We read with great interest the paper by Chesi et al regarding FGFR3 that further supports the role ofFGFR3 in the pathogenesis of multiple myeloma (MM).1 Monoclonal gammopathy of undetermined significance (MGUS) also has genetic abnormalities seen in MM, including the t(4;14)(p16.3;q32)2 3 with presumptive up-regulation of theFGFR3 oncogene. Here we provide additional data attesting that the pathogenetic pathways in MM are highly specific. Specifically, we find that in MGUS and MM the t(4;14)(p16.3;q32) is strongly associated with Δ13.
We tested 155 patients with MM and 52 patients with MGUS/SMM (50 with MGUS and 2 with SMM) for evidence of the t(4;14)(p16.3;q32) using cIg-FISH with a fusion strategy for detection of the abnormality. For IgH locus we used the previously published probes by Gabrea et al4 (VH and CH probes), and both probes were directly labeled with SpectrumGreen (Vysis, Downers Grove, IL). For the 4p16.3 locus, we used the PAC probe previously used by Chesi et al5 (FGFR3 PAC184d6/385). We also used a BAC clone containing sequences of the centromeric most cosmid (96a2) in the contig described by Chesi et al,6 retrieved with the PCR primers 5'-ACAAGACGCTACTGTTTTCC-3' and 5'-TCTAGATCTCTGCATCGAGC-3' and purchased from Incyte Genomics (human BAC Release II; Palo Alto, CA). Both 4p16.3 probes were directly labeled with SpectrumRed (Vysis). A patient was considered to have the t(4;14)(p16.3;q32) if the percent of abnormal plasma cells exceeded 10% of signals with an abnormal pattern (fusions) indicative of a t(4;14)(p16.3;q32). All of these patients were also tested for Δ13 by the previously published strategy and methods using the probes LSI13-Rb and D13S319.8
Of 155 MM patients, 16 (10.3%) had an abnormal pattern consistent with the t(4;14)(p16.3;q32). The median percentage of abnormal plasma cells (MPAPC) was 88% (range, 40%-100%). Fifteen of 16 MM patients (94%) with the t(4;14)(p16.3;q32) had Δ13 (MPAPC 99%; range, 96%-100%). Likewise, we found the t(4;14)(p16.3;q32) in a similar proportion, 5 (9.6%) of 52 patients with MGUS/SMM (3 MGUS, 1 SMM); (MPAPC 86%; range, 49%-100%). In 3 of 4 (75%) patients with MGUS/SMM and the t(4;14)(p16.3;q32), we found concurrent Δ13 (MPAPC 91%; 91%, 91%, and 98%). The incidence of Δ13 in MGUS, SMM, and MM is strikingly different from what we and others have previously reported in MM7,8 (about 50%) and MGUS (about 30%).7
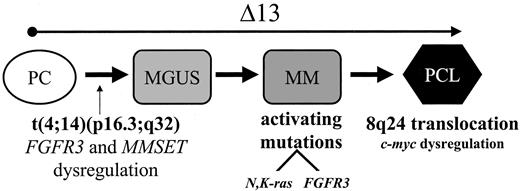
We believe this study provides important information regarding the progression pathways from MGUS to MM. First, as previously mentioned,7 we have shown in MM a striking association of the t(4;14)(p16.3;q32) with Δ13 in both MM and MGUS. This would suggest that Δ13 is an important factor in the pathogenesis of MGUS/MM with the t(4;14)(p16.3;q32) rather than being a factor promoting progression from MGUS to MM. The near-obligate presence of Δ13 in MM with the t(4;14)(p16.3;q32), but not the opposite, suggests that Δ13 occurs prior to the translocation event. Because of the low prevalence of ras mutations, the association withFGFR3 mutations, and the striking associations with Δ13, we postulate that MM with the t(4;14)(p16.3;q32) represents a unique subtype of MM. We thus propose a refinement in the model, as shown in Figure 1, that incorporates the t(4;14)(p16.3;q32), Δ13, ras, and FGFR3mutations. The results of this study also highlight the high likelihood that subgroups of MGUS patients, classified according to the underlying genetic abnormalities, may be at different risk of progression to MM. This is in need of a prospective study.
A modified model for progression of MGUS and MM with the t(4;14)(p16.3;q32).
This modified version incorporates the presence of Δ13 since early in the process of pathogenesis as a critical component of the establishment of a malignant clone.
A modified model for progression of MGUS and MM with the t(4;14)(p16.3;q32).
This modified version incorporates the presence of Δ13 since early in the process of pathogenesis as a critical component of the establishment of a malignant clone.
We thank the excellent technical assistance of S. Van Wier, R.J. Bailey, and K. Henderson.
R.F. is a Leukemia and Lymphoma Society Translational Research Awardee. R.F. is also supported by the Mayo Foundation and by the CI-5 Cancer Research Fund-Lilly Clinical Investigator Award of the Damon Runyon–Walter Winchell Foundation.
Supported in part by Public Health Service grant R01 CA83724-01 from the National Cancer Institute. The Mayo Foundation also supports this study. Supported in part by research grant CA62242 (P.R.G. and B.V.N.). Supported by the ECOG grant CA21115-25C from the National Cancer Institute (P.R.G. and N.E.K.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal