This study identified and characterized a novel δβ fusion gene in which the δ-globin gene promoter is linked to intact β-globin coding sequences in a Senegalese family. It results from a 7.4-kb deletion that removes the δ-globin coding sequences, the δβ intergenic region as well as the β-globin gene promoter and causes δ0β+ thalassemia with hemoglobin A expressed at the 11% to 15% range. The phenotype of this naturally occurring δβ hybrid gene not only clarifies, in an in vivo context, the respective strength of δ- and β-globin gene promoters, but also emphasizes the importance of β-globin intragenic sequences in the expression of β-globin chains.
Introduction
Molecular studies of various deletions within the human β-globin gene cluster have provided important insights into the regulatory mechanisms of globin gene expression and hemoglobin (Hb) switching.1-3 In δβ thalassemias (δβ thal), depending on the location and deletion size, expression of the adult δ- and β-globin genes is either abolished or reduced, resulting, respectively, in (δβ)0 or (δβ)+ thal phenotypes.1 Some of the deletions, around 7.4 kb, result in a δβ fusion gene that expresses an abnormal Hb, termed “Hb Lepore.” Hb Lepore consists of 3 variants characterized by 3 different δ-to-β sequence transitions at the fusion junction: Hb Lepore-Hollandia (δaa1-22 βaa50-146), Hb Lepore-Baltimore (δaa1-50 βaa86-146), and Hb Lepore-Boston-Washington (δaa1-87βaa116-146).
We report here identification and characterization of a new form of deletional δβ thal in a Senegalese family with an unusual phenotype of (δ0β+) thal. Like other Lepore-type deletions, this Senegalese deletion involves a DNA segment of around 7.4 kb and results in a novel fusion gene in which a δ-globin gene promoter drives the expression of an intact β-globin gene and thus provides a novel mechanism for β+ thalassemia. This naturally occurring mutation also offers a rare opportunity to evaluate, in an in vivo context, the strength of the δ-globin promoter in driving the expression of a linked β-globin gene.
Study design
Hematologic parameters, HbA2, HbS, and HbF levels were determined by standard procedures. The β-globin haplotype, the α-globin gene status, and common sequence polymorphisms within the β-globin gene (sequence framework) and mutations in the Aγ and Gγ promoters were analyzed as described previously.4-7 The deletion leading to the δβ fusion gene was identified by a polymerase chain reaction (PCR)-based procedure.8 The complete sequence of the hybrid gene was determined by amplifying a 1667-bp fragment (from the δ promoter region up to the β polyA addition signal) using a forward primer 5′GACACACATGACAGAACAGCCAAT3′ (GenBank coordinates: 54586-54610), homologous to the δ-promoter8 and a 3′β reverse primer 5′GCTCGCTTTCTTGCTGTCCA3′ (coordinates: 63611-63630) under the following conditions: denaturation, 95°C for 40 seconds; annealing, 56°C for 50 seconds; and extension, 72°C for 2 minutes, during 30 PCR cycles.
Results and discussion
Case history
The proband is a Senegalese child born and living in France, diagnosed at birth as having homozygous sickle cell anemia (SCA) based on his Hb phenotype (high HbS and HbF with no HbA) and DNA analysis. With no history of sickle cell-related symptoms nor transfusion on periodic clinical follow-up until the age of 5, his case was referred again to explore the molecular basis of his mild presentation. The DNA analysis by PCR using a forward primer in the β-promoter 5′GTACGGCTGTCATCACTTAGACCTCA3′ coordinates: 62024-62050 and a reverse primer 5′CACTGATGCAATCATTCGTC3′ coordinates: 62773-62754 within the β-globin gene, confirmed his SCA status (results not shown). His Hb analysis showed the presence of HbA (11.7%) (Figure1A-B). Given such conflicting DNA and Hb phenotype assessment (respectively, SCA and Sβ+thalassemia), family study was undertaken.
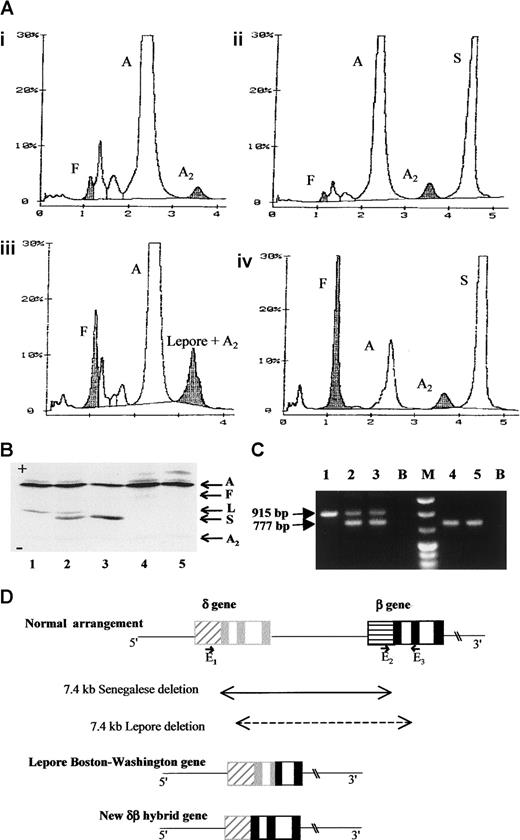
Hemoglobin and DNA analysis of the Senegalese δ0β+thalassamia and the rearranged gene structure of the novel fusion gene.
(A) High-performance liquid chromatography (variant system, Biorad Laboratories, Richmond, CA) profiles of the hemolysates from the proband's father (i), mother (ii), a control sample of Hb Lepore heterozygote (iii), and the proband (iv). In this system, Hb Lepore migrates at the position of HbA2. The level of the proband's HbA was assessed to be 11.7% of total hemoglobin. (B) Isoelectrofocusing profiles of the following hemolysates from a control sample of Hb Lepore heterozygote (1), in vitro mixed HbA/ Hb Lepore and HbA/HbS (2), mother (3), father (4), and a healthy individual (5). Hb Lepore is clearly distinguished from HbS and no Hb Lepore was detected in the father even after overloading. (C) Agarose electrophoretic profile of the PCR products. PCR products obtained by using primers8 E1, 5′GACACACATGACAGAA CAGCCAAT3′; GenBank coordinates: 54586-54610; E25′CGATCTTCAATATGCTTACCAAG3′; coordinates: 61848-61870; and E3 5′CATTCGTCTCTTTCCCATTCTA3′, coordinates: 62763-62742 are in lanes 1 to 3 and those with E1 and E3, in lanes 4 and 5. In this system, the E2-E3 pair generates a 915-bp fragment for a normal β-globin gene and the E1-E3 pair, a 777-bp fragment for Lepore-type chromosome, whereas E1-E3 are too far to produce a PCR fragment under our experimental conditions. The template DNA used is from the proband's mother (lane 1), the father (lanes 2 and 4), and an individual heterozygous for Hb Lepore (lanes 3 and 5). (D) Schematic representation of the normal human δ- and β-globin gene arrangement, the Lepore Boston-Washington gene, and the herein described δβ fusion gene. The boxes, ▨ and ░, respectively represent the δ-globin gene promoter and exons and, ▤ and ▪, respectively represent the β-globin gene promoter and exons. The position and orientation of the E1, E2, and E3 primers are also indicated. A indicates HbA; F, HbF; A2, HbA2; S, HbS; L, Hb Lepore; B, PCR blanks; M, molecular size marker.
Hemoglobin and DNA analysis of the Senegalese δ0β+thalassamia and the rearranged gene structure of the novel fusion gene.
(A) High-performance liquid chromatography (variant system, Biorad Laboratories, Richmond, CA) profiles of the hemolysates from the proband's father (i), mother (ii), a control sample of Hb Lepore heterozygote (iii), and the proband (iv). In this system, Hb Lepore migrates at the position of HbA2. The level of the proband's HbA was assessed to be 11.7% of total hemoglobin. (B) Isoelectrofocusing profiles of the following hemolysates from a control sample of Hb Lepore heterozygote (1), in vitro mixed HbA/ Hb Lepore and HbA/HbS (2), mother (3), father (4), and a healthy individual (5). Hb Lepore is clearly distinguished from HbS and no Hb Lepore was detected in the father even after overloading. (C) Agarose electrophoretic profile of the PCR products. PCR products obtained by using primers8 E1, 5′GACACACATGACAGAA CAGCCAAT3′; GenBank coordinates: 54586-54610; E25′CGATCTTCAATATGCTTACCAAG3′; coordinates: 61848-61870; and E3 5′CATTCGTCTCTTTCCCATTCTA3′, coordinates: 62763-62742 are in lanes 1 to 3 and those with E1 and E3, in lanes 4 and 5. In this system, the E2-E3 pair generates a 915-bp fragment for a normal β-globin gene and the E1-E3 pair, a 777-bp fragment for Lepore-type chromosome, whereas E1-E3 are too far to produce a PCR fragment under our experimental conditions. The template DNA used is from the proband's mother (lane 1), the father (lanes 2 and 4), and an individual heterozygous for Hb Lepore (lanes 3 and 5). (D) Schematic representation of the normal human δ- and β-globin gene arrangement, the Lepore Boston-Washington gene, and the herein described δβ fusion gene. The boxes, ▨ and ░, respectively represent the δ-globin gene promoter and exons and, ▤ and ▪, respectively represent the β-globin gene promoter and exons. The position and orientation of the E1, E2, and E3 primers are also indicated. A indicates HbA; F, HbF; A2, HbA2; S, HbS; L, Hb Lepore; B, PCR blanks; M, molecular size marker.
Table 1 summarizes the phenotype and DNA data of the Senegalese family. Three other siblings, 2, 3, and 4, also had discrepant DNA and phenotype data and none had sickle cell-related symptoms. Such a discrepancy may arise from the preferential amplification of the βS allele in PCR, with concomitant failure to amplify the βA allele. To explore further, we designed various primers within the structural gene, which all allowed amplification of both βs and βA sequences. We thus postulated that the primer/template mismatches, causal for amplification failure, lie in the promoter region of the low expressing βA allele. Indeed, we were unable to amplify this β+ thal allele with its promoter region by PCR until we used primers designed for the detection of Lepore-type deletion. In this procedure, one primer is homologous to the δ-promoter region and the other to the β-globin gene sequence. A 777-bp fragment, indicative of a 7.4-kb deletion8 was obtained (Figure 1C). Sequencing of this fragment revealed that (1) the deletion removes all the coding sequences of the δ- globin gene, the entire intergenic δβ sequences, and the β-globin gene promoter regions (the initially used upstream primer falls within this deleted region), and that (2) the deletion junction occurs on a 37-bp region within the 5′ untranslated sequence, approximately 21 nucleotides downstream from the cap site of the δ-globin gene (Figure 1D). The exact fusion point cannot be defined because of the sequence identity between δ- and β-globin genes in this region. The 7.4-kb Senegalese deletion occurs in a region known to be a hot spot for recombination (accounting for 75% of the meiotic recombination events within the β-globin locus).9 This deletion results in a novel δβ fusion gene distinct from the classical Lepore gene. This is substantiated by the following findings: (1) no Hb Lepore was found in individuals who inherited this allele, and (2) the translated regions of the fusion gene has normal β-globin gene sequence including codon 22, which confers the characteristic charge difference between Hb Lepore and HbA.
Phenotype and genotype data of the Senegalese family
| . | Sex/age (y) . | Total Hb (g/dL) . | MCV (fL) . | HbA (%) . | HbA2 (%) . | HbS (%) . | HbF (%) . | −158 Gγ . | α gene . | δ°β+ thal status . |
|---|---|---|---|---|---|---|---|---|---|---|
| Father | M/50 | 12.8 | 77 | 94.6 | 2.7 | — | 2.7 | C/C | αα/−α3.7α | +/− |
| Mother | F/39 | 11.9 | 89.2 | 59.9 | — | 39 | 1.1 | C/T | αα/αα | −/− |
| Child 1 | M/19 | 13.2 | 84.7 | 96.7 | 3 | — | 0.3 | C/C | αα/−α3.7α | −/− |
| Child 2 | M/13 | 10.9 | 76 | 15.6 | — | 70 | 12.4 | C/T | αα/−α3.7α | +/− |
| Child 3 | F/12 | 12.2 | 85 | 14 | — | 66 | 20.2 | C/T | αα/−α3.7α | +/− |
| Child 4 | F/10 | 13.4 | 78 | 13.6 | — | 66 | 18.8 | C/T | αα/αα | +/− |
| Child 5* | M/5 | 11.8 | 80 | 11.7 | — | 58 | 30.4 | C/T | αα/αα | +/− |
| . | Sex/age (y) . | Total Hb (g/dL) . | MCV (fL) . | HbA (%) . | HbA2 (%) . | HbS (%) . | HbF (%) . | −158 Gγ . | α gene . | δ°β+ thal status . |
|---|---|---|---|---|---|---|---|---|---|---|
| Father | M/50 | 12.8 | 77 | 94.6 | 2.7 | — | 2.7 | C/C | αα/−α3.7α | +/− |
| Mother | F/39 | 11.9 | 89.2 | 59.9 | — | 39 | 1.1 | C/T | αα/αα | −/− |
| Child 1 | M/19 | 13.2 | 84.7 | 96.7 | 3 | — | 0.3 | C/C | αα/−α3.7α | −/− |
| Child 2 | M/13 | 10.9 | 76 | 15.6 | — | 70 | 12.4 | C/T | αα/−α3.7α | +/− |
| Child 3 | F/12 | 12.2 | 85 | 14 | — | 66 | 20.2 | C/T | αα/−α3.7α | +/− |
| Child 4 | F/10 | 13.4 | 78 | 13.6 | — | 66 | 18.8 | C/T | αα/αα | +/− |
| Child 5* | M/5 | 11.8 | 80 | 11.7 | — | 58 | 30.4 | C/T | αα/αα | +/− |
MCV indicates mean corpuscular volume; Hb, hemoglobin; M, male; F, female.
Proband.
It is very likely that the novel fusion gene herein described results from a mechanism quite similar to the event (crossing-over between misaligned chromosomal pairs) that generates Hb Lepore. However the striking difference is that the novel fusion gene produces HbA, not Hb Lepore10 because the δ-to-β sequence transition occurs in the 5′ untranslated region. The chromosome carrying the “anti δβ fusion” counterpart should have duplicated δ-globin genes and may correspond to a reported case with unusually high HbA2 level (15%).11
In the heterozygous state, the Senegalese deletion results in a silent carrier state as observed in the father: normal range of HbA (94.6%) and HbA2 (2.7%). But a modest increase in HbF (2.7%) is similar to what is observed in Hb Lepore heterozygotes. Further increment in γ-chain output in the 4 (δ0β+) thal/βS siblings (HbF 12.4%-30.4%) may be related to the presence, in trans, of a sickle cell gene of “Senegal haplotype.”12
The new δβ hybrid gene is expressed at low level, very likely due to the weak δ-globin promoter. Indeed, the δ-promoter lacks (as compared to β- promoter) both CACCC and CAATT conserved sequences,13 which are required for appropriate expression of the β-globin gene.14 Interestingly, the hybrid gene with δ-promoter is able to produce β-globin chain 5-fold higher than δ-globin chain and matches the expression level of Hb Lepore. This suggests that sequences within the β-globin gene contribute to the overall expression of β-globin.15 16 Altogether this study brings direct in vivo evidence that the marked reduction in the expression of fusion genes including the classical Lepore genes is essentially due to the 5′ flanking sequences of the δ-globin gene and not to the δ-globin coding sequences that are present within the Lepore gene.
Benign presentation of the disease could be attributed to the presence of significant levels of HbA and HbF. This study also emphasizes the importance of confronting genotype and phenotype data in assessing an unusually mild course of SCA.
S.Z.-Z. is the recipient of a fellowship from the Association Française de Recherche Génétique (AFRG).
Supported by a grant No. TS3*-CT93-0244DG12HSMU from the European union.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rajagopal Krishnamoorthy, INSERM U 458, Hôpital Robert Debre, 48 Boulevard Sérurier, 75019 Paris, France; e-mail: krishna@infobiogen.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal