Patients with the immunodeficiency disorder Wiskott-Aldrich syndrome (WAS) have lymphocytes with aberrant microvilli, and their T cells, macrophages, and dendritic cells are impaired in cytoskeletal-dependent processes. WAS is caused by a defective or a missing WAS protein (WASP). Signal mediators interleukin-4 (IL-4) and CD40 are important for actin-dependent morphology changes in B cells. A possible function of WASP and its interacting partners, Cdc42 and Rac1, was investigated for these changes. It was found that active Cdc42 and Rac1 induced filopodia and lamellipodia, respectively, in activated B cells. Evidence is given that IL-4 has a specific role in the regulated cycling of Cdc42 because IL-4 partially and transiently depleted active Cdc42 from detergent extract of activated B cells. WASP-deficient B lymphocytes were impaired in IL-4– and CD40-dependent induction of polarized and spread cells. Microvilli were expressed on WASP-deficient B cells, but they appeared shorter and less dense in cell contacts than in wild-type cells. In conclusion, evidence is provided for the involvement of Cdc42, Rac1, and WASP in the cytoskeletal regulation of B lymphocytes. Aberrations in WASP-deficient B lymphocytes, described here, provide further evidence that WAS is a cytoskeletal disease of hematopoietic cells.
Introduction
Activation of lymphocytes is often accompanied by changes in cell morphology. Transendothelial migration requires microvilli-dependent adhesion and spreading of lymphocytes.1 The fine regulation of immune activation in lymph nodes and spleen is dependent on migratory and adhesive capacities of B and T cells.2,3 For migration and eventual contact with other immune cells to occur, adhesion receptors are essential and appear at the trailing uropod of a migrating cell. In contrast, chemoattractant receptors are expressed at the leading edge.4 Reorganization of microtubuli and dynamics of the actomyosin filaments are crucial events during migration and adhesion.5
T-cell–derived signals through interleukin-4 (IL-4) receptors or CD40 on B cells induce cytoskeletal rearrangements, altering B-cell morphology. Thymus-independent stimulus lipopolysaccharide (LPS) from gram-negative bacteria, and even more pronounced IL-4 and agonistic anti-CD40 antibodies (anti-CD40 monoclonal antibody [mAb]), mimicking the CD40 ligand, stimulate B lymphocytes to acquire a motile shape.6-9 It has been shown that anti-CD40 or LPS plus IL-4, but not LPS alone, induces the formation of dendritic protrusions when B cells are cultured on immobilized antibodies directed to B-cell surface determinants.10,11 LPS plus IL-412 or anti-CD4013 induces homotypic B-cell aggregates that are tight and spherical, whereas LPS-induced aggregates are loose and irregularly shaped.14 The induction of spread cells and tight aggregates are correlated with an increase in number and length of villous structures on the B-cell surface.9
Wiskott-Aldrich syndrome (WAS) is a severe X-linked immunodeficiency disorder caused by mutations in the gene encoding the WAS protein (WASP). Lymphocytes from patients with WAS have aberrant formation of microvilli.15-17 In addition, T cells16,17and B cells18 have been reported to have abnormal cytoarchitecture. WASP-deficient dendritic cells19 and macrophages20,21 have aberrant polarization and are impaired in stimulated migration. So far, WASP expression has only been detected in cells of the hematopoietic lineage,22 whereas its homologue N-WASP is more ubiquitously expressed.23 WASP24 and N-WASP25interact directly with the Arp2/3 complex, which is important for polymerization from barbed ends and branching of actin filaments.26 Similarly, WASP27,28 and N-WASP23 colocalize with polymerized actin. Recently, mice carrying a WASP null allele were described,29 30 and it was found that T-cell function was impaired, whereas mutant B cells proliferated normally to B-cell stimuli.
WASP was identified as a binding partner for the small GTPase Cdc42.27,31 Cdc42 and its relative Rac1 belong to the family of Rho GTPases, known to operate as molecular switches, cycling between an active guanosine triphosphate (GTP)-bound and an inactive guanosine diphosphate (GDP)-bound state. This cycling is tightly regulated by guanosine nucleotide exchange factors (GEFs), which stimulate the exchange of GDP to GTP, and GTPase-activating proteins (GAPs), which increase the intrinsic rate of GTP hydrolysis.32 Rho GTPases are involved in many cellular processes in which the cell cytoskeleton is the final target, such as migration,33,34 cytokinesis,35 and phagocytosis.36,37 Effector proteins of Cdc42 and Rac1 are numerous, and many of them, including WASP and N-WASP, have Cdc42/Rac-interactive binding (CRIB) motifs that specifically recognize the GTP-bound form.32
In this paper, we have investigated the intracellular pathways induced by LPS, IL-4, and CD40 activation in B lymphocytes. We show that active Cdc42 and Rac1 induced morphology changes in activated B cells. IL-4 partially and transiently depleted active Cdc42 from detergent extract of B cells. Furthermore, we found that WASP-deficient B cells were impaired in many morphologic processes. Interestingly, N-WASP was clearly expressed in WASP-deficient B cells. The data suggest that Cdc42, Rac1, and WASP are important for the induction of morphologic changes leading to B-cell migration and adhesion.
Material and methods
Reagents and antibodies
Recombinant murine IL-4 was either derived from supernatants of the plasmacytoma X63 Ag8-653 transfected with IL-4 cDNA38or purchased from Peprotech EC (London, United Kingdom). Endotoxin levels of the purified IL-4 were less than 0.1 ng/μg (1 EU/μg). Alone, neither X63 IL-4 nor purified IL-4 induced B-cell proliferation, as measured by 3H-thymidine incorporation, excluding an effect of contaminating endotoxins because LPS alone induced high proliferation. The amount of IL-4 inducing a half-maximal DNA synthesis response in concanavalin A–activated T cells was defined as 1 U. Unless otherwise indicated, 2 U/mL IL-4 (2% X63 supernatant or 2 ng/mL purified IL-4) was used. LPS from Escherichia coli O55:B5 was either prepared at the Department of Microbiology and Tumour Biology (Karolinska Institutet) or purchased from Sigma Aldrich (Stockholm, Sweden). Batches of fetal calf serum (FCS; Labora, Stockholm, Sweden) were selected for low endotoxin levels (less than 0.05 EU/mL). These FCS batches, when applied alone, did not induce B-cell proliferation, as measured by 3H-thymidine incorporation, or polarization (Figure 3). Rat anti–mouse CD40 (1C10)39 and CD44 (RK3G9)40 mAbs were prepared as previously described.11 Rabbit antibodies to the GTPase-binding domain (CRIB, amino acids 201-321) of human WASP were prepared as previously described.41 Rabbit anti–N-WASP antibodies were raised against a peptide (amino acids 173-189) of human N-WASP. Rabbit anti-myc and anti-Cdc42 antibodies were purchased from Santa Cruz Biotechnology (Scandinavian Diagnostic Service, Falkenberg, Sweden). Goat anti–rat immunoglobulin G (IgG; H&L) antibodies and biotinylated-F(ab′)2 donkey anti–rabbit IgG (H&L) were from Jackson ImmunoResearch Laboratories (West Grove, PA). Horseradish peroxidase–conjugated swine anti–rabbit IgG antibodies and fluorescein isothiocyanate (FITC)–coupled streptavidin were from Dako A/S (Glostrup, Denmark).
Animals
A pair of WASP-deficient animals, kindly provided by Dr Fredrick Alt (Boston, MA),29 were bred and maintained in the animal facility of the Department of Cell and Molecular Biology (Karolinska Institutet, Stockholm). Because WAS is an X-linked trait, males are hemizygous for the WASP allele (denoted as WASP−throughout the paper). WASP−/− females × WASP+ CBA male (purchased from Charles River, Uppsala, Sweden) breeding was established, followed by WASP+/−× WASP− (F1) breeding to provide litters with WASP− mutants and WASP+ sibling controls (F2). The genotype of the mice was controlled by polymerase chain reaction on tail-prepared DNA. CBA/J × C57BL/6 (F1) mice (referred to as CB throughout the paper) were purchased from Charles River. Males were used at 4 to 9 weeks.
Cell culture
Small resting B cells were prepared from splenic cell suspensions after the removal of T cells and Percoll gradient centrifugation as previously described.14 42 Cells were cultured at 0.5 × 106 cells/mL in RPMI 1640 supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate, 50 U/mL penicillin, 50 μg/mL streptomycin, 5 μM 2-mercaptoethanol (all from Gibco BRL, Life Technology, Paisley, United Kingdom) and 5% to 10% of batch-selected FCS at 37°C in a humidified atmosphere containing 5% CO2. Cell lines secreting monoclonal antibodies were cultured in the same way but with 1% to 5% batch-selected FCS.
Migration assay
To exclude contaminants of chemoattractants, both the X63-derived IL-4 and the purified IL-4 (Peprotech) were tested in a migration assay using a 2-chamber system (polycarbonate filter plates, 5-μm pore size; Transwell, Costar, New York, NY). B cells (100 000) were loaded to the upper wells, and no stimulus (5% FCS in RPMI), X63 IL-4, purified IL-4, or SDF-1 (50 ng/mL; R&D Systems, Oxon, United Kingdom) was added onto the lower wells. B cells were allowed to migrate for 2 hours, and then cells in the lower wells were collected and enumerated in triplicate. FCS induced low migration (2400 ± 670 cells, background). X63 IL-4 and purified IL-4 induced a slightly higher number of migrating cells (3300 ± 370 and 3600 ± 350 cells, respectively). Compared to background levels, these changes were nonsignificant (P > .05) using a t test. SDF-1, a potent B-cell chemoattractant, induced the migration of 8300 ± 800 cells (P < .001 compared to background). Figures indicate the mean of triplicate results ± 1 SD. Thus, the X63 supernatant and purified IL-4 seem to lack classical chemokines inducing migration with fast kinetics.
Transfections and stainings
cDNAs of myc-tagged Cdc42L61, Cdc42N17, wild-type Cdc42, RacL61, and RacN17 in the pRK5 vector (Pharmingen, Stockholm, Sweden) was used. CB B lymphocytes were stimulated with LPS for 46 to 50 hours. Fourteen micrograms DNA was electroporated (960 μF, 350 V, and 200 Ω) into activated B cells (10 × 106), then diluted in medium containing LPS or LPS plus IL-4. Cells were transferred to anti–CD44-coated coverslip and cultured for 3 to 4 hours. They were thereafter fixed in either acetone–methanol (1:1) for 20 minutes at −20°C or 4% phosphate-buffered paraformaldehyde for 10 minutes at room temperature, followed by 0.1% Triton X-100 for 1 minute. Cells were first blocked in 10% mouse serum in phosphate-buffered saline (PBS), stained with rabbit anti-myc antibodies, biotinylated antirabbit antibodies, and streptavidin-FITC. All solutions contained 10% mouse serum in PBS.
Antibody coating of plastic culture vessels or coverslips was performed as follows: goat anti–rat IgG was added and incubated for 1 hour at 37°C, followed by addition of rat anti–mouse CD44 (RK3G9) antibodies, incubated overnight at room temperature. Coverslips were washed in balanced salt solution before cells were added.
Glutathione-S-transferase pull-down assay
Residues 201-321 (CRIB) of human WASP were cloned into the pGEX-2T (Pharmacia Biotech, Uppsala, Sweden). Production and purification of recombinant GST-CRIB was described elsewhere.43 Precipitation of Cdc42-GTP was performed as previously described.43 Briefly, CB B lymphocytes (25 or 50 × 106 cells per sample) were stimulated with LPS for 48 hours, and IL-4 or FCS-containing RPMI was added in the last half hour of incubation. Cells were centrifuged at 4°C and lysed for 10 minutes on ice in RIPA buffer (100 μL/106 cells) containing 2 μg/mL aprotinin and leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 5 mM MgCl2. Cdc42-GTP was precipitated from lysates with glutathione–Sepharose beads (Pharmacia Biotech), preloaded with GST-CRIB, for 30 minutes at 4°C. The beads were washed, resuspended in reduced sample buffer (50 mM Tris, 50 mM dithiothreitol, 0.1% bromphenol blue, 10% glycerol, and 5% sodium dodecyl sulfate [SDS]), boiled, and loaded on a 15% polyacrylamide gel. Separated proteins were transferred to a nitrocellulose membrane by Western blotting.
Cell polarization assays
B lymphocytes, isolated from spleens of WASP− or WASP+ mice, were cultured in 24-well tissue culture plates in the presence of LPS, IL-4 (derived from the X63 plasmacytoma or purified from Peprotech), anti-CD40 mAb, or no stimulus for 20 to 24 hours. Cells were fixed by adding an equal volume of 2.5% phosphate-buffered glutaraldehyde and were washed twice in PBS before they were counted using an inverted light microscope. The percentage of polarized cells—cells that were not spherical but were tapered and had uropods—was determined.
Cell spreading assay
Cell spreading assays were performed in antibody-coated 96-well plates as previously described.11 Briefly, cells were stimulated with LPS, LPS plus IL-4 (derived from the X63 plasmacytoma or purified from Peprotech), or anti-CD40 mAb and were transferred to anti–CD44-coated plates for 17 to 19 additional hours of culture. Cells were thereafter fixed in 2.5% phosphate-buffered glutaraldehyde, and the percentage of spread cells—defined as having at least one dendritic process of at least one cell diameter—was determined using an inverted light microscope.
Aggregation assays
B lymphocytes, isolated from spleens of WASP− or WASP+ mice, were cultured for 46 to 50 hours. The morphology of B-cell aggregates, from cultures stimulated with LPS, LPS plus IL-4 (derived from the X63 plasmacytoma or purified from Peprotech), anti-CD40 mAb or anti-CD40 plus IL-4, was photographed directly using a camera connected to an inverted microscope. Percentage cell aggregation was measured by adding 100 μL trypan blue to 200 μL/well cultures. Cells were then resuspended by gentle pipetting up and down 6 times in a blunted disposable pipette and were transferred to a hemocytometer. Aggregated cells (2 or more cells in association) and single cells were enumerated under an inverted light microscope. The percentage of aggregated cells was then calculated.
Electron microscopy
B lymphocytes, isolated from WASP− and WASP+ mice, were stimulated with LPS or anti-CD40 mAb plus IL-4 for 48 hours. Aggregates were gently pelleted to preserve cell–cell adhesion and microvilli and then were fixed in glutaraldehyde. Specimen preparation was described previously.11 For examination, a Jeol 100CX electron microscope (Tokyo, Japan) was used at 80 kV.
Western blot analysis
B lymphocytes, isolated from WASP− and WASP+ mice, were stimulated with LPS or LPS plus IL-4 for 46 to 50 hours. Cells were harvested, lysed in buffer (50 mM Tris, 150 mM NaCl, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and 2 μg/mL aprotinin and leupeptin) at 4°C for 15 minutes. Equal protein concentrations of lysates were boiled in reduced sample buffer (described above) containing 2% SDS and were thereafter loaded on a 10% polyacrylamide gel. Separated proteins were transferred to a polyvinylidene difluoride membrane by Western blotting. Membranes were blocked in Tris-buffered saline, 0.05% Tween 20, and 5% fat-free dry milk (Blotto A) for 1 hour at 4°C and then incubated with primary antibodies in Blotto A overnight at 4°C. Horseradish peroxidase–conjugated secondary antibody in Blotto A was added and incubated for 1.5 hours at 4°C. Between steps, membranes were extensively washed in Tris-buffered saline with 0.05% Tween 20. Blots were developed with enhanced chemiluminescence (Pierce, Rockford, IL). Membranes were stained with Ponceau Red to confirm equal loading.
Results
Cdc42 and Rac1 induce filopodia and lamellipodia in activated B lymphocytes
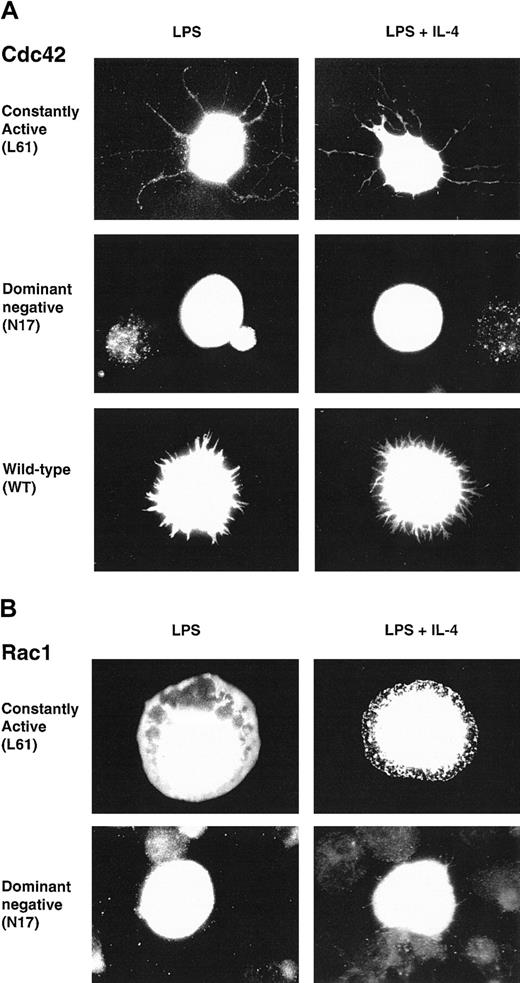
We wanted to investigate a potential role of Cdc42 and Rac1 in the cytoskeletal rearrangements of B lymphocytes. These GTPases induced morphologic changes in fibroblasts after microinjection.44,45 Notably, active Cdc42 induced filopodia, a structure that seems similar to the protrusions found on spread B cells.10,11 We therefore considered Cdc42 and Rac1 as having a potential role in IL-4–induced spreading. To elucidate the role of Cdc42 and Rac1 in B lymphocytes, we used the fact that the Rho GTPases can be converted to constitutively active (CA) and dominant negative (DN) forms using single amino acid substitutions. CA mutants are locked in the GTP-bound form, thereby preventing intrinsic and GAP-induced GTP hydrolysis.46,47 DN mutants are locked in the GDP-bound form, thus forming a nonfunctional complex with cellular GEFs and competing with endogenous proteins for binding to GEFs.48 CA, DN, or wild-type (WT) myc-tagged constructs of Cdc42 or Rac1 were transiently electroporated into LPS-activated B lymphocytes. Cells were then cultured in the presence of LPS or LPS plus IL-4 on anti-CD44 antibody layers (B cells, in contrast to fibroblasts, can only spread in the presence of antibody layers). Cells were fixed, permeabilized, and stained with anti-myc antibodies. CA Cdc42 induced long filopodia in cells stimulated with LPS or LPS plus IL-4, whereas DN Cdc42 failed to induce these structures (Figure 1A). WT Cdc42 induced short filopodia structures or lamellipodia (Figure 1A). CA Rac1 mainly induced lamellipodia in cells stimulated with LPS or LPS plus IL-4 and, rarely, short filopodia (Figure 1B). DN Rac1 did not induce these membrane structures (Figure 1B). Morphologic changes induced by CA and WT Cdc42 or Rac1 in LPS-stimulated cells were indistinguishable from those in LPS plus IL-4 stimulated cells. Thus, in this system, the LPS stimulus alone is sufficient to induce membrane alterations. Induction of morphologic changes was dependent on coating with anti-CD44 antibodies because, in the absence of antibody layers, no morphologic changes were detected and cells were spherical (not shown).
Cdc42 and Rac1 induce filopodia and lamellipodia in activated B lymphocytes.
Constitutively active (CA), dominant negative (DN), or wild-type (WT)myc-tagged constructs of Cdc42 (A) or Rac1 (B) were transiently electroporated into B lymphocytes, preactivated with LPS for 46 to 50 hours. Cells were diluted in medium containing LPS or LPS plus IL-4 and cultured on anti-CD44–coated glass coverslips for an additional 3 to 4 hours, fixed, permeabilized, and visualized with anti-myc antibodies. Cells were photographed using a camera connected to a fluorescence microscope with a × 100 objective. (A) CA Cdc42 induced filopodia, long microspikes of polymerized actin, whereas DN Cdc42 failed to induce this structure and transfected cells were spherical. WT Cdc42 induced either short filopodialike structures or lamellipodia (not shown). (B) CA Rac1 induced lamellipodia, flattened sheets of polymerized actin, whereas DN Rac1-expressing cells were spherical. Similar observations were made using LPS or LPS plus IL-4–activated B cells. These cells are representative of the morphologic phenotypes detected in more than 5 experiments, including screening of at least 300 cells in each experiment.
Cdc42 and Rac1 induce filopodia and lamellipodia in activated B lymphocytes.
Constitutively active (CA), dominant negative (DN), or wild-type (WT)myc-tagged constructs of Cdc42 (A) or Rac1 (B) were transiently electroporated into B lymphocytes, preactivated with LPS for 46 to 50 hours. Cells were diluted in medium containing LPS or LPS plus IL-4 and cultured on anti-CD44–coated glass coverslips for an additional 3 to 4 hours, fixed, permeabilized, and visualized with anti-myc antibodies. Cells were photographed using a camera connected to a fluorescence microscope with a × 100 objective. (A) CA Cdc42 induced filopodia, long microspikes of polymerized actin, whereas DN Cdc42 failed to induce this structure and transfected cells were spherical. WT Cdc42 induced either short filopodialike structures or lamellipodia (not shown). (B) CA Rac1 induced lamellipodia, flattened sheets of polymerized actin, whereas DN Rac1-expressing cells were spherical. Similar observations were made using LPS or LPS plus IL-4–activated B cells. These cells are representative of the morphologic phenotypes detected in more than 5 experiments, including screening of at least 300 cells in each experiment.
Short pulses of IL-4 partially and transiently deplete active Cdc42 from the B-cell cytosol
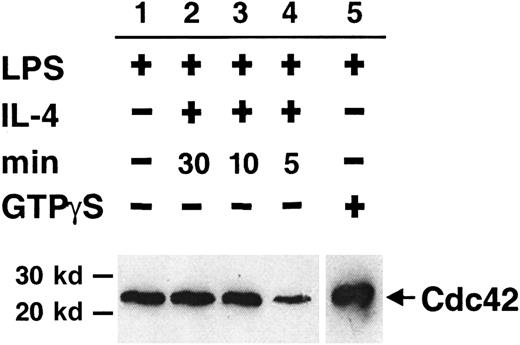
Many proteins, including specific GAPs and GEFs, regulate the cycling of Cdc42. IL-4 may have a potential role in this process, thereby inducing cytoskeletal changes in activated B cells. We have used a GST-CRIB (from WASP) fusion protein to precipitate the cytosolic fraction of active Cdc42 from detergent lysates of LPS-stimulated B lymphocytes given short pulses of IL-4. In each experiment, extract from cells stimulated with LPS alone was used as a standard for the detected level of Cdc42. A clear decrease in the level of cytosolic Cdc42-GTP was detected after IL-4 stimulation for 5 minutes (Figure2, lane 4) compared to the LPS standard (lane 1). No reduction was detected after IL-4 stimulation for 30 minutes, 10 minutes (lanes 2 and 3, respectively), or 1 minute (not shown). By adding FCS-containing medium alone, we controlled for the reduction being caused by IL-4 stimulation (not shown). Similar results were obtained in 4 experiments, suggesting that IL-4 stimulation of B cells leads to a transient depletion of active Cdc42 from the cytosol. In some other experiments, however, no difference between the groups was obtained. It is unlikely that these data were obtained by chance or the amount of active Cdc42 in the different experimental groups would have been totally variable, which was not the case. Instead, we believe this reflects some of the difficulties when working with rapidly cycling proteins.49
Short pulses of IL-4 partially and transiently deplete active Cdc42 from the B-cell cytosol.
Detergent extracts of LPS-activated B cells were stimulated with IL-4 for 5 to 30 minutes. Cdc42-GTP was removed from extracts using GST-CRIB (from WASP) coupled to glutathione–Sepharose beads. Bound proteins were identified with Western blotting using anti-Cdc42 antibodies. Experimental groups were as follows: no addition of IL-4 (lane 1), addition of IL-4 for 30 (lane 2), 10 (lane 3), or 5 (lane 4) minutes. LPS extracts preloaded with GTPγS are shown in lane 5. All lanes were on the same membrane, but the exposure time was shorter for lane 5 because the signal was strong. Data are representative of 4 experiments.
Short pulses of IL-4 partially and transiently deplete active Cdc42 from the B-cell cytosol.
Detergent extracts of LPS-activated B cells were stimulated with IL-4 for 5 to 30 minutes. Cdc42-GTP was removed from extracts using GST-CRIB (from WASP) coupled to glutathione–Sepharose beads. Bound proteins were identified with Western blotting using anti-Cdc42 antibodies. Experimental groups were as follows: no addition of IL-4 (lane 1), addition of IL-4 for 30 (lane 2), 10 (lane 3), or 5 (lane 4) minutes. LPS extracts preloaded with GTPγS are shown in lane 5. All lanes were on the same membrane, but the exposure time was shorter for lane 5 because the signal was strong. Data are representative of 4 experiments.
GTPγS, a nonhydrolyzable GTP analogue, arrests Cdc42 in its active state. B-cell lysates from LPS-stimulated cells, preloaded with GTPγS, showed a strong band of Cdc42-GTP after precipitation (Figure2, lane 5). This indicates that Cdc42-GDP is the predominant form in the cytoplasm under the experimental conditions used.
Induction of B-cell polarization is impaired in the absence of WASP
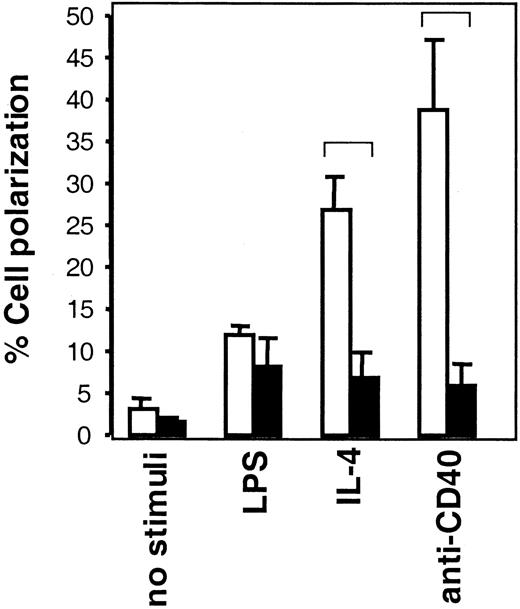
We have investigated whether the defects in polarization and migration of WAS dendritic cells and macrophages also apply to B cells from WASP-deficient mice. Lymphocytes undergoing locomotion characteristically have a polarized or prolonged cell body. Thus, assessment of cells with a motile cell shape can be used to determine locomotion capacity of lymphocytes.6,7 50 In WASP+ B cells (Figure 3, open bars), IL-4, anti-CD40 mAb, and LPS all induced a higher frequency of motile-shaped cells than noninduced cells, the 2 former stimuli giving higher responses than the latter. In WASP− B cells (Figure3, black bars), LPS stimuli induced a frequency of motile-shaped cells similar to that in WASP+ cells. However, IL-4 and anti-CD40 induction of motile-shaped WASP− B cells was impaired in comparison to WASP+ cells. This suggests that WASP has an important role for IL-4– and CD40-induced motility but that LPS-induced signaling can bypass the requirement for WASP or use completely different pathways.
Induction of B-cell polarization is impaired in the absence of WASP.
B cells from WASP− (▪) and WASP+ (■) mice were cultured with indicated stimuli for 24 hours, after which they were fixed. Polarized cells were defined as having a tapered cell body and uropods, whereas nonpolarized cells were spherical. Polarized cell frequency was determined. Results are presented as mean values of triplicate experiments, error bars represent 1 SD, and brackets indicate P < .005 using a 2-tailed t test. At least 300 cells were counted in each group, and the data are representative of at least 4 experiments.
Induction of B-cell polarization is impaired in the absence of WASP.
B cells from WASP− (▪) and WASP+ (■) mice were cultured with indicated stimuli for 24 hours, after which they were fixed. Polarized cells were defined as having a tapered cell body and uropods, whereas nonpolarized cells were spherical. Polarized cell frequency was determined. Results are presented as mean values of triplicate experiments, error bars represent 1 SD, and brackets indicate P < .005 using a 2-tailed t test. At least 300 cells were counted in each group, and the data are representative of at least 4 experiments.
Impaired spreading in the absence of WASP
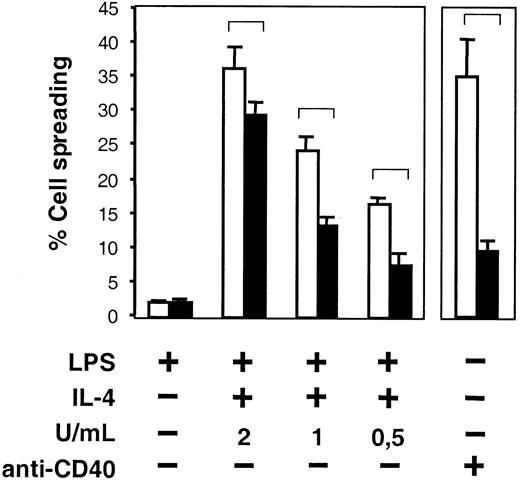
We also investigated the capacity of B-cell spreading in the absence of WASP. LPS plus IL-4 or anti-CD40 mAb induced a high number of spread WASP+ B cells (Figure4, open bars). However, WASP− B cells were impaired in spreading in response to the corresponding stimuli (Figure 4, black bars). Only occasional spread cells were seen in LPS cultures. Still, in the absence of WASP, spreading was not completely abolished because the percentage of WASP− spread cells was higher in the presence of suboptimal concentrations of IL-4 (Figure 4) or anti-CD40 (not shown) than with LPS alone. Spreading did not occur in the absence of immobilized antibodies or on surfaces coated with irrelevant antibodies (not shown).
Impaired spreading in the absence of WASP.
B cells from WASP− (▪) and WASP+ (■) mice were cultured with indicated stimuli for 46 to 50 hours and were thereafter transferred to anti-CD44–coated plates. Cells were incubated for 17 to 19 more hours and fixed. The frequency of spread cells, having at least one protrusion longer than one cell diameter, was determined. Mean values of 5 samples (LPS and LPS plus IL-4) or triplicate experiments (anti-CD40 mAb, 30 μg/mL) in each group are presented. Error bars represent 1 SD, and at least 300 cells were counted in each group. Brackets indicate P < .001 using a 2-tailed t test. This observation is representative of 3 similar experiments.
Impaired spreading in the absence of WASP.
B cells from WASP− (▪) and WASP+ (■) mice were cultured with indicated stimuli for 46 to 50 hours and were thereafter transferred to anti-CD44–coated plates. Cells were incubated for 17 to 19 more hours and fixed. The frequency of spread cells, having at least one protrusion longer than one cell diameter, was determined. Mean values of 5 samples (LPS and LPS plus IL-4) or triplicate experiments (anti-CD40 mAb, 30 μg/mL) in each group are presented. Error bars represent 1 SD, and at least 300 cells were counted in each group. Brackets indicate P < .001 using a 2-tailed t test. This observation is representative of 3 similar experiments.
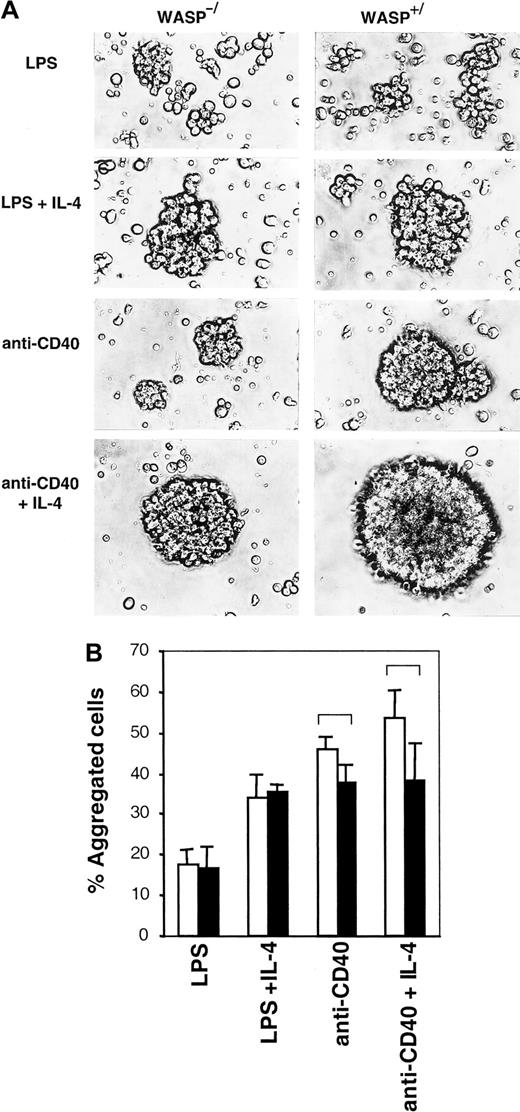
Aggregation is mildly impaired in the absence of WASP
IL-4 and CD40 are known to induce B-cell homotypic adhesion, resulting in the formation of tight, spherical aggregates.12-14 We investigated whether WASP was important for this process. LPS-stimulated WASP+ B cells formed loose, irregularly shaped aggregates, and these were indistinguishable from those of WASP− B cells (Figure5A). LPS plus IL-4 induced tight, spherical aggregates to a similar extent in WASP+ and WASP− B cells. However, anti-CD40 mAb- or anti-CD40 plus IL-4–induced WASP− aggregates were smaller, though still spherical, and less dense than WASP+ aggregates. Another method to estimate cellular aggregation is to gently resuspend aggregate cultures and then enumerate single and aggregated cells. Again, using this method, no differences in percentage of aggregated cells were detected in LPS or LPS plus IL-4 cultures (Figure 5B). In anti-CD40 or anti-CD40 plus IL-4 cultures, a mild defect was detected in WASP− B cells (black bars) compared to WASP+ B cells (open bars). Enumeration and morphologic characterization of aggregates suggest that WASP can be a component in the induction of homotypic B-cell adhesion, resulting in the formation of tight aggregates. A mild defect in aggregation can be a secondary cause of impaired polarization of WASP− B cells because motility most likely is required for the formation of big aggregates.
Aggregation is mildly impaired in the absence of WASP.
B cells from WASP− (▪) and WASP+ (■) mice were cultured for 46 to 50 hours with indicated stimuli. (A) Cells were photographed using a camera connected to an inverted microscope with a × 20 objective. Similar results were obtained in at least 5 experiments. (B) Aggregate cultures were gently resuspended, and single versus aggregated cells were enumerated. Mean values of triplicate experiments are shown. Error bars represent 1 SD, and at least 500 cells were counted in each group. Brackets indicateP < .07 using a 2-tailed t test. This observation is representative of 3 similar experiments.
Aggregation is mildly impaired in the absence of WASP.
B cells from WASP− (▪) and WASP+ (■) mice were cultured for 46 to 50 hours with indicated stimuli. (A) Cells were photographed using a camera connected to an inverted microscope with a × 20 objective. Similar results were obtained in at least 5 experiments. (B) Aggregate cultures were gently resuspended, and single versus aggregated cells were enumerated. Mean values of triplicate experiments are shown. Error bars represent 1 SD, and at least 500 cells were counted in each group. Brackets indicateP < .07 using a 2-tailed t test. This observation is representative of 3 similar experiments.
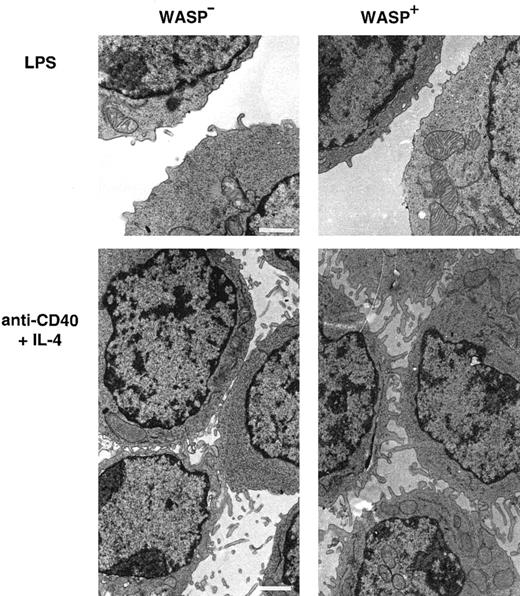
Activated WASP− B lymphocytes express short microvilli on their surfaces
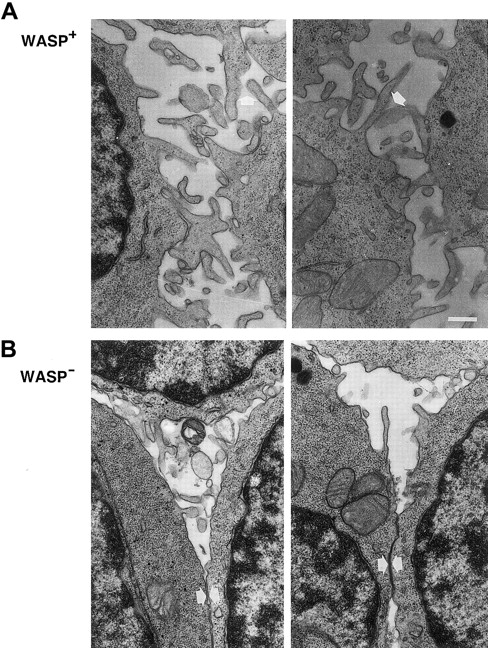
As observed in scanning electron microscopy, lymphocytes from patients with WAS have distorted microvilli on their surfaces.15-17 We used transmission electron microscopy to investigate the expression of microvilli in murine WASP− B lymphocytes. LPS-stimulated WASP− or WASP+ B cells expressed few and short microvillilike structures (Figure6). WASP− B cells stimulated with anti-CD40 mAb and IL-4 expressed more and longer microvilli on their surfaces, but they were shorter than on similarly activated WASP+ B cells. Higher magnification of cell contacts is shown in Figure 7. WASP+ B cells had extensive connections through microvilli in cell contacts (Figure 7A, white arrows). The microvilli connections in cell contacts of WASP− B cells was less dense or absent, leading to more frequent close contact through flat surfaces (Figure 7B, white arrows). In addition, WASP− B cells had smooth surfaces, whereas WASP+ B cells were more irregularly shaped. In summary, microvilli were expressed on activated WASP− B cells, but they appeared shorter and less dense in cell contacts than in wild-type cells.
Activated WASP− B lymphocytes express short microvilli.
B cells from WASP− and WASP+ mice were cultured for 48 hours with indicated stimuli, gently resuspended, then fixed and prepared for transmission electron microscopy. Note the extensive network of microvilli in cell contacts of anti-CD40 plus IL-4–stimulated cells, especially pronounced in WASP+cells. These observations are representative of 2 similar experiments. Size bar represents 1 μm. Original magnification 3800 × (LPS) and 3000 × (anti-CD40 plus IL-4).
Activated WASP− B lymphocytes express short microvilli.
B cells from WASP− and WASP+ mice were cultured for 48 hours with indicated stimuli, gently resuspended, then fixed and prepared for transmission electron microscopy. Note the extensive network of microvilli in cell contacts of anti-CD40 plus IL-4–stimulated cells, especially pronounced in WASP+cells. These observations are representative of 2 similar experiments. Size bar represents 1 μm. Original magnification 3800 × (LPS) and 3000 × (anti-CD40 plus IL-4).
Microvilli on WASP− B lymphocytes are shorter and less pronounced in cell contacts.
Higher magnification of cell contacts from the electron microscopy studies in Figure 6. IL-4– and anti-CD40–stimulated WASP− and WASP+ B cells are shown. (A) Note extensive microvilli contacts (white arrows) between WASP+B cells and the irregular cell shapes. (B) Microvilli network between WASP− B cells was less extensive or absent, and cells had smooth surfaces (white arrows). Size bar represents 0.4 μm. Original magnification 8000 ×.
Microvilli on WASP− B lymphocytes are shorter and less pronounced in cell contacts.
Higher magnification of cell contacts from the electron microscopy studies in Figure 6. IL-4– and anti-CD40–stimulated WASP− and WASP+ B cells are shown. (A) Note extensive microvilli contacts (white arrows) between WASP+B cells and the irregular cell shapes. (B) Microvilli network between WASP− B cells was less extensive or absent, and cells had smooth surfaces (white arrows). Size bar represents 0.4 μm. Original magnification 8000 ×.
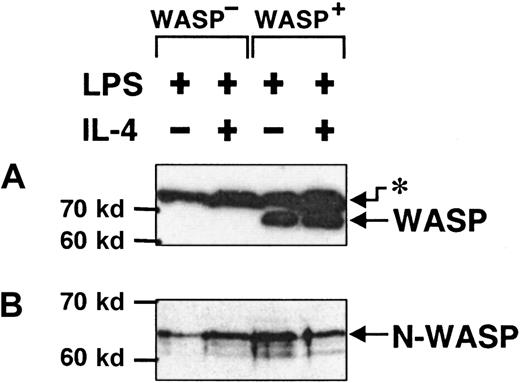
N-WASP is expressed in activated murine B lymphocytes
We have described partial morphologic defects in B cells lacking expression of WASP. The WASP-deficient mice used here, like some patients with WAS, seem to have a less severe phenotype.18 51 A likely possibility is that another protein can substitute for some of the function of WASP. We investigated the protein levels of N-WASP, a likely substitute candidate, in activated murine B cells. For this purpose, we used specific antibodies directed to either the CRIB domain of WASP or to a peptide of N-WASP. The anti-WASP antibody detected WASP in detergent lysates of WASP+ B cells, whereas WASP was completely absent in WASP− cells (Figure8A). On the other hand, N-WASP was clearly detected in both WASP+ and WASP− B cells (Figure 8B), in similar concentrations. Thus, N-WASP was expressed in activated B lymphocytes, but it does not appear that in the absence of WASP, the level of N-WASP was increased.
N-WASP is expressed in activated murine B lymphocytes.
Detergent lysates of B cells, stimulated with LPS or LPS plus IL-4 for 46 to 50 hours, were separated using SDS-PAGE, blotted, and exposed to antibodies against WASP or N-WASP. (A) Anti-WASP antibodies recognized WASP in WASP+ B cells and in an unknown, higher molecular species (asterisk) in both WASP+ and WASP− B cells. (B) Anti–N-WASP antibody recognized a band consisting of 2, perhaps 3, species that most likely represent different phosphorylation states of N-WASP. These data are representative of 3 similar experiments.
N-WASP is expressed in activated murine B lymphocytes.
Detergent lysates of B cells, stimulated with LPS or LPS plus IL-4 for 46 to 50 hours, were separated using SDS-PAGE, blotted, and exposed to antibodies against WASP or N-WASP. (A) Anti-WASP antibodies recognized WASP in WASP+ B cells and in an unknown, higher molecular species (asterisk) in both WASP+ and WASP− B cells. (B) Anti–N-WASP antibody recognized a band consisting of 2, perhaps 3, species that most likely represent different phosphorylation states of N-WASP. These data are representative of 3 similar experiments.
Discussion
Cdc42, Rac1, and WASP are all implicated as important for the reorganization of the actin cytoskeleton. WASP and N-WASP regulate actin polymerization through direct interaction with the Arp2/3 complex. We investigated morphologic changes in B lymphocytes induced by IL-4 or CD40 activation and found that Cdc42 and Rac1, when overexpressed in activated B cells, induced filopodia and lamellipodia, respectively. WASP-deficient B cells were impaired in polarization, spreading, homotypic aggregation, and microvilli formation in response to IL-4– and CD40-dependent stimuli. Partial morphologic deficiencies can be explained by functional redundancy of a WASP homologous protein. In agreement with this, we found that the WASP homologue, N-WASP, was expressed in B lymphocytes at a level similar to those in WASP knockout and wild-type cells.
In the early 1990s, Hall and coworkers44,45 performed crucial experiments to explain the function of the Rho GTPases. When active Cdc42 and Rac1 were micro-injected into the Swiss 3T3 fibroblast cell line, specific membrane structures, filopodia, and lamellipodia, respectively, were induced. When we transiently expressed these proteins in activated B cells, we could induce similar phenotypes, indicating that Cdc42 and Rac1 also induce these specific structures in B cells. This activation was dependent on the immobilization of B cells through antibodies to cell membrane molecules. It was clear from our results that LPS alone was sufficient to induce these events, because LPS- and LPS plus IL-4–stimulated cells were indistinguishable in both the morphologic phenotypes and the kinetics of induction (not shown). These results were surprising given that LPS failed to induce spreading in nontransfected B lymphocytes. One reason for this difference may be that the induction of filopodia and lamellipodia is a process separate from the induction of spreading. Another possibility is that these processes may, in part, contain common intracellular pathways but that spreading requires additional factors. However, yet another important difference is that in the transfection experiments, active Cdc42 or Rac1 were in much higher concentrations than their endogenous levels. This may influence downstream effectors and perhaps bypass the tight regulation of signaling by the GTPases. Recently, WASP was shown to have an auto-inhibitory contact between the GTPase binding domain (CRIB) and the C-terminal part, suggesting that WASP activation is also tightly regulated.52 The same mechanism most likely occurs in N-WASP.25 High levels of active Cdc42 or Rac1 can probably shift the GTPase-WASP equilibrium to the favor of GTPase binding to WASP, keeping the latter in an open, active state. Therefore, in this “simplified system,” LPS and LPS plus IL-4 can induce morphologic changes. Interestingly, when active constructs were expressed in WASP− B cells, the same phenotypes were induced as in WASP+ B cells (not shown). This suggests that Cdc42 and Rac1 can bypass the requirement for WASP, either through action through WASP-independent pathways or through functional redundancy perhaps by N-WASP. In a model of neutrophil activation, Cdc42 and Rac1 could bypass the requirement for WASP and instead activate actin nucleation through N-WASP.53
Our results show that IL-4 induces a transient decrease in the level of active cytosolic Cdc42. This observation can be explained by an altered endogenous localization of Cdc42. In the cytoplasm, RhoGDI (guanosine nucleotide dissociation inhibitor) seems to have a major role in sequestering the GDP-bound GTPases, thereby inhibiting the spontaneous exchange of GDP to GTP.32,54 By way of a posttranslationally added geranylgeranyl lipid modification, GTP-bound proteins are able to translocate to membranes.55 For example, phorbol myristate acetate (PMA)-triggered activation of NADPH in human neutrophils correlates with the translocation of Rac2-GTP to the plasma membrane.56 In this system, the translocation is rapid, occurring after a 5-minute stimulation. In a cell-free system, using isolated neutrophil membranes and purified proteins, Rac2 and Cdc42 were rapidly translocated to membranes after GTPγS activation.57 It is tempting to speculate that IL-4 may have a similar role for Cdc42 translocation in B cells. Recent experiments in neutrophils indicate that PMA or the chemoattractant fMet-Leu-Phe (fMLP) induces an increase in the level of active cytosolic Cdc42 and Rac2.58 In a keratinocytic cell line, IL-4 alters the level of active cytosolic Cdc42.59 One explanation for the discrepancy between different results may be found in the experimental procedures. Conditions for cell lysis are important for the solubility of membrane-bound proteins that take part in multimolecular complexes. Together, results of all the experiments mentioned indicate that distinct stimuli transiently and rapidly influence the cytosolic levels of active GTPases.
We also investigated a function for WASP in IL-4– and CD40-induced slow processes, such as the induction of motile and spread cells. WASP− B cells were impaired in IL-4– and anti-CD40-induced cell polarization and spreading. These observations suggest that WASP− B lymphocytes had intrinsic defects in processes that require a dynamic actin cytoskeleton, such as motility. In contrast, LPS-induced motility can bypass the requirement for WASP or use completely different pathways. Together with defects in stimulated migration and polarization of WAS dendritic cells19 and macrophages,20,21 evidence is accumulating for WASP as important for cell trafficking.60Slow morphologic changes in response to IL-4 and CD40 most likely require gene transcription. IL-4–induced morphology is at least partly mediated by the signal transducer and activator of transcription Stat-6 protein because Stat-6−/− B cells are deficient in the induction of motile and spread cells and also in microvilli formation.9 We are investigating a possible link between Stat-6–activated genes and WASP-dependent signaling pathways.
As judged by scanning electron microscopy, nonactivated human blood lymphocytes have fine villous projections. WAS blood lymphocytes have fewer microvilli, and those expressed are usually abnormally shaped in that they are short and blunted.15-17 In our experiments using transmission electron microscopy, microvilli were clearly induced on anti-CD40 mAb plus IL-4–activated murine WASP− B cells. However, the microvilli were shorter than those of activated wild-type B cells. We have previously observed that cell contacts often take place through microvilli and that tight aggregation correlates well with the expression of many long microvilli9 (data not shown). It is interesting that the activated WASP− B cells more often contacted each other by way of flat surfaces rather than the wild-type cells. Although a comparison is difficult, it is possible that activated murine WASP− B cells have more microvilli than do human lymphocytes from patients with WAS. However, some conditions differ between the present study and published studies. For example, in published studies of human cells, total lymphocytes or T cells were isolated from peripheral blood, whereas we investigated splenic B cells. In addition, in published studies human cells were nonactivated or activated with T-cell stimuli, whereas we studied activated B cells.
In some patients with WAS of less severe phenotype, truncated WASP transcripts and proteins have been detected.18,51 We could confirm that shorter WASP transcripts were completely absent in B cells from WASP-deficient mice (data not shown).29 Thus, partial morphologic deficiencies in murine WASP− B cells cannot be explained by truncated WASP. However, we identified similar levels of N-WASP in WASP+ and WASP− B cells. Because WASP and N-WASP share 50% homology at the protein level,23 it is tempting to speculate that N-WASP can partially substitute for WASP. Perhaps this is also true for patients with WAS who exhibit a less severe disease phenotype.
Increased surface area through filopodia, lamellipodia, and microvilli is a prerequisite for many cells that make extensive cell-to-cell or cell-to-matrix contacts. For example, in the immunologic synapse,5 a T cell forms filopodia and lamellipodia and clusters membrane molecules on these to sense and contact an antigen-presenting cell. During an immune response, B and T cells encounter the antigen in the spleen and lymph nodes. There, the collaboration between migratory and adhesive capacities is instrumental for B-cell activation to take place.2,3 We suggest that T-cell–derived signals by IL-4 and CD40 can induce morphologic alterations necessary for B-cell migration and interaction with T cells, follicular dendritic cells, and other B cells in B-cell foci and germinal centers (GCs). The importance of CD40–CD40 ligand interactions in GCs was revealed in CD40-deficient mice, which exhibit impaired GC formation.61 Earlier reports, in which electron microscopy was used to examine antigen-specific interaction between B and T cells, revealed extensive membrane contacts between cells.62 Later on, polarized expression of IL-4 in contact areas was shown to enhance conjugate formation.63,64Impaired migration and contact between immune cells from patients with WAS, because of reduced surface plasticity, can most likely explain some of the severity of the disease. In addition, the antigen presentation capacity of WAS B cells may be impaired because antigen presentation on B cells seems to be dependent on CD40–CD40 ligand interaction, whereas other cells can present antigen in a CD40-independent fashion.65 We are investigating how LPS, IL-4, and anti-CD40 activation influences the antigen-presenting capacity of B cells. It will be interesting to include WASP-deficient B cells and to examine whether WASP-dependent morphology changes are important for antigen presentation by B cells.
We have described impairment in the cytoskeletal regulation of WASP-deficient B cells. This is, to our knowledge, the first time a defect in murine B-cell function is shown. Facchetti et al18 showed that Epstein-Barr virus–transformed B-cell lines from patients with WAS are impaired in F-actin regulation. Together, these reports suggest that impaired B-lymphocyte responses may contribute to the immunodeficiency of WAS.
We thank Dr Johan Thyberg for excellent guidance with the electron microscopy and Karin Blomgren for assistance in specimen preparation. We thank Dr Fredrick Alt for providing the WASP knockout mice. We also thank Dr Edward J Davey for stimulating and helpful discussions.
Supported by grants from the Swedish Natural Science Research Council, the Swedish Cancer Foundation, the Network for Inflammation Research funded by the Swedish Foundation for Strategic Research, the Fifth Framework Programme of the European Commission, the Åke Wiberg Foundation, and the Karolinska Institutet.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lisa Westerberg, Department of Cell and Molecular Biology, Karolinska Institutet, von Eulers väg 3, 171 77 Sockholm, Sweden; e-mail: lisa.westerberg@cmb.ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal