Granulocyte colony-stimulating factor (G-CSF) is a major cytokine that regulates proliferation and differentiation of myeloid cells, although the underlying mechanisms by which G-CSF controls myeloid differentiation are largely unknown. Differentiation of hematopoietic cells is regulated by lineage-specific transcription factors, and gene-targeting studies previously revealed the critical roles of CCAAT/enhancer-binding protein (C/EBP) α and C/EBPε, respectively, in the early and mid-late stages of granulocyte differentiation. The expression of C/EBPε in 32Dcl3 cells and FDCP1 cells expressing mutant G-CSF receptors was examined and it was found that G-CSF up-regulates C/EBPε. The signal for this expression required the region containing the first tyrosine residue of G-CSF receptor. Dominant-negative signal transducers and activators of transcription 3 blocked G-CSF–induced granulocytic differentiation in 32D cells but did not block induction of C/EBPε, indicating that these proteins work in different pathways. It was also found that overexpression of C/EBPε greatly facilitated granulocytic differentiation by G-CSF and, surprisingly, that expression of C/EBPε alone was sufficient to make cells differentiate into morphologically and functionally mature granulocytes. Overexpression of c-myc inhibits differentiation of hematopoietic cells, but the molecular mechanisms of this inhibition are not fully understood. In 32Dcl3 cells overexpressing c-myc that do not differentiate by means of G-CSF, induction of C/EBPε is completely abrogated. Ectopic expression of C/EBPε in these cells induced features of differentiation, including changes in nuclear morphologic characteristics and the appearance of granules. These data show that C/EBPε constitutes a rate-limiting step in G-CSF–regulated granulocyte differentiation and that c-myc antagonizes G-CSF–induced myeloid differentiation, at least partly by suppressing induction of C/EBPε.
Introduction
Development of hematopoietic cells is controlled by lineage-specific transcription factors.1 In the myeloid lineage, a family of CCAAT/enhancer-binding proteins (C/EBPs) plays a key role in differentiation. C/EBPα is expressed in early myeloid progenitors, and its expression decreases as these progenitors differentiate into mature granulocytes.2 In turn, C/EBPε is up-regulated at the promyelocyte or myelocyte stage and expressed thereafter.3,4 C/EBPβ and C/EBPδ are expressed basically in all stages of myeloid differentiation, although their expression is somewhat higher in mature myeloid cells.2
Corresponding to a broad pattern of expression, mice deficient in C/EBPα have multiple abnormalities, including neonatal death due to impaired glycogenesis and lung abnormalities.5,6 During hematopoiesis, these mice have complete differentiation arrest at the myeloid progenitor stage, resulting in an absence of mature neutrophils and eosinophils in peripheral blood.7 The critical role of C/EBPβ in the immune response was revealed in studies in C/EBPβ-deficient mice, which have macrophage dysfunction, impaired bacterial killing, and lymphoproliferative disorders.8C/EBPε knockout mice have impaired granulopoiesis and myelodysplasia.9 Although these mice produce mature granulocytes, they are morphologically and functionally abnormal, with hyposegmented nuclei and an impaired oxidative burst. In addition, bone marrow hyperplasia resembling myelodysplasia becomes apparent after 3 months. Importantly, these knockout mice have no developmental defects in other hematopoietic lineages, including the erythrocyte, megakaryocyte, and lymphocyte. Thus, C/EBPα and C/EBPε are critical, nonredundant regulators in the early and late stages of myelopoiesis, respectively, and their sequential, coordinated expression orchestrates the complex gene regulation involved in producing functional mature neutrophils and eosinophils.
Granulocyte colony-stimulating factor (G-CSF) plays a pivotal role in myeloid development. Mice deficient in either G-CSF or its receptor genes have a reduced neutrophil count (∼ 20% of normal).10,11 Despite the fundamental role of G-CSF in granulopoiesis, there is controversy regarding whether G-CSF acts as an active differentiation inducer or just a survival factor for committed granulocyte precursors. In the latter model, the differentiation pathways of each precursor are internally predetermined in a stochastic fashion and cytokines simply prevent them from undergoing apoptosis by means of their cognate pre-expressed receptor (stochastic model). This model hypothesis is supported by the observation that a hematopoietic progenitor cell line overexpressing bcl-2 has spontaneous differentiation to multiple lineages without adding any cytokines.12 On the other hand, many studies showing that cytokines such as erythropoietin (Epo) and thrombopoietin induce differentiation by inducing specific factors, such as signal transducers and activators of transcription (STAT) or cyclin-dependent kinase (CDK) inhibitors,13 14 support the former model, which grants cytokines an active role in cellular differentiation. Yet none of those studies linked the signals from cytokine receptor to activation of transcriptional regulators that is crucial for differentiation to a specific lineage, and they therefore failed to identify the precise role of cytokines in hematopoietic differentiation.
The differentiation process is often accompanied by cell-cycle arrest and down-regulation of c-myc, a critical component of cellular proliferation15,16 that drives transition from G1 to S by activating its target genes, such as ornithine decarboxylase and Cdc25B.17,18 In general, differentiation and proliferation are mutually exclusive processes and enforced expression of c-myc inhibits differentiation by both preventing irreversible withdrawal from the cell cycle and inhibiting commitment that leads to a terminally differentiated state.19-21However, the precise mechanism by which c-myc prevents cells from committing to a specific differentiation pathway is unclear. One possibility is that c-myc suppresses expression of specific genes, such as transcription factors that critically regulate differentiation to specific lineage. For example, in preadipocytes, c-myc suppresses differentiation to mature adipocytes by inhibiting transcription of C/EBPα, a key regulator of adipogenesis, and overexpression of C/EBPα overrides this suppression.22 Likewise, in myeloid cells, ectopic expression of c-myc in 32Dcl3 cells suppresses granulocytic differentiation by G-CSF, although the mechanism by which this occurs is unknown.
In this study, we found that G-CSF induces C/EBPε in 32Dcl3 cells and that this induction strongly correlates with granulocytic differentiation. The signal for this induction depends on the first tyrosine (Tyr) residue of G-CSF receptor but does not depend on STAT3. We also found that expression of C/EBPε alone is sufficient for morphologic and functional differentiation in 32Dcl3 cells and that ectopic expression of C/EBPε overcomes the differentiation block in 32D/myc cells. Our data show the essential, rate-limiting role of C/EBPε in G-CSF–induced myeloid differentiation, and support the concept that the balance of c-myc and C/EBPε determines whether cells proliferate or differentiate into granulocytes.
Materials and methods
Cell lines
The study used 32Dcl3 cells and FDCP1 cells expressing mutant G-CSF receptor. Cells were cultured in RPMI1640 supplemented with 10% fetal-bovine serum (FBS; Gibco BRL, Rockville, MD), 100 U/mL penicillin G, 100 μg/mL streptomycin, 2 mM l-glutamine, and 25 U/mL recombinant murine interleukin (IL)–3 at 37°C in a humidified atmosphere with 5% carbon dioxide. FDCP1 cells expressing various G-CSF receptor truncation mutants were described previously.23
Plasmids and establishment of 32D transfectants
The expression vector pcEpsilon32 containing human C/EBPε under the control of cytomegalovirus promoter was a generous gift from Dr K. G. Xanthopoulos (National Institutes of Health, Bethesda, MD). Dominant-negative STAT3 was created by polymerase chain reaction using Pfu polymerase (Stratagene, La Jolla, CA) and subcloned intoEcoRI and BamHI sites of pcDNA3 (Invitrogen, Carlsbad, CA). The integrity of amplified sequences was confirmed by DNA sequencing. To obtain 32D cells stably expressing C/EBPε or dominant-negative STAT3, 1 × 107 32Dcl3 cells were electroporated with 20 μg expression vector and selected in the presence of G418 (750 μg/mL) for 14 days. The clones were obtained by limiting dilution, and expression of C/EBPε was examined using Western blot analysis (see below). Three independent clones that showed high levels of expression were analyzed further. All clones had essentially the same phenotype, and representative data from each experiment are shown here. For transfection into 32D/myc cells, a pcZeo-CEBPε expression vector was constructed by subcloning full-length complementary DNA (cDNA) of C/EBPε excised from pcEpsilon32 by HindIII and BamHI into the same sites of pcDNA3-Zeo vector (Invitrogen). Transfection was done as described above, and selection with 750 μg/mL zeomycin was started after 48 hours of transfection. The morphologic characteristics of transfected cells were examined after 6 days of transfection.
Northern blot analysis
Total RNA was extracted from 1 × 107 cells by using RNAzol-B according to the manufacturer's protocol (Tel-test, Friendswood, TX). The RNA samples (20 μg/lane) were separated on 1.0% formaldehyde-denaturing agarose gel and transferred to Hybond N+ membrane (Amersham, Piscataway, NJ). Full-length cDNA of C/EBPε, myeloperoxidase (MPO), c-myc, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as probes. All probes were labeled using a Rediprime kit (Amersham). Hybridizations with phosphorus 32–labeled probes were done in ExpressHyb buffer (Clontech, Palo Alto, CA) according to the manufacturer's protocol. The membranes were washed in washing buffer of 2 × standard saline citrate (SSC) and 0.1% sodium dodecyl sulfate (SDS) for 30 minutes at room temperature, with several buffer changes, and this was followed by 2 washes in 0.1 × SSC and 0.1% SDS for 15 minutes at 42°C. The membranes were exposed on XAR film (Kodak, Rochester, NY) at −80°C for 1 to 5 days.
Immunoprecipitation and Western blotting
Cells (1 × 107) were lysed in extraction buffer (10 mM Tris-hydrochloric acid (HCl), 50 mM sodium chloride (NaCl), 5 mM EDTA, 50 mM sodium fluoride, 30 mM sodium pyrophosphate, 100 μM sodium orthovanadate, 1% Triton-X 100, and 1 mM phenylmethylsulfonyl fluoride). Lysates were centrifuged at 12 000g for 15 minutes at 4°C to remove debris, and protein concentrations were measured using the bicinchoninic acid method (Pierce, Rockford, IL). For immunoprecipitation, lysates were incubated with primary antibodies and protein A–Sepharose beads for several hours at 4°C. The beads were washed extensively with lysis buffer, and recovered proteins were eluted with sample buffer (50 mM Tris [pH 6.8], 2% SDS, 10% glycerol, 1 mM dithiothreitol, and 0.1% bromophenol blue). The immune complexes or 50 μg of each cell extract were/was resolved on 4% to 20% SDS-polyacrylamide gel and transferred to Hybond ECL (Amersham). The membrane was blocked in 5% nonfat milk in TBS-T (20 mM Tris-HCl, 137 mM NaCl, and 0.1% Tween 20) hybridized sequentially with primary antibodies and horseradish peroxidase–conjugated anti-immunoglobulin secondary antibody (Amersham). Bound antibodies were detected by an enhanced-chemiluminescence Western blotting kit (Amersham). Rabbit anti-CRP1 (C/EBPε) polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Morphologic analyses, immunohistochemical studies, and nitroblue tetrazolium reduction assays
Samples of 32Dcl3 and 32D/ε cells treated with IL-3 or G-CSF were prepared on glass slides using the cytospin method. Morphologic features were evaluated with use of Wright-Giemsa staining. Immunohistochemical staining of MPO was done with an MPO detection kit (Sigma, St Louis, MO). For NBT assays, 1 × 106 cells were centrifuged, suspended in 500 μL phosphate-buffered saline (PBS), and incubated at 37°C for 30 minutes with 1 mg/mL NBT and 30 ng/mL 12-O-tetradecanoylphorbol-13-acetate. Cytospin preparations were made and stained with Wright staining. The percentage of cells with formazan deposits in the cytoplasm was determined by microscopical examination.
Flow cytometry
Cells were resuspended in PBS containing 1% FBS and 0.1% sodium azide. Nonspecific antibody binding to surface Fc receptors was blocked by incubating cells with Fc Block (Pharmingen, San Diego, CA) for 15 minutes at 4°C. Cells were then stained with phycoerythrin-conjugated anti–Mac-1α antibody (Pharmingen) for 30 minutes at 4°C. Analysis was done with a FACS Calibur flow cytometer using CellQuest software (Becton Dickinson, Franklin Lakes, NJ).
Results
G-CSF induces C/EBPε in 32Dcl3 cells, and its expression highly correlates with myeloid differentiation
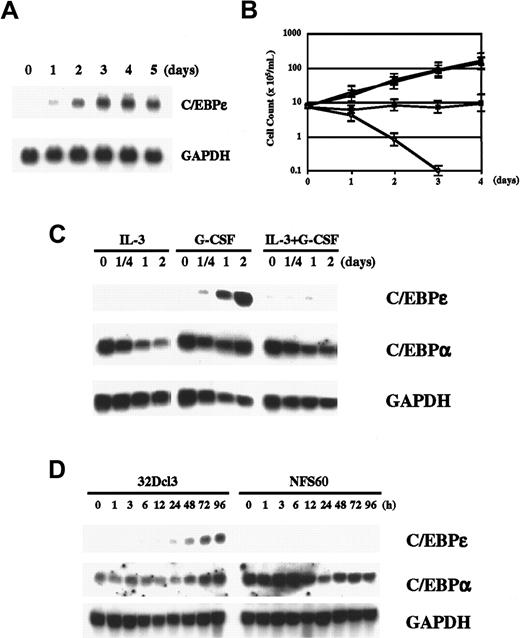
G-CSF induces granulocytic differentiation in 32Dcl3 cells (murine myeloid cell line). Because G-CSF is a key cytokine in myeloid differentiation, we wondered whether G-CSF induces C/EBPε in 32Dcl3 cells. As illustrated in Figure 1A, we found that G-CSF clearly induced C/EBPε expression in 32Dcl3 cells after one day of stimulation. Expression reached the maximum level in 3 days and remained unchanged thereafter for up to 5 days. Up-regulation of C/EBPε protein was also demonstrated by Western blot analysis (Figure 2A). This induction cannot have been due to withdrawal of IL-3 because such withdrawal itself did not induce C/EBPε expression (data not shown), and examination of other cell lines expressing various truncation mutants of G-CSF receptor showed that a signal from G-CSF receptor is required for induction of C/EBPε (see below). Taken together, these results indicate that C/EBPε is a downstream target of G-CSF signaling.
G-CSF regulates C/EBPε.
(A) Induction of C/EBPε by G-CSF. Total RNA extracted from 32Dcl3 cells (20 μg) treated with 50 ng/mL recombinant human G-CSF for the indicated times was subjected to Northern blot analysis. (B) Growth curve for 32Dcl3 cells cultured in various cytokines. The 32Dcl3 cells maintained in the presence of IL-3 were washed extensively with PBS and transferred to medium containing 25 U/mL IL-3 (●), 50 ng/mL G-CSF (▪), IL-3 and G-CSF (▴), or medium without cytokines (○). Viable cells were counted using the trypan blue dye exclusion method at the times indicated. Cells were diluted with each medium to keep cell density within 2 to 10 × 105/mL. (C) Expression of C/EBPε and C/EBPα in 32Dcl3 cells treated with IL-3, G-CSF, or both. Cells growing in IL-3 were washed twice with PBS and transferred to medium containing the indicated cytokines. Northern blot analysis was done as described above. (D) Comparison of expression of C/EBPε and C/EBPα in 32Dcl3 cells and NFS60 cells. Cells were starved in RPMI 1640 with 10% FBS lacking cytokines for 14 hours and then stimulated with 50 ng/mL G-CSF for the times indicated. Each total RNA (20 μg) was then analyzed by Northern blot analysis.
G-CSF regulates C/EBPε.
(A) Induction of C/EBPε by G-CSF. Total RNA extracted from 32Dcl3 cells (20 μg) treated with 50 ng/mL recombinant human G-CSF for the indicated times was subjected to Northern blot analysis. (B) Growth curve for 32Dcl3 cells cultured in various cytokines. The 32Dcl3 cells maintained in the presence of IL-3 were washed extensively with PBS and transferred to medium containing 25 U/mL IL-3 (●), 50 ng/mL G-CSF (▪), IL-3 and G-CSF (▴), or medium without cytokines (○). Viable cells were counted using the trypan blue dye exclusion method at the times indicated. Cells were diluted with each medium to keep cell density within 2 to 10 × 105/mL. (C) Expression of C/EBPε and C/EBPα in 32Dcl3 cells treated with IL-3, G-CSF, or both. Cells growing in IL-3 were washed twice with PBS and transferred to medium containing the indicated cytokines. Northern blot analysis was done as described above. (D) Comparison of expression of C/EBPε and C/EBPα in 32Dcl3 cells and NFS60 cells. Cells were starved in RPMI 1640 with 10% FBS lacking cytokines for 14 hours and then stimulated with 50 ng/mL G-CSF for the times indicated. Each total RNA (20 μg) was then analyzed by Northern blot analysis.
Protein expression and growth characteristics of 32Dcl3 cells expressing C/EBPε.
(A) Parental 32Dcl3 cells were washed and transferred from medium containing IL-3 to G-CSF, and cell lysates were prepared at the times indicated. Lysates of 32D/ε cells were obtained from cells growing in IL-3. Each protein extract (50 μg) was resolved on 4% to 20% gradient SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was probed with polyclonal antibody against C/EBPε. (B) Parental 32Dcl3 cells (solid symbols) and 32D/ε cells (open symbols) maintained in IL-3 were washed with PBS and transferred to medium containing 25 U/mL IL-3 (circle), 50 ng/mL G-CSF (square), or no cytokines (triangle). Viable cells were counted using the trypan blue dye exclusion method. Cells were diluted with each medium to keep cell density within 2 to 10 × 105/mL.
Protein expression and growth characteristics of 32Dcl3 cells expressing C/EBPε.
(A) Parental 32Dcl3 cells were washed and transferred from medium containing IL-3 to G-CSF, and cell lysates were prepared at the times indicated. Lysates of 32D/ε cells were obtained from cells growing in IL-3. Each protein extract (50 μg) was resolved on 4% to 20% gradient SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was probed with polyclonal antibody against C/EBPε. (B) Parental 32Dcl3 cells (solid symbols) and 32D/ε cells (open symbols) maintained in IL-3 were washed with PBS and transferred to medium containing 25 U/mL IL-3 (circle), 50 ng/mL G-CSF (square), or no cytokines (triangle). Viable cells were counted using the trypan blue dye exclusion method. Cells were diluted with each medium to keep cell density within 2 to 10 × 105/mL.
We next examined the correlation between C/EBPε expression and granulocytic differentiation. In medium containing IL-3, 32Dcl3 cells grow and maintain blast-like morphologic characteristics. They also grow in medium containing both IL-3 and G-CSF (Figure 1B). If C/EBPε is crucial in G-CSF–induced granulocytic differentiation, C/EBPε should not be expressed in cells stimulated with both IL-3 and G-CSF. As expected, we found that induction of C/EBPε was completely suppressed by costimulation with IL-3 and G-CSF (Figure 1C). Likewise, IL-3 alone did not induce C/EBPε. We also examined another C/EBP family member, C/EBPα, which is expressed at high levels in 32D cells, regardless of added cytokines. Expression of C/EBPα was unchanged after G-CSF stimulation, whereas it was slightly suppressed by IL-3 alone or IL-3 and G-CSF. This finding suggests that C/EBPε, rather than C/EBPα, is an important regulator in G-CSF–induced granulocytic differentiation and that IL-3 may prevent differentiation by inhibiting the induction signal for C/EBPε from G-CSF receptor.
We also examined NFS60 cells, which do not differentiate but rather proliferate in response to G-CSF. As shown in Figure 1D, G-CSF did not induce C/EBPε in these cells. In contrast to our findings with C/EBPε, C/EBPα was highly expressed in NFS60 cells, as it was in 32D cells. This observation confirms the correlation between expression of C/EBPε and G-CSF–regulated differentiation.
C/EBPε facilitates morphologic and functional differentiation to granulocytes
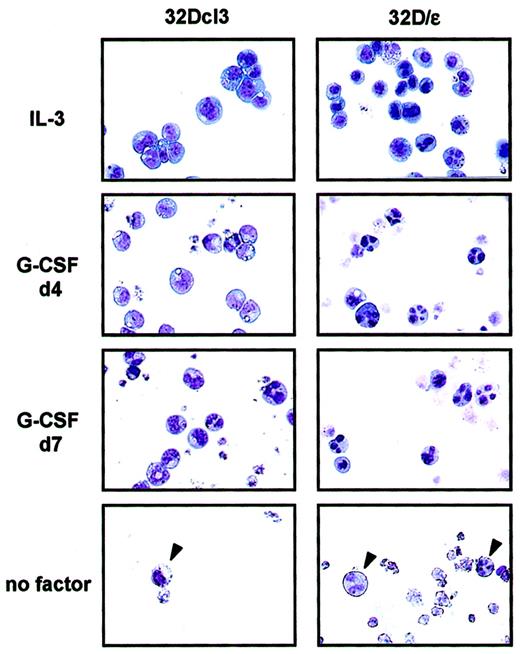
To investigate the role of C/EBPε in granulocytic differentiation, we stably expressed C/EBPε in 32Dcl3 cells. As shown in Figure 2A, these cells (32D/ε) expressed C/EBPε protein at a level comparable to that in a parental cell line treated with G-CSF for 2 days. Interestingly, 32D/ε cells grew at a slower rate than parental 32Dcl3 cells in IL-3 (Figure 2B). In addition, they showed spontaneous differentiation to granulocytes in IL-3, at a significantly higher rate than parental cells (Figure 3and Table 1). Surprisingly, when 32D/ε cells were transferred to medium containing G-CSF, they immediately started to differentiate to granulocytes, and 4 days later, almost all cells looked like segmented neutrophils (Table 1 and Figure 3). In contrast, parental 32Dcl3 cells still showed immature blast–like morphologic features after 4 days of treatment with G-CSF and they took 14 days to fully differentiate (Table 1 and Figure 3). Moreover, even though both these cell lines underwent apoptosis in a few days after removal of IL-3, few surviving 32D/ε cells showed spontaneous differentiation to granulocytes, whereas parental 32Dcl3 cells maintained their immature blast–like morphologic characteristics under the same conditions (Figure 3).
Priming of 32D/ε cells for granulocyte differentiation.
Shown are the morphologic characteristics of parental 32Dcl3 cells and 32D/ε cells maintained in IL-3, treated with 50 ng/mL G-CSF for the period indicated (d4 indicates 4 days; and d7, 7 days), and cultured in medium without cytokines (no factor) for 4 days. The morphologic features of the cells were visualized using Wright-Giemsa staining (original magnification, ×400).
Priming of 32D/ε cells for granulocyte differentiation.
Shown are the morphologic characteristics of parental 32Dcl3 cells and 32D/ε cells maintained in IL-3, treated with 50 ng/mL G-CSF for the period indicated (d4 indicates 4 days; and d7, 7 days), and cultured in medium without cytokines (no factor) for 4 days. The morphologic features of the cells were visualized using Wright-Giemsa staining (original magnification, ×400).
Differential counts of 32Dcl3 and 32D/ε cells treated with interleukin 3, granulocyte colony-stimulating factor, or both
| Cells . | IL-3 . | G-CSF . | IL-3 + G-CSF . | ||||
|---|---|---|---|---|---|---|---|
| Day 4 . | Day 7 . | Day 10 . | Day 14 . | Day 4 . | Day 7 . | ||
| 32Dcl3 | |||||||
| Myeloblasts | 96.6 ± 1.5 | 87.0 ± 3.6 | 3.7 ± 4.0 | 3.3 ± 1.5 | 0.3 ± 0.6 | 92.0 ± 2.6 | 93.7 ± 3.1 |
| Myelocytes | 5.0 ± 2.0 | 11.3 ± 3.5 | 72.6 ± 8.0 | 29.0 ± 3.6 | 21.0 ± 5.6 | 8.0 ± 2.6 | 6.3 ± 3.1 |
| Band (seg) | 0 ± 0 | 2.3 ± 1.2 | 23.7 ± 11.1 | 67.7 ± 4.6 | 78.3 ± 5.5 | 0 ± 0 | 0 ± 0 |
| 32D/ε | |||||||
| Myeloblasts | 85.0 ± 7.0 | 1.0 ± 1.0 | 0 ± 0 | — | — | 83.7 ± 3.2 | 83.7 ± 6.0 |
| Myelocytes | 11.0 ± 5.3 | 25.7 ± 4.0 | 21.0 ± 3.6 | — | — | 9.3 ± 2.1 | 11.3 ± 3.2 |
| Band (seg) | 4.0 ± 2.6 | 73.3 ± 3.8 | 79.0 ± 3.6 | — | — | 7.0 ± 3.6 | 5.0 ± 3.5 |
| Cells . | IL-3 . | G-CSF . | IL-3 + G-CSF . | ||||
|---|---|---|---|---|---|---|---|
| Day 4 . | Day 7 . | Day 10 . | Day 14 . | Day 4 . | Day 7 . | ||
| 32Dcl3 | |||||||
| Myeloblasts | 96.6 ± 1.5 | 87.0 ± 3.6 | 3.7 ± 4.0 | 3.3 ± 1.5 | 0.3 ± 0.6 | 92.0 ± 2.6 | 93.7 ± 3.1 |
| Myelocytes | 5.0 ± 2.0 | 11.3 ± 3.5 | 72.6 ± 8.0 | 29.0 ± 3.6 | 21.0 ± 5.6 | 8.0 ± 2.6 | 6.3 ± 3.1 |
| Band (seg) | 0 ± 0 | 2.3 ± 1.2 | 23.7 ± 11.1 | 67.7 ± 4.6 | 78.3 ± 5.5 | 0 ± 0 | 0 ± 0 |
| 32D/ε | |||||||
| Myeloblasts | 85.0 ± 7.0 | 1.0 ± 1.0 | 0 ± 0 | — | — | 83.7 ± 3.2 | 83.7 ± 6.0 |
| Myelocytes | 11.0 ± 5.3 | 25.7 ± 4.0 | 21.0 ± 3.6 | — | — | 9.3 ± 2.1 | 11.3 ± 3.2 |
| Band (seg) | 4.0 ± 2.6 | 73.3 ± 3.8 | 79.0 ± 3.6 | — | — | 7.0 ± 3.6 | 5.0 ± 3.5 |
Values are the mean ± SD percentages of cells from 3 independent experiments. Myelocytes includes promyelocytes, myelocytes, and metamyelocytes.
G-CSF, granulocyte colony-stimulating factor; IL-3, interleukin-3; band (seg), band and segmented neutrophils.
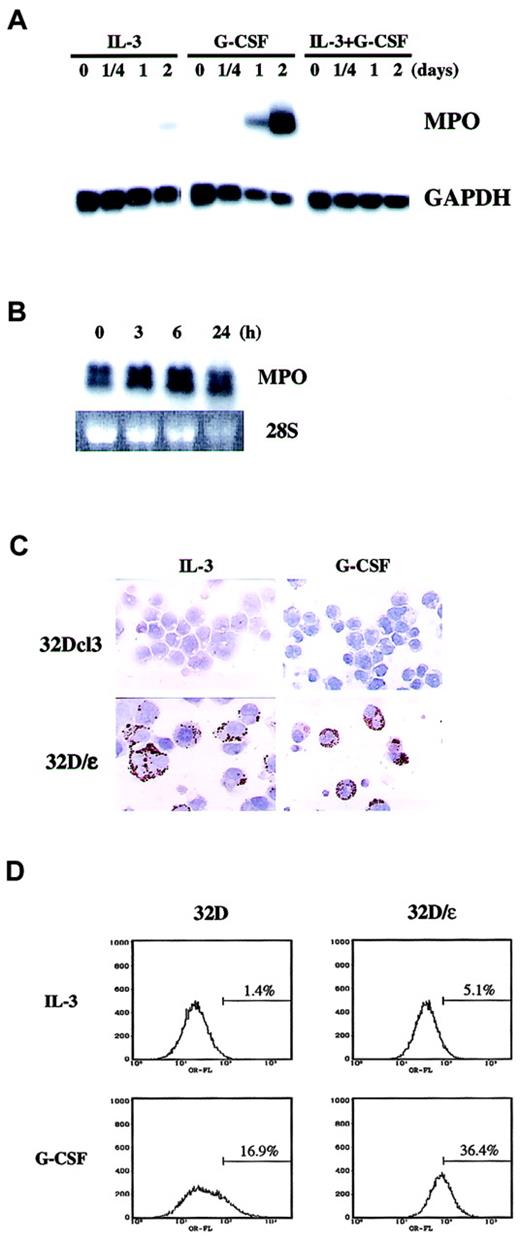
We then examined whether the morphologic change observed in 32D/ε cells was accompanied by functional maturation. It was previously reported that C/EBPε knockout mice have a defective oxidative burst, a finding that suggests that C/EBPε is critical in the generation of reactive oxygen. To test whether C/EBPε is sufficient to elicit an oxidative burst, we conducted an NBT reduction test in 32D/ε cells. The number of NBT-positive cells in medium containing either IL-3 or G-CSF was much higher among 32D/ε cells than parental 32Dcl3 cells (Table 2). We also examined expression of MPO as a differentiation marker. In parental 32D cells, neither IL-3 alone nor IL-3 and G-CSF induced MPO expression (Figure4A). In contrast, G-CSF strongly induced MPO in these cells in 2 days. Surprisingly, we observed constitutive MPO expression in 32D/ε cells even in the presence of IL-3, and G-CSF treatment marginally increased this expression (Figure 4B). We confirmed this observation by immunohistochemical staining of MPO in vivo (Figure 4C and Table 2). In this setting, G-CSF significantly increased the percentage of MPO-positive 32D/ε cells (Table2). MPO is a primary granule protein known to be induced by C/EBPα, but this observation suggests that C/EBPε can also induce MPO. We did not observe any MPO activity in G-CSF–treated parental 32D cells on immunohistochemical examination, whereas Northern blotting analysis found that G-CSF induced MPO messenger RNA (mRNA) in 2 days. This discrepancy could be due to either delayed protein synthesis of MPO or the different sensitivity of the assays.
Myeloperoxidase and nitroblue tetrazolium reducing activity in 32Dcl3 and 32D/ε cells
| Cells . | MPO (%) . | NBT (%) . |
|---|---|---|
| 32Dcl3 | ||
| IL-3 | 0 ± 0 | 10.3 ± 2.1 |
| G-CSF | 0 ± 0 | 50.3 ± 2.5 |
| 32D/ε | ||
| IL-3 | 46.0 ± 4.6 | 60.0 ± 7.2 |
| G-CSF | 86.7 ± 4.2 | 95.3 ± 2.5 |
| Cells . | MPO (%) . | NBT (%) . |
|---|---|---|
| 32Dcl3 | ||
| IL-3 | 0 ± 0 | 10.3 ± 2.1 |
| G-CSF | 0 ± 0 | 50.3 ± 2.5 |
| 32D/ε | ||
| IL-3 | 46.0 ± 4.6 | 60.0 ± 7.2 |
| G-CSF | 86.7 ± 4.2 | 95.3 ± 2.5 |
Values are the mean ± SD percentages of cells positive for MPO or NBT from 3 independent experiments. Cells were treated with G-CSF for 3 days.
MPO indicates myeloperoxidase; NBT, nitroblue tetrazolium. See Table 1for other abbreviations.
Constitutive MPO and enhanced Mac-1α expression in 32D/ε cells.
(A) MPO mRNA expression in 32Dcl3 cells. The blot shown in Figure 1C was reprobed with MPO. (B,C) 32D/ε cells cultured in IL-3 were washed twice with PBS and then stimulated with 50 ng/mL G-CSF. Cells were harvested at the times indicated and subjected to Northern blot analysis (B). Expression of MPO was confirmed by immunohistochemical staining of 32Dcl3 and 32D/ε cells cultured in IL-3 or treated with 50 ng/mL G-CSF for 3 days (C; original magnification, ×400). (D) FACS analysis of Mac-1α in 32D and 32D/ε cells cultured in IL-3 or treated with 50 ng/mL G-CSF for 3 days.
Constitutive MPO and enhanced Mac-1α expression in 32D/ε cells.
(A) MPO mRNA expression in 32Dcl3 cells. The blot shown in Figure 1C was reprobed with MPO. (B,C) 32D/ε cells cultured in IL-3 were washed twice with PBS and then stimulated with 50 ng/mL G-CSF. Cells were harvested at the times indicated and subjected to Northern blot analysis (B). Expression of MPO was confirmed by immunohistochemical staining of 32Dcl3 and 32D/ε cells cultured in IL-3 or treated with 50 ng/mL G-CSF for 3 days (C; original magnification, ×400). (D) FACS analysis of Mac-1α in 32D and 32D/ε cells cultured in IL-3 or treated with 50 ng/mL G-CSF for 3 days.
The enhanced differentiation capacity of 32D/ε cells was also confirmed by flow cytometric analysis of Mac-1α expression. As shown in Figure 4D, 32D/ε cells expressed about 3 times more Mac-1α than parental cells when cultured in IL-3. Moreover, compared with parental cells, 32D/ε cells had enhanced Mac-1α expression in response to G-CSF. All these data strongly indicate that C/EBPε is the principal downstream target of G-CSF, whose expression alone is sufficient to support terminal granulocyte differentiation.
Tyr 703 of G-CSF receptor is important for C/EBPε induction
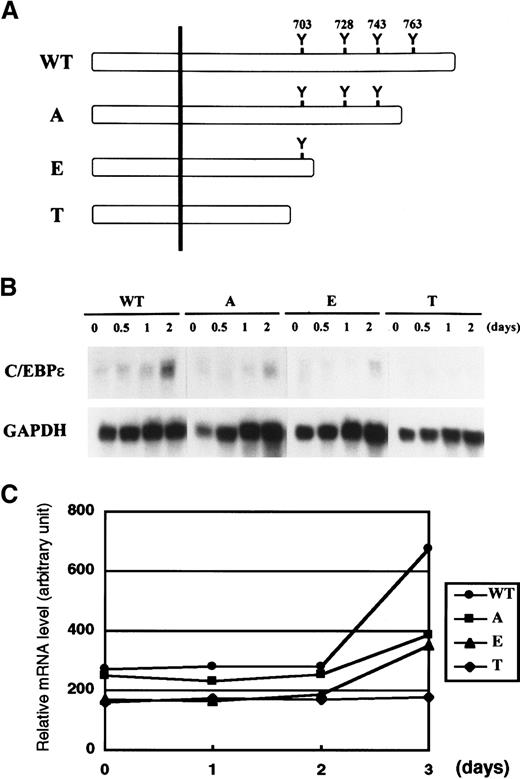
Tyr residues on the cytoplasmic domain of cytokine receptors are phosphorylated by Janus kinase and serve as docking sites for various signaling molecules that contain the Src homology 2 domain. Yoshikawa et al24 showed that the first Tyr residue (Tyr 703) of G-CSF receptor is important for a myeloid differentiation signal that leads to morphologic change, induction of MPO, and growth arrest. On the basis of this observation, we speculated that Tyr 703 might be important for induction of C/EBPε by G-CSF. We addressed this question by using FDCP1 cells expressing various truncation mutants of G-CSF receptor (Figure5A).23 As shown in Figure5B, C/EBPε was induced by G-CSF in FDCP1–G-CSF receptor wild-type (WT) cells. Induction of C/EBPε was reduced but was significant in A and E mutant cells, both of which retain Tyr 703. However, T mutant cells, which lack all Tyr residues on the receptor, showed no C/EBPε induction. A densitometric analysis of relative C/EBPε mRNA expression compared with GAPDH is shown in Figure 5C. Although the differences in C/EBPε induction among mutants were subtle, the data were highly reproducible. This result suggests that the region surrounding Tyr 703 is sufficient to generate the induction signal for C/EBPε and that a C-terminal portion of the receptor including Tyr 763 contributes to this induction.
Induction of C/EBPε by mutant G-CSF receptor.
(A,B) FDCP1 cells expressing various truncation mutants of G-CSF receptor described in panel A were analyzed for expression of C/EBPε (B). Cells growing in IL-3 were washed twice with PBS and transferred to medium containing 50 ng/mL G-CSF. Cells were harvested at the times indicated and subjected to Northern blot analysis. (C) Relative mRNA induction of C/EBPε. Northern blotting film shown in panel B in the linear exposure was subjected to densitometric analysis. The graph is plotted as the signal ratio of C/EBPε compared with GAPDH. WT indicates wild-type G-CSF receptor; detailed constructions of A, E, and T mutants are described in Fukunaga et al.23
Induction of C/EBPε by mutant G-CSF receptor.
(A,B) FDCP1 cells expressing various truncation mutants of G-CSF receptor described in panel A were analyzed for expression of C/EBPε (B). Cells growing in IL-3 were washed twice with PBS and transferred to medium containing 50 ng/mL G-CSF. Cells were harvested at the times indicated and subjected to Northern blot analysis. (C) Relative mRNA induction of C/EBPε. Northern blotting film shown in panel B in the linear exposure was subjected to densitometric analysis. The graph is plotted as the signal ratio of C/EBPε compared with GAPDH. WT indicates wild-type G-CSF receptor; detailed constructions of A, E, and T mutants are described in Fukunaga et al.23
STAT3 is critical in G-CSF–induced myeloid differentiation but acts in a pathway different from that of C/EBPε
Previously, it was shown that Tyr 703 is a major docking site for STAT3.25 This raised the possibility that C/EBPε could be regulated by STAT3. To explore this possibility, we created 32D cells expressing a carboxyl-truncated STAT3 that lacked 55 amino acids including the transactivation domain, which acts in a dominant- negative fashion with respect to endogenous STAT3 (Figure6A).26 In contrast to parental cells, 32D cells expressing dominant- negative STAT3 (32D/DN-STAT3) proliferated in G-CSF (Figure 6B), and G-CSF could support their long-term growth over 2 months (data not shown). Morphologic analysis showed that 32D/DN-STAT3 cells maintained immature morphologic characteristics in G-CSF, without evidence of differentiation (Figure 6C). These observations are consistent with previous reports showing that dominant-negative STAT3 inhibits G-CSF– or IL-6–induced differentiation and growth arrest in other hematopoietic cell lines26-28; and this indicates that the dominant-negative construct is working properly.
The 32D cells expressing dominant-negative STAT3 show differentiation block but normal C/EBPε induction.
(A) Construction of dominant-negative STAT3. (B) Growth curve of 32D/DN-STAT3. Cells were cultured in 25 U/mL IL-3 (○), 50 ng/mL G-CSF (▴), or without cytokines (▪). (C) Morphologic features of 32D/DN-STAT3 cells cultured in IL-3 or G-CSF and stained with Wright-Giemsa stain (original magnification, ×400). (D) Expression of C/EBPε and MPO mRNA in 32D/DN-STAT3 cells. Cells were transferred from IL-3 to G-CSF and subjected to Northern blot analysis at the times indicated.
The 32D cells expressing dominant-negative STAT3 show differentiation block but normal C/EBPε induction.
(A) Construction of dominant-negative STAT3. (B) Growth curve of 32D/DN-STAT3. Cells were cultured in 25 U/mL IL-3 (○), 50 ng/mL G-CSF (▴), or without cytokines (▪). (C) Morphologic features of 32D/DN-STAT3 cells cultured in IL-3 or G-CSF and stained with Wright-Giemsa stain (original magnification, ×400). (D) Expression of C/EBPε and MPO mRNA in 32D/DN-STAT3 cells. Cells were transferred from IL-3 to G-CSF and subjected to Northern blot analysis at the times indicated.
We then examined expression of C/EBPε and MPO in 32D/DN-STAT3 cells and found that C/EBPε and MPO were both normally induced in response to G-CSF (Figure 6D). This result indicates that STAT3 and C/EBPε work in different pathways and that C/EBPε is regulated by a signaling molecule other than STAT3.
c-myc blocks myeloid differentiation by inhibiting expression of C/EBPε but not C/EBPα
Aberrant proliferation control leads to altered differentiation and ultimately to malignant transformation. c-myc is one of the critical regulators of cellular proliferation, and it is known that overexpression of c-myc blocks differentiation in many cell types.19-21 In 32Dcl3 cells, overexpression of c-myc also blocks differentiation by G-CSF, but the underlying molecular mechanism of this effect is not well understood. We speculated that c-myc may block differentiation by inhibiting genes critical for differentiation, and this led us to examine expression of C/EBPε and C/EBPα in 32Dcl3 cells stably expressing c-myc (32D/myc).29
As expected, basal expression of C/EBPε in cultures containing IL-3 was completely abrogated in 32D/myc cells on Northern blot analysis, whereas expression of C/EBPα was unaffected. Moreover, G-CSF treatment did not cause any measurable increase in C/EBPε expression, whereas expression of C/EBPα was unchanged (Figure7A). We also observed no induction of MPO by G-CSF (Figure 7A). These data suggest that c-myc inhibits G-CSF–induced differentiation by inhibiting transcription of C/EBPε in 32D/myc cells.
C/EBPε is critical in myc-induced differentiation block in 32D/myc cells.
(A) Expression of C/EBPε, C/EBPα, and MPO in 32D/myc cells. Cells maintained in IL-3 were washed and transferred to medium containing 50 ng/mL G-CSF. The cells were harvested at the times indicated and subjected to Northern blot analysis. (B) Ectopic expression of C/EBPε induces differentiation in 32D/myc cells. C/EBPε expression vector (20 μg) with a cistronic zeomycin-resistance marker (pcDNA3Zeo-C/EBPε) was electroporated into 32D/myc cells, and transfected cells were selected with 750 μg/mL zeomycin for 6 days. The empty vector was used as a negative control (i indicates negative control; and ii-iv, C/EBPε-transfected cells). Morphologic features of cells were visualized by using Wright-Giemsa staining (original magnification, ×400).
C/EBPε is critical in myc-induced differentiation block in 32D/myc cells.
(A) Expression of C/EBPε, C/EBPα, and MPO in 32D/myc cells. Cells maintained in IL-3 were washed and transferred to medium containing 50 ng/mL G-CSF. The cells were harvested at the times indicated and subjected to Northern blot analysis. (B) Ectopic expression of C/EBPε induces differentiation in 32D/myc cells. C/EBPε expression vector (20 μg) with a cistronic zeomycin-resistance marker (pcDNA3Zeo-C/EBPε) was electroporated into 32D/myc cells, and transfected cells were selected with 750 μg/mL zeomycin for 6 days. The empty vector was used as a negative control (i indicates negative control; and ii-iv, C/EBPε-transfected cells). Morphologic features of cells were visualized by using Wright-Giemsa staining (original magnification, ×400).
We hypothesized that if c-myc blocks granulocytic differentiation by suppressing expression of C/EBPε, introduction of C/EBPε into 32D/myc cells would overcome this differentiation block. To test this hypothesis, we transiently overexpressed C/EBPε in 32D/myc cells, selected transfected cells by means of drug resistance, and examined their morphologic features. As shown in Figure 7B, enforced expression of C/EBPε induced a change in nuclear morphologic characteristics and the appearance of cytoplasmic granules in 32D/myc cells (ii-iv), whereas the control vector did not (i). Expression of C/EBPε in transfected cells was confirmed by immunofluorescence analysis (data not shown). These data indicate that c-myc suppresses C/EBPε expression in myeloid cells, thereby blocking differentiation in response to G-CSF.
Discussion
Unlike expression of other members of the C/EBP family, expression of C/EBPε was found to be highly restricted to myeloid cells and lymphoid cells.3,4 Although human primary CD34+ cells do not express substantial levels of C/EBPε, expression of C/EBPε was induced dramatically when such cells were induced to differentiate to granulocytes by G-CSF.4However, it was not clear whether expression of C/EBPε is regulated directly by G-CSF. In this study, we showed that G-CSF induces C/EBPε in myeloid progenitor cells and that expression of C/EBPε alone is sufficient to support both morphologic and functional differentiation. Because of these data and the phenotype of C/EBPε-deficient mice that revealed the essential role of C/EBPε in myeloid development, we conclude that C/EBPε is the key downstream effector in G-CSF–induced granulocyte differentiation.
There is controversy about whether the only role of cytokines in hematopoiesis is to support survival of the progenitors that express its cognate receptor on their surface and receptor-expressing cells are destined to execute an intrinsically preset differentiation program (stochastic model). In contrast, an instructive model suggests active roles for lineage-specific cytokines in directing various differentiation processes. Our data show that G-CSF actively regulates the myeloid differentiation program by inducing C/EBPε. Previously, mutant mice whose cytoplasmic domain of G-CSF receptor was replaced with the cytoplasmic domain of the Epo receptor were generated, and surprisingly, the mice had no apparent defect in lineage commitment.30 This finding suggests that signaling to induce C/EBPε generated from G-CSF receptor can be replaced by Epo signaling and thus should be a common pathway used by both Epo and G-CSF. Our data showing that STAT3 is not involved in this pathway also fit into this model, because Epo and G-CSF activate different STAT proteins.
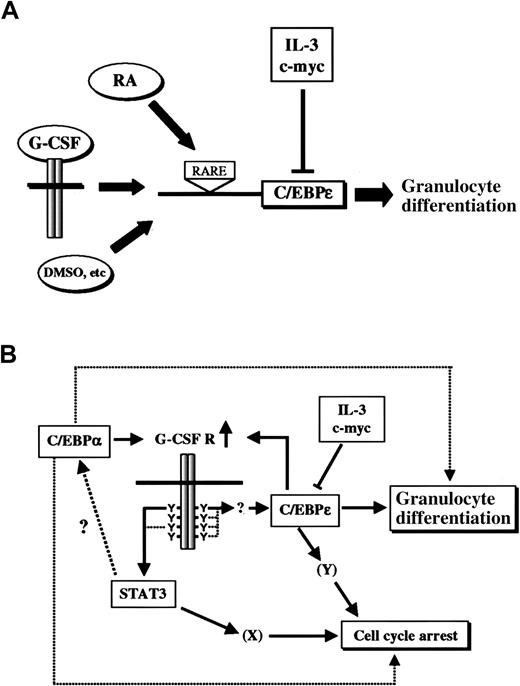
Although our results place C/EBPε downstream of G-CSF signaling, the phenotypes of G-CSF and C/EBPε knockout mice were apparently different, suggesting that C/EBPε could also be regulated by other pathways. In fact, C/EBPε can be induced by other growth factors or differentiation inducers, such as retinoic acid, stem cell factor (SCF), PIXY321 (an engineered recombinant fusion protein of IL-3 and granulocyte-macrophage colony-stimulating factor) and dimethyl sulfoxide (DMSO), in leukemic cell lines or immature myeloid cells, thereby suggesting that C/EBPε is a common integrator of various differentiation signals (Figure8A).3,31,32 In addition, Park et al32 showed that promoter of C/EBPε is regulated directly by retinoic acid through the retinoic acid responsive element. It is not clear whether other cytokines or chemicals such as SCF or DMSO directly affect the promoter activity of C/EBPε. In the case of G-CSF, induction of C/EBPε does not occur in an immediate-early fashion, indicating that multiple signaling steps are involved in this process. In fact, new protein synthesis is required for induction of C/EBPε by G-CSF, because such induction is blocked by cycloheximide (H. N., J. N. I., unpublished data, November 1998).
C/EBPε is an integrator of multiple signaling pathways that induce myeloid differentiation.
(A) Expression of C/EBPε is up-regulated not only by G-CSF but also by retinoic acid and other differentiation-inducing chemicals. Retinoic acid (RA) acts through the retinoic acid responsive element (RARE) on the promoter of C/EBPε. IL-3 and c-myc suppress C/EBPε expression at the transcriptional level. (B) A model of G-CSF–induced granulocytic differentiation. C/EBPα up-regulates G-CSF receptor and enhances signals to induce STAT3 and C/EBPε. Expression of G-CSF receptor can also be induced by C/EBPε as a positive feedback regulation. C/EBPε induces morphologic and functional differentiation to granulocytes and may be involved in cell-cycle arrest. STAT3 is critical for cell-cycle arrest, cooperatively or independently with C/EBPε. STAT3 may also be essential for differentiation, and C/EBPα is one possible downstream target of the STAT3-dependent pathway.
C/EBPε is an integrator of multiple signaling pathways that induce myeloid differentiation.
(A) Expression of C/EBPε is up-regulated not only by G-CSF but also by retinoic acid and other differentiation-inducing chemicals. Retinoic acid (RA) acts through the retinoic acid responsive element (RARE) on the promoter of C/EBPε. IL-3 and c-myc suppress C/EBPε expression at the transcriptional level. (B) A model of G-CSF–induced granulocytic differentiation. C/EBPα up-regulates G-CSF receptor and enhances signals to induce STAT3 and C/EBPε. Expression of G-CSF receptor can also be induced by C/EBPε as a positive feedback regulation. C/EBPε induces morphologic and functional differentiation to granulocytes and may be involved in cell-cycle arrest. STAT3 is critical for cell-cycle arrest, cooperatively or independently with C/EBPε. STAT3 may also be essential for differentiation, and C/EBPα is one possible downstream target of the STAT3-dependent pathway.
MPO was previously shown to be the target gene of C/EBPα33; however, the existence of constitutive expression of MPO in 32D/ε cells indicates that MPO can also be induced by C/EBPε. The previous observation that MPO is present in C/EBPε knockout mice34 but not in C/EBPα knockout mice7 seems to indicate that C/EBPα is the only regulator of MPO. However, because of the lack of C/EBPε expression in C/EBPα knockout mice that results from differentiation block at the myeloid progenitor stage, it is possible that MPO could also be regulated by C/EBPε. Hence, the best interpretation of our data is that both C/EBPα and C/EBPε can regulate MPO during myeloid differentiation.
Previous investigations found that C/EBPα is critical for early granulocyte differentiation.33,35 In those studies, induction of C/EBPα induced morphologic differentiation and expression of granulocyte-specific genes, such as G-CSF receptor, lactoferrin, neutrophil collagenase, and C/EBPε.35 Our study showed that C/EBPε is also capable of facilitating granulocytic differentiation. Taken together, the data from all these studies suggest that both C/EBPα and C/EBPε regulate granulocytic differentiation, cooperatively or independently, in vivo. Because C/EBPα induces expression of G-CSF receptor,35 it is intriguing to speculate that C/EBPα not only directs granulocytic differentiation by itself but also by enhancing C/EBPε expression by up-regulating G-CSF receptor. In addition, C/EBPε also enhances expression of G-CSF receptor,36 which then creates a positive feedback regulation on G-CSF signaling. On the other hand, our study showed that STAT3 plays an essential role in G-CSF–induced differentiation by means of a pathway distinct from that of C/EBPε. The molecular mechanism by which STAT3 regulates granulocyte differentiation is not currently understood, but one possibility is regulation through C/EBPα. A proposed model for G-CSF signaling pathways to granulocytic differentiation is shown in Figure 8B.
A previous study showed the importance of Tyr 703 of G-CSF receptor in differentiation signaling.24 In that investigation, mutation of Tyr 703 abolished the ability of G-CSF to induce morphologic differentiation, MPO expression, and cell-cycle arrest. The downstream molecule responsible for these effects was not identified, but our study suggests that C/EBPε and STAT3 are the 2 principal candidates. With regard to cell-cycle arrest, we observed a compromised growth rate in 32D/ε cells compared with parental cells, indicating that C/EBPε may be involved in growth inhibition. In fact, C/EBPα, another C/EBP family member, was previously found to inhibit proliferation of adipocytes and hepatocytes by stabilizing protein levels of p21/WAF1/CIP1.37,38 Therefore, an intriguing possibility is that C/EBPε may also inhibit progression of the cell cycle by means of p21 or other CDK inhibitors. However, because IL-3 can support long-term growth (> 5 months; unpublished data) of 32D/ε cells, though at a slower rate, it is possible that some additional mechanisms are involved in cell-cycle arrest. Data showing involvement of STAT3 in growth arrest of M1 cells by IL-6, G-CSF receptor–transfected LGM-1 cells by G-CSF, and macrophages by IL-10 suggest that STAT3 has a role in cell-cycle arrest associated with differentiation.26,27 39 Similarly, we observed G-CSF–dependent proliferation in 32Dcl3 cells expressing dominant-negative STAT3, a finding that also suggests that STAT3 sends a growth-inhibitory signal. All these data indicate that both C/EBPε and STAT3 are negative regulators of cell proliferation. Studies to address this issue are currently under way.
NF-M, the chicken homologue of C/EBPβ, plays an important role in avian myelomonocytic and eosinophilic differentiation.40,41 NF-M was first discovered as a critical transcription factor required for expression of chicken myelomonocytic growth factor, which is distantly related to mammalian G-CSF and IL-6.42 Expression of NF-M is restricted to the myelomonocytic lineage and is turned on at some point before the myeloblast stage. Conditional expression of NF-M in a hematopoietic progenitor cell line induced eosinophilic differentiation and apoptosis.41 Although NF-M is structurally most related to mammalian C/EBPβ, studies of its expression pattern and function suggested that it is more closely related to C/EBPε. In view of these findings on NF-M, it is intriguing to speculate that C/EBPε might activate transcription of the G-CSF gene, which further facilitates the differentiation program to granulocytes.
c-myc controls proliferation and differentiation of the cell through its capacity as a transcription factor to promote the activity of the cyclin E–CDK2 complex or to induce apoptosis.29Expression of c-myc increases when quiescent cells are induced to proliferate43 and decreases when the cells exit the cell cycle.15,16,44,45 In many cases, expression of c-myc overrides differentiation signals generated by differentiation inducers,19-21 suggesting that c-myc may negatively regulate differentiation by means of unknown mechanisms. We here observed that c-myc inhibited expression of C/EBPε in a myeloid differentiation system. This finding raises the possibility that the overall balance of expression of c-myc and differentiation-regulating genes such as C/EBP determines the fate of cells, that is, whether they proliferate or differentiate under various conditions. Because the reciprocal pattern of regulation—inhibition of myc by differentiation-promoting genes—has not been reported, it can be speculated that myc is the dominant regulator over differentiation; thus, explaining the regulation of myc expression is essential for understanding the complex network of growth and differentiation control.
In summary, this study revealed the essential, rate-limiting role of C/EBPε in G-CSF–induced granulocytic differentiation and illuminated the role of C/EBPε as a key integrator of various differentiation signals during myeloid differentiation (Figure 8A).
We thank Dr Kleanthis Xanthopoulos for providing pcEpsilon32 vector, Dr Shigekazu Nagata for FDCP1 cells expressing G-CSF receptor mutants, and Dr John Cleveland for 32D/myc cells.
Supported by Cancer Center CORE grant CA21765, grant RO1 DK42932 to J.N.I., grant PO1 HL53749, and the American Lebanese Syrian Associated Charities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hideaki Nakajima, Blood Center, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: hnakajim@med.keio.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal