Akt was detected. This low-level activation was due to low levels of endogenous Akt in the Cos cells (Figure 3B). To enhance expression levels of Akt, an expression vector harboring CCR9 and human Akt together was constructed, and these were overexpressed in Cos7 cells. When these cells were stimulated with TECK, activation of Akt was observed in a few seconds and peaked at 20 minutes. A substantial difference in total Akt was noted between the mock-transfected and the expression vector–transfected cells. At this time, we do not know whether MOLT4 cells express functional CCR11. Taken together, these data strongly suggest that CCR9/TECK interaction leads to the activation of Akt in a PI-3 kinase– and Gαi protein–dependent manner.
Phosphorylation of Akt via the CCR9/TECK interaction.
(A) At different time points, 1 × 107 MOLT4 cells were serum starved and stimulated with 50 nM TECK. Phosphorylated Akt was detected by means of phospho-specific Akt antibody (S473). Subsequently, the used blot was stripped and reprobed with pan-Akt antibody. For inhibitor experiment, the serum-starved MOLT4 cells were pretreated with WM or PT for 1 hour and stimulated with 50 nM TECK for 30 minutes. Western blot analysis was conducted by means of phospho-specific Akt antibody (S473). Positive control is cell lysate prepared from NIH3T3 cells stimulated with platelet-derived growth factor (PDGF), whereas negative control is cell lysate prepared from unstimulated NIH3T3 cells. (B) Cos7 cells were transiently transfected with CCR9 alone or with CCR9 and Akt coexpression vector and stimulated with 50 nM TECK. Phosphorylated Akt or total Akt was detected as described above. The bottom pictures in each panel represent expression levels of total Akt. These results are 1 of at least 3 reproducible experiments.
Phosphorylation of Akt via the CCR9/TECK interaction.
(A) At different time points, 1 × 107 MOLT4 cells were serum starved and stimulated with 50 nM TECK. Phosphorylated Akt was detected by means of phospho-specific Akt antibody (S473). Subsequently, the used blot was stripped and reprobed with pan-Akt antibody. For inhibitor experiment, the serum-starved MOLT4 cells were pretreated with WM or PT for 1 hour and stimulated with 50 nM TECK for 30 minutes. Western blot analysis was conducted by means of phospho-specific Akt antibody (S473). Positive control is cell lysate prepared from NIH3T3 cells stimulated with platelet-derived growth factor (PDGF), whereas negative control is cell lysate prepared from unstimulated NIH3T3 cells. (B) Cos7 cells were transiently transfected with CCR9 alone or with CCR9 and Akt coexpression vector and stimulated with 50 nM TECK. Phosphorylated Akt or total Akt was detected as described above. The bottom pictures in each panel represent expression levels of total Akt. These results are 1 of at least 3 reproducible experiments.
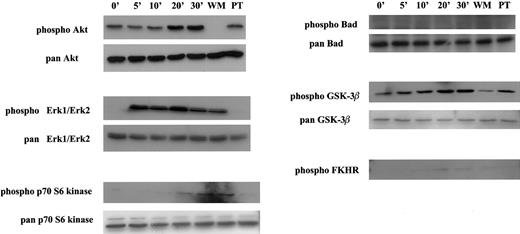
Activation of multiple signal transducers via the CCR9/TECK interaction.
MOLT4 cells were serum starved overnight and stimulated with 50 nM TECK for different amounts of time. For inhibitor experiments, the serum-starved MOLT4 cells were pretreated with WM or PT prior to stimulation and subsequently stimulated with TECK for 30 minutes. Activated signal transducers were detected by respective phospho-specific antibodies. This is 1 of at least 2 reproducible experiments.
Activation of multiple signal transducers via the CCR9/TECK interaction.
MOLT4 cells were serum starved overnight and stimulated with 50 nM TECK for different amounts of time. For inhibitor experiments, the serum-starved MOLT4 cells were pretreated with WM or PT prior to stimulation and subsequently stimulated with TECK for 30 minutes. Activated signal transducers were detected by respective phospho-specific antibodies. This is 1 of at least 2 reproducible experiments.
CCR9/TECK interaction results in activation of multiple signaling components
In the case of the β-adrenergic receptor, the chemokine receptor CXCR2, or the fMLPR receptor (single-letter amino acid codes), it has been shown that PI-3 kinase γ, a subclass of PI-3 kinase which is believed to be coupled to G protein–coupled receptors including chemokine receptors, is required for activation of MAPKs, Erk1/Erk2.23 24 Since we demonstrated that Akt is activated via a PI-3-kinase–dependent pathway upon stimulation of MOLT4 cells with TECK, this gave us an opportunity to test whether Erk1/Erk2 is activated by the CCR9/TECK interaction. As seen in Figure4, a robust activation of Erk1/Erk2 was noticed in 5 minutes, and the activated state was sustained for 30 minutes. However, in contrast to Akt, wortmannin did not block the activation of Erk1/Erk2 whereas pertussis toxin effectively blocked the activation. This suggests that unlike CXCR2 or the fMLPR in neutrophils, PI-3 kinase γ may not be involved in CCR9-mediated activation of Erk1/Erk2 in MOLT4 cells. As in activation of MAPK, p70 S6 kinase was phosphorylated. However, its phosphorylation was not inhibited by wortmannin. Rather, it was inhibited by pertussis toxin.
Activated Akt has been shown to phosphorylate multiple downstream targets, most of which are involved in cell survival.20These include Bad, GSK-3β, FKHR (a member of the forkhead family of transcription factors), and caspase 9. To determine whether Akt activated via the CCR9/TECK interaction results in phosphorylation of these targets, Western blots were performed with phopho-specific antibodies that recognize these phosphorylated targets. As seen in Figure 4, GSK-3β and FKHR, but not Bad, were phosphorylated. In 3 experiments, the phosphorylation of FKHR was very modest. Because pan-FKHR antibody gave us a high background, we were unable to measure total FKHR properly.
The CCR9/TECK interaction blocks CHX-induced or Fas-mediated apoptosis
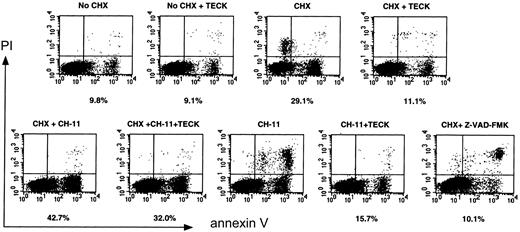
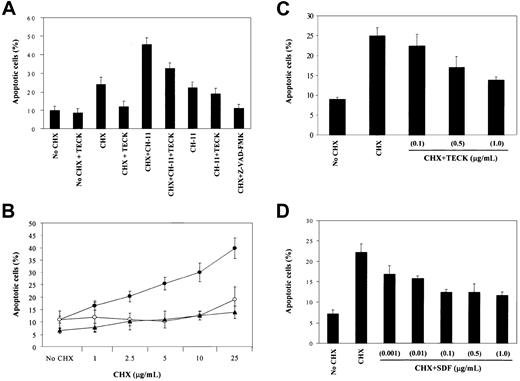
In addition to the implication of Akt, MAPK, GSK-3β, and FKHR in cell survival signaling,20 it has also been shown that Akt and MAPK are involved in resistance to Fas-mediated cell death.25,26 MOLT4 cells express Fas on the cell surface and are killed by agonistic anti-Fas, CH-11.27 Because Akt and MAPK are activated via CCR9/TECK interaction, we evaluated whether Fas-mediated cell death in these cells could be blocked by CCR9-mediated signaling. MOLT4 cells were cultured in serum-free medium overnight and stimulated with CH-11 alone, or along with TECK in the presence of CHX. CHX is known to facilitate Fas-mediated cell death or, by itself, to be able to kill some T cells.28 The CXCR4/stromal-derived factor (SDF)–1 interaction was used for comparison. Apoptotic cells were measured by annexin-V staining. CH-11 treatment was done in the absence of serum, because MOLT4 cells were highly resistant to CH-11 in the presence of serum (data not shown), and we hoped to maximize activities of the signaling components described in Figure 4 upon stimulation of MOLT4 cells with TECK. The percentage listed under each flow analysis reflects the percentage of apoptotic cells, as seen in the lower right-hand quadrant. As seen in Figure 5(an example of flow analysis), CHX (5 μg/mL) or CH-11 (0.5 μg/mL) induced a rapid apoptosis of MOLT4 cells. Notably, TECK blocked CHX-induced apoptosis by 80% to 90% and partially rescued the cells from Fas-mediated cell death. As reported, CHX potentiated Fas-mediated cell death, which was significantly attenuated by TECK. Because Z-VAD-FMK, a potent caspase inhibitor, completely blocked both CHX-induced and Fas-mediated apoptosis, it seemed likely that the blocking activity displayed by TECK was due to the inhibition of caspase activity. The in-depth analysis of 3 experiments is presented in Figure 6A. TECK was capable of blocking CHX-induced apoptosis at varying concentrations of CHX (Figure6B). The blocking activity was dependent on the dose of TECK. Efficient blockade of CHX-induced cell death occurred at 1 μg/mL TECK, whereas the efficiency rapidly declined at 100 ng/mL TECK (Figure 6C). This blocking activity was not unique to TECK because SDF-1 also efficiently blocked CHX-induced apoptosis in a dose-dependent manner. Half-maximal blocking activity was seen even at 10 ng/mL SDF-1. However, SDF-1 did not appear to effectively block Fas-mediated or CHX-potentiated Fas-mediated apoptosis (data not shown).
Effect of the CCR9/TECK or CXCR4/SDF-1 interaction on CHX-induced apoptosis as well as on Fas-mediated apoptosis.
MOLT4 cells were serum starved overnight and treated with CHX (5 μM) or CH-11 (500 ng/mL), alone or in combination, in the presence or absence of TECK (1 μg/mL), for 6 hours. As a control, Z-VAD-FMK, a caspase inhibitor, was used for blocking apoptosis. Apoptotic cells were measured by annexin-V staining by means of flow cytometry. The percentage listed under each flow analysis is the percentage of apoptotic cells, as seen in the lower right-hand quadrant. Shown are representative data from 3 different experiments.
Effect of the CCR9/TECK or CXCR4/SDF-1 interaction on CHX-induced apoptosis as well as on Fas-mediated apoptosis.
MOLT4 cells were serum starved overnight and treated with CHX (5 μM) or CH-11 (500 ng/mL), alone or in combination, in the presence or absence of TECK (1 μg/mL), for 6 hours. As a control, Z-VAD-FMK, a caspase inhibitor, was used for blocking apoptosis. Apoptotic cells were measured by annexin-V staining by means of flow cytometry. The percentage listed under each flow analysis is the percentage of apoptotic cells, as seen in the lower right-hand quadrant. Shown are representative data from 3 different experiments.
Blocking of CHX-induced apoptosis by TECK or SDF-1.
(A) MOLT4 cells were treated as described in Figure 5. Apoptotic cells were measured by annexin-V staining. (B) Ability of TECK to block CHX-induced apoptosis at varied concentrations of CHX. MOLT4 cells were induced to undergo apoptosis by CHX (●) in the presence of TECK (○) or Z-VAD-FMK (▴). Data are expressed as the mean with SD obtained from at least 3 different experiments. (C and D) Dose-dependent effect of TECK or SDF-1 on CHX-induced apoptosis. Data presented are from at least 3 different experiments.
Blocking of CHX-induced apoptosis by TECK or SDF-1.
(A) MOLT4 cells were treated as described in Figure 5. Apoptotic cells were measured by annexin-V staining. (B) Ability of TECK to block CHX-induced apoptosis at varied concentrations of CHX. MOLT4 cells were induced to undergo apoptosis by CHX (●) in the presence of TECK (○) or Z-VAD-FMK (▴). Data are expressed as the mean with SD obtained from at least 3 different experiments. (C and D) Dose-dependent effect of TECK or SDF-1 on CHX-induced apoptosis. Data presented are from at least 3 different experiments.
CHX-mediated apoptosis of MOLT4 cells is c-FLIPL–independent, and down-regulation of caspase 3 may account for the antiapoptotic activity displayed by chemokines
It has been suggested that CHX induced or potentiated apoptosis by down-regulation of c-FLIPL, an antiapoptotic protein.29,30 This prompted us to investigate whether CHX down-regulated c-FLIPL and, if so, whether TECK up-regulated c-FLIPL. As shown in Figure7A, CHX-induced or CHX-potentiated Fas-mediated cleavage of PARP was significantly blocked by TECK. However, CHX did not alter expression levels of c-FLIPL. Down-regulation of c-FLIPL was not seen at higher concentrations of CHX with prolonged incubation in which the cleavage of PARP was evident but was not significantly inhibited by TECK (Figure7B). Combined treatment of MOLT4 cells with CHX and CH-11 induced a cleavage of ICAD and Bcl-XL. Although cleavage of caspase 8 is not evident in this Figure, prolonged exposure revealed that CHX alone or along with CH-11 indeed cleaved caspase 8 and that the cleavage was blocked by TECK (data not shown). Regardless of treatments, TECK did not alter expression levels of Bcl-2 or Bcl-XL. We conclude that CHX may induce apoptosis of MOLT4 cells via a novel pathway, presumably independently of c-FLIPL, and that antiapoptotic Bcl-2 family members were not involved in CCR9- or CXCR4-mediated resistance to CHX-induced apoptosis. Some studies suggest that activation of NF-κB leads to antiapoptosis and that Akt is involved in the activation process.31,32 It has been shown that SDF-1/CXCR4 interaction results in activation of NF-κB.33 We therefore transfected C3H 10T1/2 (a mouse embryonic fibroblast cell line) with CCR9 and human immunodeficiency virus (NF-κB)3 luciferase reporter gene along with vector control. The transfected cells were stimulated with TECK (1 μg/mL) or tumor necrosis factor (TNF)–α (100 ng/mL) as a control for 6 hours. Luciferase assay was performed. It was found that TECK was unable to induce activation of NF-κB, but TNF-α dramatically induced the activity, suggesting that activation of NF-κB does not account for the CCR9-mediated cell survival against CHX (data not shown).
Blocking of c-FLIPL–independent CHX-induced apoptosis by TECK.
(A) MOLT4 cells were stimulated with given treatments for 6 hours. Protein lysates were prepared, and Western blot analysis was conducted with the use of specific antibodies. For the detection of cleaved poly (adenosine 5′-diphosphate–ribose) polymerase (PARP), cleavage-specific antibody was used. (B) MOLT4 cells were treated with CHX at varied concentrations in the presence of TECK or Z-VAD-FMK for 8 hours. Western blots were performed with the use of anti-cleavage–specific PARP and anti–c-FLIPL. This is one of 2 reproducible experiments.
Blocking of c-FLIPL–independent CHX-induced apoptosis by TECK.
(A) MOLT4 cells were stimulated with given treatments for 6 hours. Protein lysates were prepared, and Western blot analysis was conducted with the use of specific antibodies. For the detection of cleaved poly (adenosine 5′-diphosphate–ribose) polymerase (PARP), cleavage-specific antibody was used. (B) MOLT4 cells were treated with CHX at varied concentrations in the presence of TECK or Z-VAD-FMK for 8 hours. Western blots were performed with the use of anti-cleavage–specific PARP and anti–c-FLIPL. This is one of 2 reproducible experiments.
Recently, it was demonstrated that bone marrow neutrophils from caspase-3–deficient mice were resistant to CHX-induced apoptosis, indicating that caspase-3 expression is essential for this type of cell death.34 Expression levels of activated caspase 3 were measured by intracellular staining of MOLT4 cells with the use of cleavage-specific caspase-3 antibody. As shown in Figure8, both TECK and SDF-1 significantly down-regulated cleaved caspase 3 in CHX-treated MOLT4 cells, but did not affect the levels of cleaved caspase 3 in MOLT4 cells stimulated by combined treatment with CHX and CH-11. CH-11 comparably augmented the levels of cleaved caspase 3 to CHX. Interestingly, TECK, but not SDF-1, significantly down-regulated cleaved caspase 3 in Fas-mediated apoptosis, suggesting that CCR9 and CXCR4 exert differential effects on Fas signaling. This is consistent with the previous annexin-V data.
TECK and SDF-1 suppress CHX- and CH-11–induced caspase-3 activation.
Cells were treated as described in Figure 5. They were then permeabilized and fixed as indicated in “Materials and methods” and subsequently stained for intracellular activated (cleaved) caspase 3. Activated caspase-3–specific fluorescence intensity was measured by flow cytometry. The percentage of cells with activated caspase 3 was quantitated from relative-frequency histograms (panels A and B) and is shown in panels C and D. Panels A and B are representative of 2 separate experiments, whereas panels C and D are an average of the 2 separate experiments.
TECK and SDF-1 suppress CHX- and CH-11–induced caspase-3 activation.
Cells were treated as described in Figure 5. They were then permeabilized and fixed as indicated in “Materials and methods” and subsequently stained for intracellular activated (cleaved) caspase 3. Activated caspase-3–specific fluorescence intensity was measured by flow cytometry. The percentage of cells with activated caspase 3 was quantitated from relative-frequency histograms (panels A and B) and is shown in panels C and D. Panels A and B are representative of 2 separate experiments, whereas panels C and D are an average of the 2 separate experiments.
Discussion
Here, we report that PI-3 kinase plays a more important role than MAPK in CCR9-mediated chemotaxis and is important for survival signaling against Fas-mediated apoptosis. In the case of growth factor receptors, MAPK has been strongly implicated in both cell migration and cell survival.18 However, although evidence suggests that activation of chemokine receptors leads to activation of MAPK, the role for MAPK in chemotaxis is controversial.35 It has been shown that the chemotaxis of CXCR2-expressing 293 cells in response to interleukin-8 (IL-8) is not inhibited by PD98059, whereas the chemotaxis of monocytes and eosinophils in response to MCP-1 and eotaxin-1, respectively, is inhibited by PD98059.8,9,19 35This suggests that cell type is an important factor for determining the role of MAPK in chemokine-mediated chemotaxis.
PI-3 kinase represents a multi-enzyme complex that plays a central role in relaying proximal signals upon activation of growth factor receptors or G protein–coupled receptors (GPCRs) to downstream signaling components. Class I PI-3 kinases (PI-3Ks) have been implicated in many cellular responses downstream of tyrosine kinases. Such cellular responses involve proliferation, antiapoptosis, and cytoskeletal rearrangements and cell migration.36 Class IA PI-3K is a heterodimeric lipid kinase consisting of a p85 regulatory subunit and a p110 catalytic subunit (α, β, and δ). PI-3K is activated by the interaction of the p85 regulatory subunit with phosphorylated tyrosine residues on activated growth factor receptors. The binding of the p85 regulatory subunit to upstream signaling molecules causes the p110 catalytic subunit to be membrane localized and activated. Class IB PI-3K (PI-3K γ) is a newly identified enzyme that does not require the p85 regulatory subunit but is activated in the presence of G proteins, implying that PI-3K γ is linked to GPCRs. Three different groups of researchers recently reported the phenotypes of PI-3K γ–deficient mice.23,37,38 It is agreed that PI-3K γ plays an essential, nonredundant role in the chemotaxis of neutrophils and macrophages. The most interesting feature is that class IA PI-3Ks are precluded in terms of production of PIP3 upon activation of chemoattractant receptors, providing strong in vivo evidence that PI-3K γ is a unique species activated by chemokine receptors, at least in neutrophils. However, it has been reported that other class I PI-3Ks are involved in T-cell migration in response to SDF-1.18This study demonstrated that p85 was physically associated with CXCR4 in a ligand-dependent manner and that expression of dominant-negative (DN) p85 dampens the responsiveness of the T-cell line to SDF-1. In the case of the β2-adrenergic receptor, agonist-dependent activation of MAPK is sensitive to wortmannin, indicating that PI-3Ks are involved. Indeed, it has been shown that expression of DN PI-3K γ abolishes an agonist-dependent activation of MAPK.23 This was the first indication that PI-3K γ is linked to GPCR signaling. Furthermore, a study using PI-3K γ–deficient mice shows that CXCR2- or fMLPR-mediated MAPK activation depends on PI-3K γ in neutrophils.24 In contrast, our study shows that CCR9-mediated MAPK activation is not affected by wortmannin, whereas activation of Akt is completely blocked. These observations suggest that there may be differences between neutrophils and T cells in the roles of PI-3K γ in chemotaxis and activation of MAPK.
Akt is a serine/threonine kinase that is activated upon ligation of several cell surface receptors, including the receptors for insulin and PDGF.39 The biological importance of Akt activation has been demonstrated by its ability to protect cells from apoptosis.20 In order for Akt to be activated, Akt needs to be membrane localized and phosphorylated on a specific serine (S473) and a specific threonine (T308). Akt contains an amino-terminal PH domain, which binds PIP3. Binding of PIP3 to the PH domain has been proposed to facilitate targeting of Akt to membrane compartments. Because PIP3 is an end product of PI-3K, Akt activation necessarily depends on PI-3K. Several lines of evidence have suggested that chemokine receptor signaling activates Akt. These include IL-8, SDF-1, MIP-1α, and RANTES.40-42 However, the biological meaning of chemokine receptor–mediated Akt activation remains unclear. It has been demonstrated that upon exposure of differentiated HL60 cells to IL-8, Akt is recruited selectively to the membrane at the cells' leading edge.43 Although that study did not provide direct evidence for the participation of Akt in chemotaxis, polarized distribution of Akt upon the activation of chemokine receptor suggested involvement in extension of lamellipodia. We have now demonstrated that the CCR9/TECK interaction leads to PI-3K– and Gαi protein–dependent activation of Akt. To investigate whether Akt was involved in CCR9-mediated chemotaxis, we tried to stably express DN Akt, which is a kinase-dead mutant in MOLT4 cells, by using transfection or retroviruses. However, we were not successful. Because the major function of activated Akt is considered to be provision of a cell survival signal,20 it is possible that CCR9-mediated activation of Akt is involved in resistance to certain death stimuli. Activated Akt phosphorylates multiple downstream targets that are involved in cell survival.20 These include GSK-3β, FKHR, and caspase 9. We were able to demonstrate that the CCR9/TECK interaction resulted in phosphorylation of GSK-3β and FKHR in a PI-3K– and Gαi protein–dependent manner. To our knowledge, this is the first demonstration that activation of a chemokine receptor leads to phosphorylation of GSK-3β and FKHR. In addition to activation of these proteins, we have shown that p70 S6 kinase is phosphorylated upon stimulation of MOLT4 cells with TECK. It has been reported that activation of p70 S6 kinase is responsible for preventing cell death induced by cisplatin in many transformed cell lines.44Taken together, these suggest that CCR9-mediated signaling is involved in cell survival. To test this hypothesis, we chose to evaluate CHX and Fas because Fas is a physiologic death stimulus that appears to be involved in thymic T-cell development, CCR9 is preferentially expressed in double-negative T cells in thymus,14,28 and CHX is known to induce apoptosis of some cells.45 Because it has been previously shown that Akt or MAPK is responsible for resistance to Fas-mediated cell death25,26 and we have now shown that the CCR9/TECK interaction causes robust activation of these kinases, this was a testable hypothesis. Stimulation of MOLT4 cells with CH-11 or with CHX led to a significant increase in apoptosis. But on the basis of annexin-V staining, treatment of the stimulated cells with TECK, a CCR9 ligand, significantly reduced apoptosis. The nature of cells appearing in the upper right quadrant in Figure 5 stimulated with CH-11 or CHX plus Z-VAD-FMK is currently unknown. Because the appearance of these cells was inconsistent in other experiments and what we were interested in was the cell population appearing in the right lower quadrant, namely propidium iodide–negative and annexin-V–positive cells, we believe that the cells in the upper right quadrant in Figure5 do not change the data interpretation. Moreover, the percentage of propidium iodide–negative and annexin-V–positive population was very consistent in 3 experiments. Also, TECK blocked the cleavage of PARP. Concomitant with blocking of apoptosis, expression levels of caspase 3 were significantly diminished by TECK. SDF-1, whose receptor, CXCR4, is expressed on MOLT4 cells, exerted the same effect on CHX-mediated cell death. Therefore, this effect is not restricted to a TECK/CCR9 interaction. However, we consistently observed that the extent of the antiapoptotic effect displayed by TECK on Fas-mediated cell death was much greater than that of SDF-1. Also, these 2 chemokines apparently exhibited different abilities to down-regulate activated caspase 3 in response to stimulation with CH-11. Because a study shows that CC and CXC chemokine receptors seem to be differentially coupled to Gαi proteins,45 this may explain the functional disparity exhibited by TECK and SDF. Among other GPCR family members, differential coupling of Gα proteins seems to regulate survival or death of certain cell types.46-49 However, the general consensus is that Gαi protein–coupled receptors, such as the glucagonlike peptide-2 receptor or M1 muscarinic receptors, provide antiapoptotic signal to the receptor-expressing cells, whereas Gαs or Gαq protein-coupled receptors, such as beta-adrenergic receptor or parathyroid hormone–related protein receptor, give apoptotic signaling. The major function of activated Gαi proteins is to decrease the level of intracellular cAMP, resulting in down-regulation of cAMP-dependent kinase, protein kinase A (PKA).11 Because CCR9 is coupled to Gαi, it seems likely that a PKA-independent pathway is responsible for down-regulation of caspase 3 in response to CHX- or Fas-mediated apoptosis. A line of evidence suggests that CHX induces cell death by lowering the extent of translation of certain proteins, such as c-FLIPL, that are responsible for cell survival.30 50 This prompted us to investigate whether CHX reduced the level of c-FLIPL and whether TECK could block this down-regulation in MOLT 4 cells. CHX did not down-regulate c-FLIPL nor did TECK alter the level of c-FLIPL, suggesting that TECK blocks CHX-mediated cell death in a c-FLIPL–independent manner. Also, neither CHX nor TECK altered expression levels of Bcl-2 and Bcl-XL. Because CCR9 is expressed largely in thymic T cells that undergo extensive apoptosis for their differentiation, it is likely that the CCR9/TECK interaction will play a role in regulating this apoptosis.
The authors thank Audrey Carson for typing the manuscript and Dr Byoung H. Kim for instruction on how to use the Excel program.
Supported by US Public Health Service grants R01 HL56416 and RO1 DK53674 from the National Institutes of Health (H.E.B), and a Young Investigator Award of the Core Centers of Excellence in Molecular Hematology (P30 DK49218) to Indiana University School of Medicine (B.-S.Y.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Byung-S. Youn, KOMED Institute for Life Science, Graduate School of Biotechnology, Korea University, Rm 640, 1,5-ka, Anam-dong, Sungbuk-ku, Seoul, Korea.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal