Administration of 5-fluorouracil (5-FU) to mice results in a marked increase in the level of circulating platelets in 10 days. Mice lacking Mpl, the receptor for thrombopoietin (TPO), are thrombocytopenic. To gain insight into the mechanism by which 5-FU produces such a substantial stimulation of platelet production, this study investigated whether 5-FU (150 mg/kg) produced thrombocytosis in c-mpl−/− mice, thus establishing whether TPO was required for this response. A 5- to 6-fold increase in platelet levels in c-mpl−/− mice (to approximately 1000 × 109/L) was observed on days 20 and 25 after 5-FU injection. Thus, at the peak of the response, c-mpl−/− mice had platelet levels comparable to those in normal mice. Administration of 5-FU also produced thrombocytosis in previously splenectomized c-mpl−/− mice. Comparison of the platelet response to 5-FU in young (6-12 weeks) and old (33-46 weeks) c-mpl−/− mice found that older mice produced a much more marked response than younger mice, with a mean maximum platelet level of approximately 1700 × 109/L. To determine whether this increase in circulating platelets was preceded by an increase in hematopoietic progenitors, serial cultures of bone marrow and spleen were evaluated. A considerable increase in all colony types studied was observed on days 15 and 20 in spleens of c-mpl−/− mice, but no similar elevations were detected in bone marrow. These results indicate that c-mpl−/− mice can achieve a normal level of platelets after 5-FU injection, by means of a TPO-independent mechanism, and that they respond to 5-FU myelosuppression by producing large numbers of megakaryocytic, myeloid, and erythroid progenitors.
Introduction
The myelosuppressive agent fluorouracil (5-FU) produces a unique effect on megakaryocytopoiesis. Administration to mice of a single dose of 150 mg/kg results in gradual development of moderate thrombocytopenia, with the nadir in platelet count occurring approximately 4 to 8 days after administration.1-3Subsequently, the level of circulating platelets increases rapidly and thrombocytosis develops by day 10.1-3 Platelet levels as high as 3000 × 109/L to 3500 × 109/L are reached (normal platelet count in mice is 1000 × 109/L to 1200 × 109/L), and thrombocytosis persists from approximately day 10 to day 19.
Changes in platelet levels are associated with a marked increase in the number of megakaryocytes (MKs) in both bone marrow and spleen, with the increase occurring in the bone marrow after 7 or 8 days3,4but in the spleen only after 12 days.4 A marked increase in the frequency and total numbers of MK colony-forming cells (MK-CFCs) occurs in the spleen concurrent with the period of thrombocytosis.2 Despite the increase in spleen size and numbers of splenic MK-CFCs, it was shown that the spleen is not required for production of the marked thrombocytosis after administration of 5-FU.3 5
The mechanism by which 5-FU produces marked thrombocytosis is unknown. The minimal brief reduction in the concentration of recognizable MKs in the bone marrow3,4 makes it unlikely that a reduced MK mass provides this stimulus. However, it has been postulated that the killing of a population of MKs or a subset of their progenitors provides a powerful stimulus for proliferation of normally quiescent earlier progenitors, which eventually results in generation of a greatly enlarged population of MKs.6 Importantly, the persistence of marked thrombocytosis indicates that once this stimulus occurs, feedback mechanisms that normally control the number and maturation of MKs in bone marrow (and, in mice, the spleen) are overridden.
Thrombopoietin (TPO), the principal humoral regulator of megakaryocytopoiesis and levels of circulating platelets, controls not only the number and ploidy levels of recognizable MKs but also supports the full maturation of MKs into platelet-producing cells.7-9 TPO also stimulates in vitro development of MK colonies from marrow progenitors.10 Administration of TPO to mice elevates the number of MKs in bone marrow and spleen and significantly increases platelet levels 4 fold to 6 fold.11-13 In addition, TPO was shown to enhance proliferation of erythroid progenitors in normal animals both in vivo and in vitro14-16 and to accelerate recovery of erythroid and myeloid progenitors if administered after myelosuppressive therapy.13,16,17 TPO acts through a specific receptor, designated Mpl, which was first recognized as the transforming oncogene v-mpl of the myeloproliferative leukemia virus.18 The normal receptor was shown to be expressed not only in MKs and platelets but also on CD34+ cells.19 The importance of TPO in megakaryocytopoiesis and thrombopoiesis was confirmed by generation of mice deficient in the Mpl receptor20,21 or TPO.22 Mice lacking Mpl are deficient not only in committed proliferative precursors for the megakaryocytic lineage but also for the erythroid and myeloid lineages and in hematopoietic stem cells (HSCs).21-24 Interestingly, although Mpl-deficient mice have an approximately 85% reduction in peripheral platelet levels, they do not have spontaneous bleeding. Furthermore, their MKs and platelets appear normal on ultrastructural analysis, and platelets of Mpl-deficient mice were shown to be functionally normal.25 The thrombocytopenia in c-mpl−/−mice and their deficiency in hematopoietic progenitors are not exacerbated in mpl−/−–interleukin (IL) 6−/−, mpl−/−–leukemia inhibitory factor (LIF)−/−, or mpl−/−–IL-11Rα−/− double-mutant mice.26
To gain insight into the mechanism by which 5-FU produces a marked stimulation of platelet production after a relatively small decrease in platelet levels, we sought to determine whether 5-FU would produce thrombocytosis in mpl−/− mice, thereby establishing whether TPO is required for this response.
Materials and methods
General techniques
Heterozygous c-mpl+/− mouse pairs (C57/Bl6J background; provided by F. de Sauvage, Genentech, South San Francisco, CA) were interbred to generate homozygous c-mpl−/− mice, which were used subsequently to generate a colony of mpl−/− mice. Mouse genotypes were determined routinely by platelet counts in blood samples and polymerase chain reaction analyses of genomic DNA extracted from tail biopsy specimens. C-mpl−/− mice (25 to 35 g) were 6 to 8 weeks, 16 weeks, or 33 to 46 weeks of age at initiation of different experiments. Normal male C57/Bl6J mice (25 to 30 g; Harlan France, Gannat, France) were used as normal controls.
Blood samples were obtained from the retro-orbital venous plexus by using Pasteur pipettes coated with Sigmacot (Sigma, Saint Quentin Fallavier, France) and wet welted with EDTA, on selected days after injection of 5-FU. Blood samples were then placed in plastic vials containing EDTA (Sarsted, Orsay, France). Mice were killed by cervical dislocation.
Reagents
The 5-FU (50 mg/mL; Roche Laboratories, Nutley, NJ) was diluted with sterile bacteriostatic 0.9% saline to a final concentration of 15 mg/mL. Mice received a single intraperitoneal injection of this concentration of 5-FU in a dose of 150 mg/kg in a volume of approximately 0.3 mL on day 1. Rabbit anti–mouse platelet antiserum (PAS) was administered intraperitoneally to produce acute, severe thrombocytopenia as described previously.27 28
Blood cell counts and splenectomy
Platelet counts, white blood cell counts, and hematocrit values were determined in whole blood anticoagulated with EDTA and analyzed with an automated flow cytometric whole blood cell counter (Cell-Dyn 3500; Abbott Laboratories, Rungis, France). Six-week-old c-mpl−/− and control C57/Bl6 mice underwent splenectomy under general anesthesia (Hypnomidate; Roche-Boehringer, Meylan, France) and were allowed to recover for 5 weeks before experiments began.
Cell cultures
Hematopoietic progenitor cell assays.
On each culture day, femurs and spleens were removed from c-mpl−/− and normal C57/Bl6 mice treated with 5-FU. Clonogenic culturing was done as described previously29with the following slight modifications: 5 × 105 and 5 × 104 cells from spleen and bone marrow single-cell suspensions, respectively, were plated in 1 mL 1% methylcellulose in Iscoves modified Dulbecco medium (Gibco-Life Technologies, Cergy-Pontoise, France) supplemented with 30% fetal-calf serum (FCS; Biological Industries, Kibbutz Beit Haemek, Israel), 1% crystallized bovine serum albumin (BSA; Sigma), and 100 μM beta-mercaptoethanol in the presence of 10 ng/mL mouse recombinant IL-3 (PeproTech, Rocky Hill, NJ), 2 U/mL erythropoietin (EPO; kindly provided by Roche-Boehringer), and 200 U/mL TPO (PeproTech) for the clonogenic progenitor assays, and 2 U/mL EPO for the erythroid colony-forming unit (CFU-E) assays. Samples were cultured in duplicate for each experimental point. Cultures were then incubated in modular incubator chambers (Billups-Rothenberg, Del Mar, CA) at 37°C in 100% humidity in a gas mixture composed of 5% carbon dioxide (CO2) and 6% oxygen (Air Liquide France, Mitry-Mory, France). Colonies were scored on day 2 for CFU-Es and on day 7 for granulocyte-macrophage colony-forming units (CFU-GMs), mature erythroid burst-forming units (BFU-Es), erythroid-megakaryocyte colony-forming units (CFU-EMKs), and mixed colony-forming units (CFUs-MIX). The total numbers of colonies per organ (femur or spleen) were calculated from their respective frequencies in cultures and the total nucleated cell count per femur or spleen.
MK colony-forming unit (CFU-MK) assays.
Soft agar cultures of spleen and bone marrow cells from c-mpl−/− and normal C57/Bl6 mice that had received 5-FU were prepared for quantification of CFU-MKs as described previously,27,30 except for the following modifications: 20% horse serum was used instead of FCS, and 0.1 mL (instead of 0.2 mL) pokeweed mitogen, spleen cell–conditioned medium was used as the source of growth factors in each 1 mL culture. Bone marrow cultures contained 5 × 104 to 4 × 105 cells/mL, and spleen cultures contained 2.5 × 105 to 2 × 106 cells /mL. Samples were cultured in triplicate for each of the 3 concentrations of cells cultured at each experimental point. After 7 days of incubation at 37°C in 100% humidity and 5% CO2 in air, cultures were scored for CFU-MKs. Triplicate dishes were dried with filter paper (Whatman; Polylabo, Strasbourg, France), fixed with 1% paraformaldehyde for 10 minutes at room temperature, and stained for acetylcholinesterase to histochemically identify CFU-MKs, defined as a group of at least 3 acetylcholinesterase-positive cells.31
MK DNA distribution
Marrow and spleen MK DNA distribution was determined as follows. Bone marrow (from 2 femurs) and spleen suspensions were prepared in phosphate-buffered saline (PBS)–citrate–BSA buffer (0.38% sodium citrate in PBS and 0.5% BSA) in plastic tubes. Cells (1-5 × 106) were labeled for 30 minutes at 4°C with 1.25 μg fluorescein isothiocyanate–conjugated (FITC) monoclonal antibody CD41 (rat anti–mouse glycoprotein IIb; Pharmingen, San Diego, CA) and washed twice gently with PBS-citrate. The pellet was resuspended in 200 μL PBS, and 4 mL of a cold solution of 70% ethanol in PBS was added. After 1 hour of incubation at 4°C, the suspension was centrifuged and cells were resuspended in 100 μL PBS. Propidium iodide (2 mL [50 μg/mL]) and RNAase (100 μg/mL) in PBS were added. After 30 minutes at 37°C, 105 cells were analyzed by 2-color flow cytometry on a flow cytometer (FACScalibur; Becton Dickinson, San Jose, CA).
Detection of Mpl in platelets
For platelet preparations, blood was collected into polypropylene tubes containing 10% final volume of ACD buffer (38 mM citric acid, 75 mM trisodium citrate, and 100 mM dextrose). Platelets were prepared according to the protocol described by Berger et al.32 Whole cell extracts prepared by lysis of 2 × 106 platelets in Laemli buffer (1 ×) were loaded on sodium dodecyl sulfate–polyacrylamide gels. After electrophoresis, proteins were transferred to nitrocellulose membranes (Protran BA 85; Schleicher and Schuell, Cera Labo, Ecquevilly, France), blocked with Tris-buffered saline (20 mM Tris [pH 7.4] and 150 mM sodium chloride) containing 0.1% Tween 20 and 5% skim milk, and probed with a polyclonal rabbit anti– mouse Mpl antibody. Proteins were visualized by using enhanced chemiluminescence (Amersham, Les Ulis, France) after incubation with a secondary horseradish peroxidase–conjugated antibody.
Platelet preparation and aggregation
Platelets were prepared as described by Bugaud et al.33 Platelet concentration was adjusted to 108/μL. Platelets (250 μL) were then preincubated at 37°C, without stirring. Platelet aggregation was initiated by adding 0.1 U/mL bovine thrombin and 1 mM calcium chloride, with constant stirring (1200 rpm) in an aggregometer cuvette (Chronolog dual-beam aggregometer; Beckman Coulter France, Villepinte, France).
Statistical analysis
Statistical analysis was done by using 2-tailed Studentt tests and Microsoft Excel (Redmond, WA) software.
Results
Effects of 5-FU administration in c-mpl−/−mice
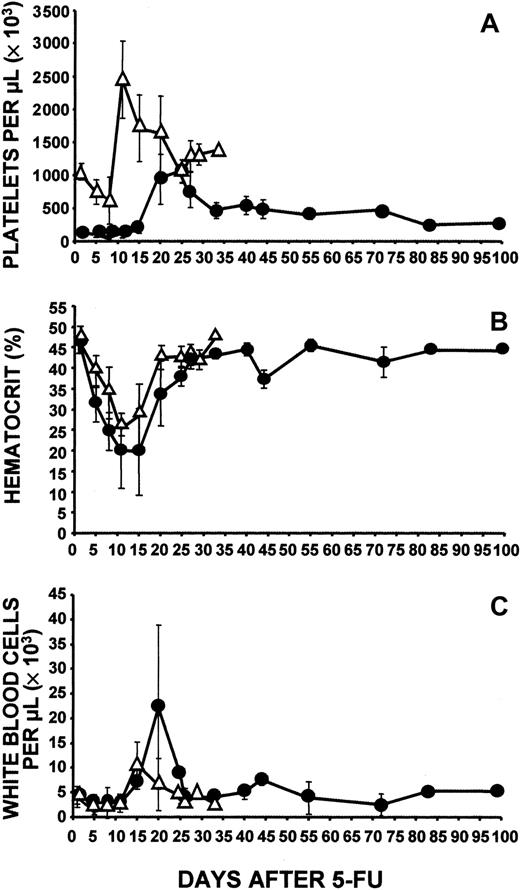
Administration of a single dose of 5-FU (150 mg/kg) produced the expected marked thrombocytosis in normal C57/Bl6 mice. Surprisingly, a marked increase in platelet levels was also produced in c-mpl−/− mice, but the maximum elevation of platelet levels in those mice did not occur until 10 to 15 days later than the peak in the normal mice (Figure 1A). Although the peak level in C57/Bl6 mice was much higher than that in c-mpl−/− mice, it represented an increase of only 2 to 3 fold over platelet counts in normal controls, whereas there was an increase of approximately 5 to 6 fold over the baseline level in the thrombocytopenic c-mpl−/− mice. Even more noteworthy was the observation that at the peak of the response, c-mpl−/− mice were able to reach normal platelet levels. No Mpl-positive platelets were detected by Western blot analysis, even at the peak of the response (data not shown).
Effects of 5-FU.
Shown are effects of intraperitoneal administration of 150 mg/kg 5-FU (on day 1) on levels of circulating platelets (A), hematocrit (B), and white blood cell counts (C) in wild-type C57/Bl6 mice (▵) and c-mpl−/− mice (●). Baseline blood cell counts were obtained before injection of 5-FU. C-mpl−/− mice had delayed development of thrombocytosis, which did not reach the level observed in wild-type mice. Data were derived from 5 independent experiments and based on a total of 83 c-mpl−/− mice and 31 C57/Bl6 mice. One day before administration of 5-FU and 7 days afterward, blood samples were obtained from all mice, thereby allowing determination of baseline platelet counts and confirmation of the response to 5-FU. Thereafter, blood samples were obtained from groups of 2 to 10 mice. Normal mean ± SE values (n = 17) were platelets, 1053 × 109/L ± 176 × 109/L; hematocrit, 0.47 ± 0.2; and white blood cells, 4.3 ± 2.0 × 109/L.
Effects of 5-FU.
Shown are effects of intraperitoneal administration of 150 mg/kg 5-FU (on day 1) on levels of circulating platelets (A), hematocrit (B), and white blood cell counts (C) in wild-type C57/Bl6 mice (▵) and c-mpl−/− mice (●). Baseline blood cell counts were obtained before injection of 5-FU. C-mpl−/− mice had delayed development of thrombocytosis, which did not reach the level observed in wild-type mice. Data were derived from 5 independent experiments and based on a total of 83 c-mpl−/− mice and 31 C57/Bl6 mice. One day before administration of 5-FU and 7 days afterward, blood samples were obtained from all mice, thereby allowing determination of baseline platelet counts and confirmation of the response to 5-FU. Thereafter, blood samples were obtained from groups of 2 to 10 mice. Normal mean ± SE values (n = 17) were platelets, 1053 × 109/L ± 176 × 109/L; hematocrit, 0.47 ± 0.2; and white blood cells, 4.3 ± 2.0 × 109/L.
Platelet function in c-mpl−/− mice was previously found to be normal.25 To determine whether platelets obtained from c-mpl−/− mice when their platelet counts were elevated (day 19 after 5-FU administration) were also functionally normal, we measured thrombin-induced aggregation by incubating platelet preparations with 0.1 U/mL thrombin for 2 minutes at 37°C. We found that 46% ± 2.1% of the platelets from the c-mpl−/−mice aggregated. This was similar to the level of aggregation obtained with platelets from control untreated C57/Bl6 mice (45.5% ± 21.9%) and from C57/Bl6 mice 14 days after 5-FU administration (49.2% ± 18.8%; maximum platelet levels), a finding indicating that not only could c-mpl−/− mice achieve platelet levels comparable to those in normal mice after 5-FU injection but also that the platelets produced were functional. Furthermore, in these mice, the increase in circulating platelets above baseline values persisted for a longer period than in control mice and did not return to normal for more than 2 months after administration of 5-FU. Although the c-mpl−/− mice were not initially anemic, they had a greater decrease in hematocrit level than the normal mice and recovery occurred more slowly (Figure 1B). On day 20 after administration of 5-FU, some c-mpl−/− mice had a marked leukocytosis, a condition not observed in normal mice (Figure 1C). The preceding period of leukopenia was not more severe in c-mpl−/− mice than in normal controls.
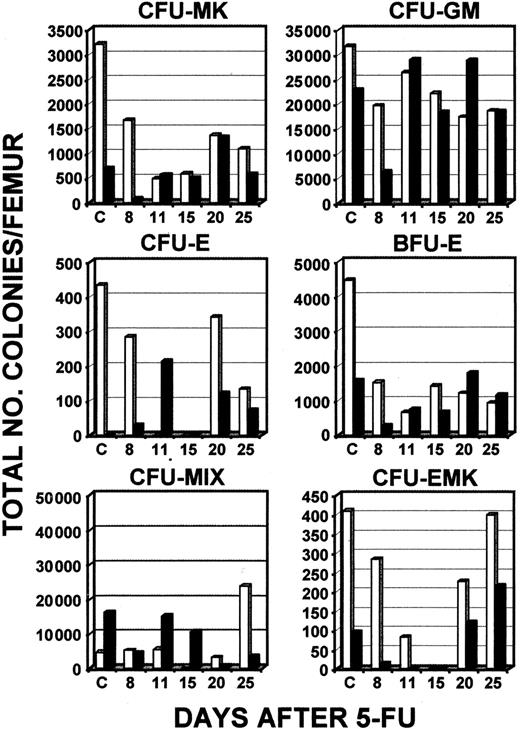
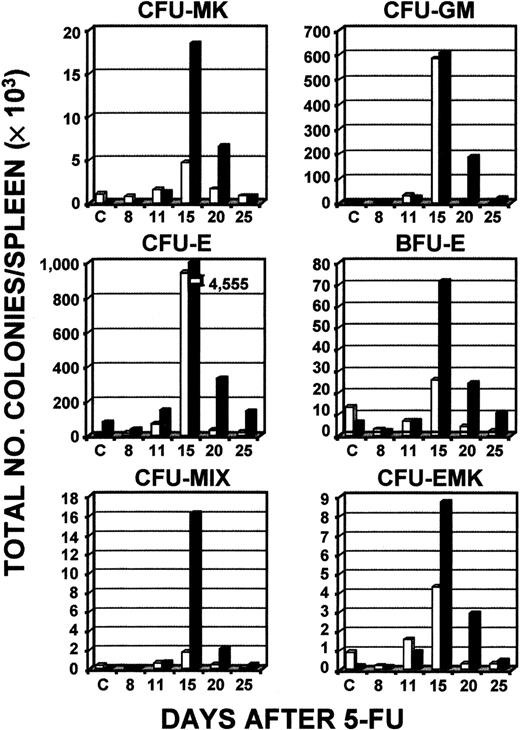
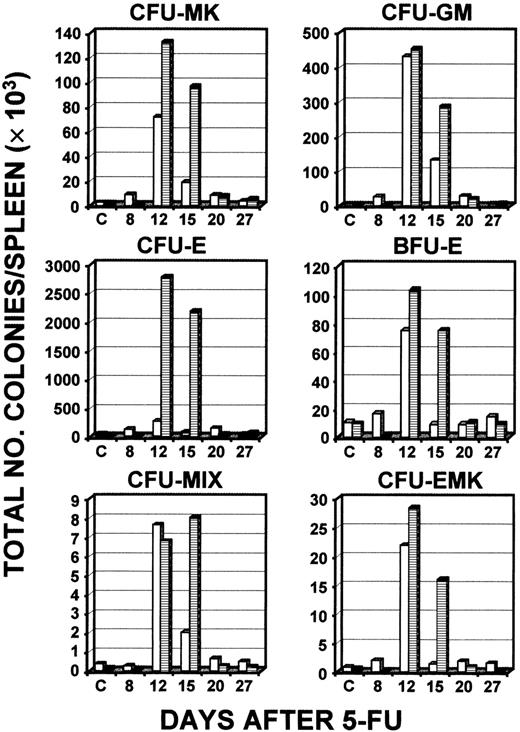
To determine whether the increase in circulating platelets and white blood cells was preceded by an increase in hematopoietic progenitors, serial culturing of bone marrow and spleen samples was done (Figure2). Analysis of the response of CFUs in bone marrow revealed that during the 25-day period after administration of 5-FU, levels of a wide range of CFUs did not increase significantly to above baseline levels in normal controls or c-mpl−/−mice, except for CFU-E (day 11) and CFU-EMK (day 25) in the c-mpl−/− mice and CFUs-MIX (day 25) in the normal controls (Figure 2). Overall, there was no indication of a consistent relationship between levels of platelet response in wild-type and c-mpl−/− mice and an increase in bone marrow CFUs. In striking contrast, on days 15 and 20 there was a marked increase in all colony types studied in spleens of c-mpl−/− mice, with a considerable elevation above normal levels observed uniformly on day 15 (Figure 3). Peak levels of splenic colonies in c-mpl−/− mice always occurred at the same time as in normal mice and were consistently greater than levels in normal controls. The baseline numbers of CFU-MKs per spleen for control and c-mpl−/− mice were 1091 and 75, respectively, and the mean peak level was 4651 for controls (4-fold increase), and 18 386 for c-mpl−/− mice (245- fold increase).
Total numbers of types of CFCs in bone marrow after administration of 5-FU.
Serial determinations of different types of hematopoietic colonies compared levels in wild-type C57/Bl6 mice (■) and c-mpl−/− mice (▪). Mice were 6- to 8-weeks old at initiation of these experiments. The results of a single experiment representative of 3 other independent experiments are shown, except for the finding for CFU-MKs, which is the mean result of 2 experiments representative of 2 other independent experiments.
Total numbers of types of CFCs in bone marrow after administration of 5-FU.
Serial determinations of different types of hematopoietic colonies compared levels in wild-type C57/Bl6 mice (■) and c-mpl−/− mice (▪). Mice were 6- to 8-weeks old at initiation of these experiments. The results of a single experiment representative of 3 other independent experiments are shown, except for the finding for CFU-MKs, which is the mean result of 2 experiments representative of 2 other independent experiments.
Total numbers of types of CFCs in the spleen after administration of 5-FU.
Serial determinations of different types of colonies compared levels in wild-type C57/Bl6 mice (■) and c-mpl−/− mice (▪). Mice were 6- to 8-weeks old at initiation of these experiments. The results of a single experiment representative of 3 other independent experiments are shown, except for the finding for CFU-MKs, which is the mean result of 2 experiments representative of 2 other independent experiments.
Total numbers of types of CFCs in the spleen after administration of 5-FU.
Serial determinations of different types of colonies compared levels in wild-type C57/Bl6 mice (■) and c-mpl−/− mice (▪). Mice were 6- to 8-weeks old at initiation of these experiments. The results of a single experiment representative of 3 other independent experiments are shown, except for the finding for CFU-MKs, which is the mean result of 2 experiments representative of 2 other independent experiments.
Analysis of the ploidy of MKs after 5-FU treatment
Previous in vivo experiments showed that TPO is essential for full ploidy development.34,35 Because 5-FU has been found to cause a ploidy shift in MKs,3 6 we investigated whether ploidy was altered in c-mpl−/− mice or whether the increase in circulating platelets observed in these animals after 5-FU treatment was just a consequence of platelet production by an increased number of MKs, without a ploidy shift. We first conducted a ploidy analysis of the MKs in spleens of c-mpl−/− and C57/Bl6 mice at different times after 5-FU injection. As shown in Table1, MKs in the spleens of C57/Bl6 mice had a slightly higher mean ploidy level than those in c-mpl−/− mice, although the ploidy distribution was overall very similar. However, 64N, 128N, and 256N MKs were detected only in spleens of C57/Bl6 mice. A ploidy shift was observed in MKs from c-mpl−/− mice as soon as day 7 after 5-FU injection and persisted until day 10 to 14 (Table 1). This shift occurred at the same time as the shift observed in C57/Bl6 mice. A marked, 2- to 3-fold increase in the frequency of MKs in the 8N and 16N ploidy classes was observed concomitantly with the appearance of the 64N and 128N classes of MKs in c-mpl−/− mice on days 7 and 10. A ploidy shift was also observed in MKs from bone marrow of c-mpl−/−mice, but it was less pronounced than that in MKs from the spleen (data not shown).
Megakaryocyte ploidy distribution in spleens of c-mpl−/− and C57/B16 mice on selected days after treatment with 5-fluorouracil
| Mice/day . | 2N . | 4N . | 8N . | 16N . | 32N . | 64N . | 128N . | 256N . |
|---|---|---|---|---|---|---|---|---|
| C-mpl−/− | ||||||||
| 0 (control) | 44.9 ± 12.8 | 26.3 ± 2.8 | 13.5 ± 4.6 | 9.4 ± 4.0 | 6.0 ± 3.4 | — | — | — |
| 7 | 9.4 ± 7.5 | 22.1 ± 7.1 | 39.6 ± 7.5 | 17.5 ± 2.4 | 7.5 ± 2.7 | 2.6 ± 2.7 | 1.4 ± 1.6 | — |
| 10 | 13.2 ± 10.4 | 17.5 ± 6.7 | 32.2 ± 7.1 | 24.0 ± 7.0 | 11.5 ± 3.1 | 1.1 ± 2.1 | 0.6 ± 1.1 | — |
| 14 | 44.4 ± 14.6 | 34.5 ± 4.4 | 13.3 ± 7.6 | 7.7 ± 6.2 | 3.2 ± 2.6 | 1.8 ± 1.6 | 1.0 ± 0.9 | — |
| 18 | 45.2 ± 11.6 | 22.7 ± 3.7 | 13.5 ± 3.5 | 10.5 ± 4.5 | 4.4 ± 2.4 | 2.7 ± 2.0 | 1.2 ± 1.4 | — |
| C57/B16 | ||||||||
| 0 (control) | 33.0 ± 7.6 | 29.6 ± 0.5 | 17.8 ± 2.5 | 9.8 ± 2.8 | 4.8 ± 1.4 | 2.7 ± 0.8 | 1.8 ± 0.5 | 0.5 ± 0.7 |
| 7 | 21.0 ± 8.9 | 29.1 ± 2.8 | 27.5 ± 2.2 | 12.3 ± 2.3 | 5.7 ± 1.6 | 2.6 ± 0.7 | 1.1 ± 0.6 | — |
| 10 | 51.6 ± 5.0 | 26.6 ± 1.8 | 11.9 ± 1.8 | 5.2 ± 0.9 | 2.4 ± 0.3 | 1.4 ± 0.3 | 0.7 ± 0.2 | 0.2 ± 0.2 |
| 14 | 47.4 ± 12.8 | 34.5 ± 12.3 | 10.4 ± 2.9 | 5.1 ± 1.7 | 1.7 ± 0.5 | 1.0 ± 0.4 | — | — |
| 18 | 60.2 ± 6.6 | 23.8 ± 2.9 | 8.9 ± 1.9 | 4.0 ± 1.1 | 1.7 ± 0.4 | 0.7 ± 0.1 | 0.7 ± 0.5 | — |
| Mice/day . | 2N . | 4N . | 8N . | 16N . | 32N . | 64N . | 128N . | 256N . |
|---|---|---|---|---|---|---|---|---|
| C-mpl−/− | ||||||||
| 0 (control) | 44.9 ± 12.8 | 26.3 ± 2.8 | 13.5 ± 4.6 | 9.4 ± 4.0 | 6.0 ± 3.4 | — | — | — |
| 7 | 9.4 ± 7.5 | 22.1 ± 7.1 | 39.6 ± 7.5 | 17.5 ± 2.4 | 7.5 ± 2.7 | 2.6 ± 2.7 | 1.4 ± 1.6 | — |
| 10 | 13.2 ± 10.4 | 17.5 ± 6.7 | 32.2 ± 7.1 | 24.0 ± 7.0 | 11.5 ± 3.1 | 1.1 ± 2.1 | 0.6 ± 1.1 | — |
| 14 | 44.4 ± 14.6 | 34.5 ± 4.4 | 13.3 ± 7.6 | 7.7 ± 6.2 | 3.2 ± 2.6 | 1.8 ± 1.6 | 1.0 ± 0.9 | — |
| 18 | 45.2 ± 11.6 | 22.7 ± 3.7 | 13.5 ± 3.5 | 10.5 ± 4.5 | 4.4 ± 2.4 | 2.7 ± 2.0 | 1.2 ± 1.4 | — |
| C57/B16 | ||||||||
| 0 (control) | 33.0 ± 7.6 | 29.6 ± 0.5 | 17.8 ± 2.5 | 9.8 ± 2.8 | 4.8 ± 1.4 | 2.7 ± 0.8 | 1.8 ± 0.5 | 0.5 ± 0.7 |
| 7 | 21.0 ± 8.9 | 29.1 ± 2.8 | 27.5 ± 2.2 | 12.3 ± 2.3 | 5.7 ± 1.6 | 2.6 ± 0.7 | 1.1 ± 0.6 | — |
| 10 | 51.6 ± 5.0 | 26.6 ± 1.8 | 11.9 ± 1.8 | 5.2 ± 0.9 | 2.4 ± 0.3 | 1.4 ± 0.3 | 0.7 ± 0.2 | 0.2 ± 0.2 |
| 14 | 47.4 ± 12.8 | 34.5 ± 12.3 | 10.4 ± 2.9 | 5.1 ± 1.7 | 1.7 ± 0.5 | 1.0 ± 0.4 | — | — |
| 18 | 60.2 ± 6.6 | 23.8 ± 2.9 | 8.9 ± 1.9 | 4.0 ± 1.1 | 1.7 ± 0.4 | 0.7 ± 0.1 | 0.7 ± 0.5 | — |
5-FU indicates 5-fluorouracil.
Ploidy analysis of splenic megakaryocytes from c-mpl−/−mice and C57/B16 mice treated with a single injection of 5-FU (150 mg/kg) is shown. For each indicated time after injection, spleen cells were collected from 4 mice (5 mice for days 0 and 7) and stained sequentially with fluorescein isothiocyanate–conjugated anti-CD41 and the DNA intercalating fluorescent dye propidium iodide. Results are mean ± SD values from 2 independent experiments. Cells were analyzed by 2-color flow cytometry.
Effect of 5-FU administration on c-mpl−/−splenectomized mice
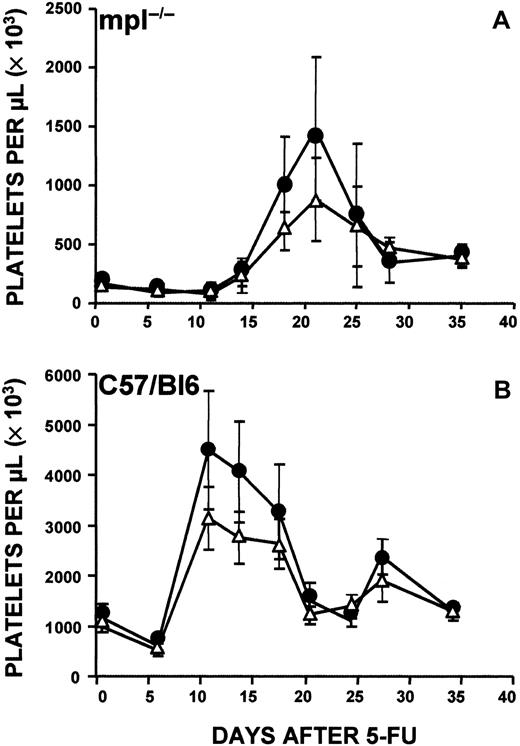
Because a marked increase in splenic CFU-MKs and all colony types was observed in c-mpl−/− mice after 5-FU administration, we determined whether the spleen played a role in the rebound thrombocytosis observed in these mice. Previous studies showed that even though the increase of CFU-MKs is observed mainly in the spleen, the organ is not required for production of thrombocytosis in normal mice after 5-FU injection.3 We injected 5-FU (150 mg/kg) into 11-week-old c-mpl−/− mice and control C57Bl6 mice that had been splenectomized 5 weeks earlier. As shown in Figure4A, on day 21, splenectomized c-mpl−/− mice also had a marked thrombocytosis that was greater than that in eusplenic c-mpl−/− mice after 5-FU administration (1480 × 109/L ± 607 × 109/L platelets versus 883 × 109/L ± 351 × 109/L platelets). Higher rebound thrombocytosis was also observed in splenectomized C57/Bl6 mice (Figure 4B).
Platelet response to 5-FU in eusplenic and splenectomized mice.
(A) Comparison of platelet response to administration of 5-FU (150 mg/kg) in eusplenic c-mpl−/− mice (▵) and splenectomized c-mpl−/− (●) mice. Elevation of platelet counts in splenectomized c-mpl−/− mice was greater than that in eusplenic c-mpl−/− mice, and the counts were higher than normal. (B) Response in eusplenic C57/Bl6 mice (▵) and splenectomized C57/Bl6 mice (●). Mice were given injections of 5-FU 5 weeks after splenectomy. Baseline platelet counts were obtained before injection. Data are based on a total of 20 mice analyzed in each group.
Platelet response to 5-FU in eusplenic and splenectomized mice.
(A) Comparison of platelet response to administration of 5-FU (150 mg/kg) in eusplenic c-mpl−/− mice (▵) and splenectomized c-mpl−/− (●) mice. Elevation of platelet counts in splenectomized c-mpl−/− mice was greater than that in eusplenic c-mpl−/− mice, and the counts were higher than normal. (B) Response in eusplenic C57/Bl6 mice (▵) and splenectomized C57/Bl6 mice (●). Mice were given injections of 5-FU 5 weeks after splenectomy. Baseline platelet counts were obtained before injection. Data are based on a total of 20 mice analyzed in each group.
Comparison of the effect of 5-FU in young and older c-mpl−/− mice
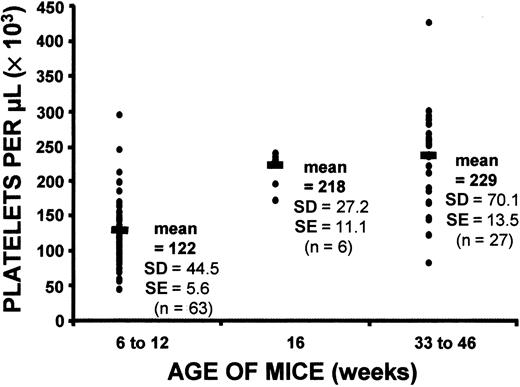
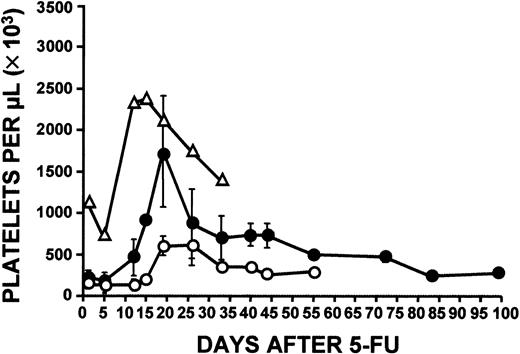
Initial observations suggested that the response of c-mpl−/− mice to 5-FU might differ according to the age of the mice. We had already found a difference between baseline platelet counts in young and old c-mpl−/− mice (Figure5). As the c-mpl−/− mice aged, their platelet levels rose significantly, and after 4 months, they had platelet counts higher than those in 6- to 12-week old c-mpl−/− mice. However, even the oldest mice studied (33-46 weeks) remained markedly thrombocytopenic. There was no effect of age on normal hematocrit levels and total white blood cell counts (data not shown). Comparison of the platelet response in young (6-12 weeks) and old (33-46 weeks) c-mpl−/− mice to administration of 5-FU showed that although both old and young mice had an elevation of platelet levels, the older mice had a much more marked response, with platelet counts beginning to increase on day 12 and reaching peak level on day 20, at which time the mean platelet level in older mice was approximately 1700 × 109/L (Figure6). This represented a 7-fold increase in platelets. Thus, although the older c-mpl−/− mice were initially markedly thrombocytopenic, they eventually developed thrombocytosis, a condition that reached its peak 10 days later than in normal controls. Equally striking was the persistence of platelet levels above 500 × 109/L in the older c-mpl−/− mice until day 72 (Figure 6). Platelet levels in this group did not return to baseline values until after 72 days. Older c-mpl−/− mice also had higher white blood cell counts than younger mice on day 15 (23×109/L±8.9×109/L versus 13.6 × 109/L ± 3.6 × 109/L).
Comparison of baseline platelet counts in c-mpl−/− mice at different ages.
Platelet counts in older mice (both 16 weeks and 33-46 weeks) were significantly higher than those in young mice (6-12 weeks) (P < .0001 for 16 weeks versus 6-12 weeks;P < .0001 for 33-46 weeks versus 6-12 weeks). The mean platelet count for each group is indicated by the black horizontal bar. Each point represents one mouse. The number of mice in each age group is shown in parentheses. The normal mean platelet count was 1053 × 109/L ± 176 × 109/L (n = 17).
Comparison of baseline platelet counts in c-mpl−/− mice at different ages.
Platelet counts in older mice (both 16 weeks and 33-46 weeks) were significantly higher than those in young mice (6-12 weeks) (P < .0001 for 16 weeks versus 6-12 weeks;P < .0001 for 33-46 weeks versus 6-12 weeks). The mean platelet count for each group is indicated by the black horizontal bar. Each point represents one mouse. The number of mice in each age group is shown in parentheses. The normal mean platelet count was 1053 × 109/L ± 176 × 109/L (n = 17).
Platelet response.
Young (6-12 weeks; ○) and old (33-46 weeks; ●) c-mpl−/− mouse platelet responses to administration of 5-FU (150 mg/kg) were compared. Elevation of platelet counts in older c-mpl−/− mice was much greater than in young mice, and counts were higher than normal. The platelet response in control C57/Bl6 mice (▵) is also shown. Data were derived from 3 independent experiments and based on a total of 68 young and 68 old c-mpl−/− mice and 12 C57/Bl6 mice. One day before 5-FU administration and 7 days afterward, blood samples were obtained from all mice, thereby allowing determination of baseline platelet counts and confirmation of the response to 5-FU. Thereafter, blood samples were obtained from groups of 5 to 7 young and 4 to 15 old c-mpl−/− mice and 1 to 4 C57/Bl6 mice.
Platelet response.
Young (6-12 weeks; ○) and old (33-46 weeks; ●) c-mpl−/− mouse platelet responses to administration of 5-FU (150 mg/kg) were compared. Elevation of platelet counts in older c-mpl−/− mice was much greater than in young mice, and counts were higher than normal. The platelet response in control C57/Bl6 mice (▵) is also shown. Data were derived from 3 independent experiments and based on a total of 68 young and 68 old c-mpl−/− mice and 12 C57/Bl6 mice. One day before 5-FU administration and 7 days afterward, blood samples were obtained from all mice, thereby allowing determination of baseline platelet counts and confirmation of the response to 5-FU. Thereafter, blood samples were obtained from groups of 5 to 7 young and 4 to 15 old c-mpl−/− mice and 1 to 4 C57/Bl6 mice.
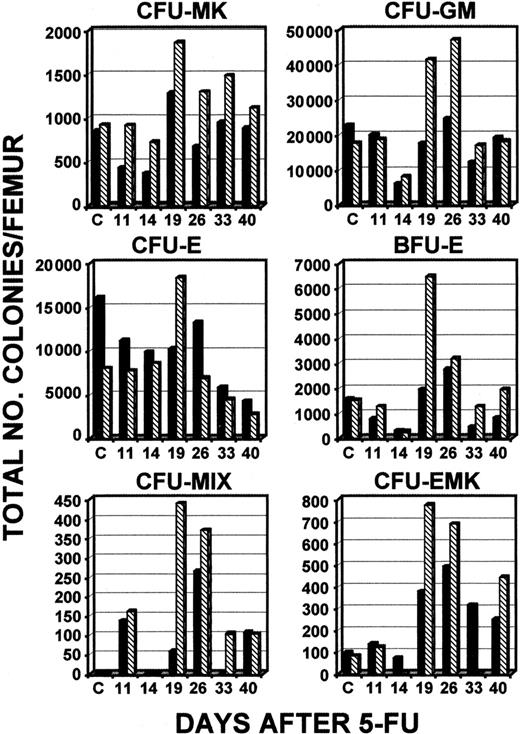
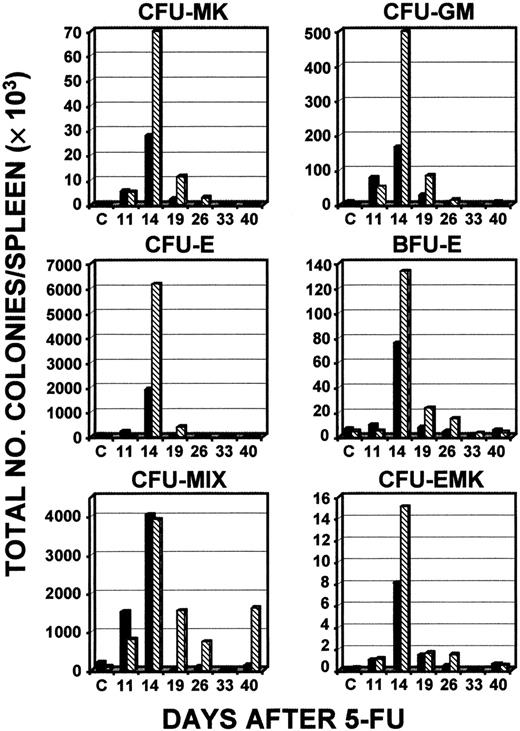
Additional experiments were done to assess differences in the responses of young and old c-mpl−/− mice to 5-FU. A direct comparison between 6- to 12-week old and 33- to 46-week old c-mpl−/− mice found little evidence that CFUs in the bone marrow of young mice were elevated above baseline levels at any time after administration of 5-FU, except for CFU-MKs, CFU-EMKs, and CFUs-MIX (Figure 7). However, in the bone marrow of c-mpl−/− mice that were 33 to 46 weeks old, a peak was observed on days 19 to 26 for all colonies studied (Figure 7). In spleen samples, as was observed in other experiments, total numbers of each type of colony reached levels much higher than normal (Figure8). Peak levels occurred on day 14, and maximum levels were always higher in old c-mpl−/− mice than in younger c-mpl−/− mice, except for CFU-MIX colonies, for which the levels were the same (Figure 8). A 373-fold increase in the number of CFU-MKs was observed on day 14 in the older mice, whereas younger animals had only a 90-fold increase in this experiment (the baseline numbers of CFU-MKs per spleen for young and old c-mpl−/− mice were 308 ± 98 and 381 ± 164, respectively).
Total numbers of types of CFCs in bone marrow after administration of 5-FU.
Serial determinations of different types of colonies compared levels in young c-mpl−/− mice (6-8 weeks; ▪) and old c-mpl−/− mice (33-46 weeks; ▧). The results of a single experiment representative of 2 independent experiments are shown.
Total numbers of types of CFCs in bone marrow after administration of 5-FU.
Serial determinations of different types of colonies compared levels in young c-mpl−/− mice (6-8 weeks; ▪) and old c-mpl−/− mice (33-46 weeks; ▧). The results of a single experiment representative of 2 independent experiments are shown.
Total numbers of types of CFCs in the spleen after administration of 5-FU.
Serial determinations of different types of colonies compared levels in young c-mpl−/− mice (6-8 weeks; ▪) and old c-mpl−/− mice (33-46 weeks; ▧). The results of a single experiment representative of 2 independent experiments are shown.
Total numbers of types of CFCs in the spleen after administration of 5-FU.
Serial determinations of different types of colonies compared levels in young c-mpl−/− mice (6-8 weeks; ▪) and old c-mpl−/− mice (33-46 weeks; ▧). The results of a single experiment representative of 2 independent experiments are shown.
Effect of 5-FU in young and older normal C57/Bl6 mice
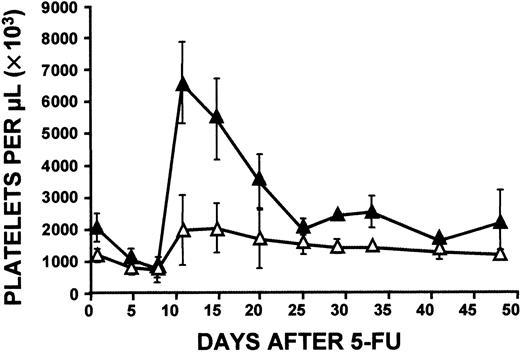
To determine whether the difference in response between old and young mice was a characteristic only of c-mpl−/− mice or a general phenomenon linked to age, we compared the platelet response to administration of 5-FU of young (6-12 weeks) and older (33-46 weeks) wild-type C57/Bl6 mice. Surprisingly, at the peak of the response, older C57/Bl6 mice had much higher platelet levels than younger normal mice (6000 × 109/L versus 2000 × 109/L, respectively; Figure9). Some older mice had platelet levels of 8000 × 109/L. However, in both groups of wild-type mice, platelet levels returned to normal on about day 25 (baseline platelet counts were 1190 × 109/L ± 159 × 109/L [n = 7] in young and 2031 × 109/L ± 428 × 109/L [n = 39] in older mice). White blood cell counts in older C57/Bl6 mice exactly paralleled those in younger mice at all times studied after 5-FU injection (data not shown). Serial analysis of hematopoietic progenitors in bone marrow did not detect a significant increase in any of the wide range of colonies studied, regardless of the age of the mice (data not shown). In spleen samples, peak colony levels occurred between day 12 and day 15 in both groups of mice (Figure 10). As we observed in c-mpl−/− mice, maximum levels of splenic colonies were higher in older mice, although the differences between peak levels in the 2 groups were less marked.
Platelet response.
Young (6-12 weeks; ▵) and old (33-46 weeks; ▴) wild-type C57/Bl6 mouse platelet responses to administration of 5-FU (150 mg/kg) were compared. Elevation of platelet counts in old C57/Bl6 mice was much greater than in young mice. Data were derived from serial observations in groups of mice in 5 independent experiments (a total of 23 young and 55 old C57/Bl6 mice). During the initial 25-day observation period, there were 5 to 15 young and 8 to 43 old C57/Bl6 mice.
Platelet response.
Young (6-12 weeks; ▵) and old (33-46 weeks; ▴) wild-type C57/Bl6 mouse platelet responses to administration of 5-FU (150 mg/kg) were compared. Elevation of platelet counts in old C57/Bl6 mice was much greater than in young mice. Data were derived from serial observations in groups of mice in 5 independent experiments (a total of 23 young and 55 old C57/Bl6 mice). During the initial 25-day observation period, there were 5 to 15 young and 8 to 43 old C57/Bl6 mice.
Total numbers of types of CFCs in the spleen after administration of 5-FU in C57/Bl6 mice.
Serial determinations of different types of colonies compared levels in young wild-type C57/Bl6 mice (6-8 weeks; ■) and old wild-type C57/Bl6 mice (33-46 weeks; ▤). The results of a single experiment representative of 2 independent experiments are shown.
Total numbers of types of CFCs in the spleen after administration of 5-FU in C57/Bl6 mice.
Serial determinations of different types of colonies compared levels in young wild-type C57/Bl6 mice (6-8 weeks; ■) and old wild-type C57/Bl6 mice (33-46 weeks; ▤). The results of a single experiment representative of 2 independent experiments are shown.
Effect of PAS or recurrent hemorrhage on platelet levels in c-mpl−/− mice
Finally, we investigated whether the increase in platelet levels in c-mpl−/− mice was due specifically to 5-FU treatment or whether other known stimuli for thrombocytosis would produce the same effect. We first investigated whether the rebound thrombocytosis typically produced 4 to 7 days after production of acute, severe thrombocytopenia with PAS would occur after a single intraperitoneal injection of PAS in c-mpl−/− mice. In contrast to results in normal mice, after production of acute, severe thrombocytopenia (mean platelet level 1 day after PAS, 48 × 109/L), platelet levels in c-mpl−/− mice increased gradually and returned slowly to baseline values by day 15. Rebound thrombocytosis was not observed in c-mpl−/− mice (data not shown).
We next examined whether chronic intermittent hemorrhage would result in an elevation of platelet levels in c-mpl−/− mice, because hemorrhage is a well-established stimulus for production of thrombocytosis.36 37 We found that repeated bleeding that produced a recurrent reduction in hematocrit level to 0.3 did not result in thrombocytosis (data not shown).
Discussion
An important experimental model for the study of platelet production uses the myelosuppressive agent 5-FU. A single dose of 5-FU (150 mg/kg) depletes the marrow of rapidly cycling hematopoietic cells38 and leads ultimately to a prolonged period of thrombocytosis 10 to 15 days after 5-FU administration.1,4The mechanism induced by administration of 5-FU and responsible for these effects is unknown. Stem cell factor (SCF) was found to have an important role in the compensatory thrombocytosis that follows 5-FU treatment, since SCF-deficient mice do not develop rebound thrombocytosis after administration of 5-FU.39,40 The inability of these mice to respond to 5-FU–induced thrombocytopenia can be corrected by treating them with SCF.40 TPO has been found to be the principal humoral regulator of megakaryocytopoiesis and platelet production.7 9 Therefore, in this study, we investigated whether thrombocytosis would occur in c-mpl−/− mice after administration of 5-FU, thus establishing whether the TPO–Mpl interaction is involved in this response.
We found that 5-FU treatment produced a marked increase in platelet levels in c-mpl−/− mice (Figure 1A). More noteworthy is our observation that at the peak of this response, the mice could acieve normal levels of platelets. Furthermore, the increase in the levels of circulating platelets above baseline values persisted for a longer period in c-mpl−/− mice than in control C57/Bl6 mice and did not return to baseline levels for more than 2 months after administration of 5-FU (Figure 1A and Figure 6). The increase in circulating platelets was preceded by a dramatic increase (245 fold) in splenic megakaryocytic progenitors (CFU-MKs; Figure 3). However, as was shown previously for normal mice,3 5 the spleen did not have any role in this phenomenon, because splenectomized c-mpl−/− mice also developed a marked thrombocytosis. Analysis of histological sections of spleen and bone marrow at different times after 5-FU administration also clearly demonstrated an increase in MKs in both tissues (data not shown).
Importantly, our data, which demonstrated that the ploidy distribution of MKs in the spleen differed markedly from that of MKs in bone marrow, are essentially identical to those previously reported for DNA levels in splenic MKs.41 Analysis of the DNA content of these MKs indicated that despite the absence of any TPO-based stimulus, their ploidy increased in response to 5-FU (Table 1). In contrast, production of moderate thrombocytopenia in C57/Bl6 mice that resulted from bone marrow ablation by strontium 90 did not cause a ploidy shift in splenic MKs.41 Therefore, our results indicate that the rebound megakaryocytopoiesis and thrombocytosis observed after 5-FU treatment can be produced by a mechanism independent of TPO and Mpl. Previous observations that a dose of 5-FU insufficient to produce thrombocytopenia results in an increase in splenic CFU-MKs6and that 5-FU causes a ploidy shift in MKs after only a moderate reduction in platelet levels,3 which would be insufficient to produce a ploidy shift if due to PAS,28 also strongly suggest that a TPO-Mpl–independent mechanism is involved in production of thrombocytosis by 5-FU. This conclusion is further supported by our observations that production of acute thrombocytopenia with PAS did not result in rebound thrombocytosis in c-mpl−/−mice. This latter physiologic response is thought to be mediated by TPO. Similarly, chronic intermittent hemorrhage sufficient to repeatedly reduce the hematocrit level to 0.3 did not produce an elevation of platelet levels (data not shown), although hemorrhage is a well-established stimulus for production of thrombocytosis.36 37
The existence of a modified or alternative regulation of megakaryocytopoiesis in c-mpl−/− or TPO−/−mice was previously suggested by the fact that these mice can produce sufficient platelets to prevent spontaneous hemorrhage20,21and by the finding that qualitatively normal platelets and MKs are produced.25 IL-3, one of the cytokines previously postulated to play a role in megakaryocytopoiesis, does not contribute to the residual thrombopoiesis in c-mpl−/−mice,42,43 a finding that is consistent with previous observations that nude mice (which lack T cells and therefore are deficient in IL-3) have normal megakaryocytopoiesis both in the baseline state and during the response to the stimulation produced by acute, severe thrombocytopenia.44 Gainsford et al26 demonstrated that IL-6, IL-11, and LIF are similarly not required for the residual MK and platelet production in c-mpl−/− mice. These observations, in conjunction with our current findings, strongly indicate the existence of an unknown regulator of megakaryocytopoiesis and thrombopoiesis.
A similar pattern of response to 5-FU (ie, delayed appearance of rebound thrombocytosis associated with lower maximum platelet levels) was also observed in mice that had undergone bone marrow transplantation 90 days before administration of 5-FU.45In that model, mice given transplants had a lesser increase in MK concentration and platelet numbers than normal mice, a finding that can be attributed to a limitation of the proliferative capacity of the population of primitive hematopoietic cells.
Unexpectedly, we found that although c-mpl−/− mice are deficient in progenitor cells of multiple hematopoietic lineages,21,22 they could respond to 5-FU–induced myelosuppression by producing large numbers of all myeloid progenitors (Figure 3 and Figure 10). Furthermore, not only did the peak levels of colonies in these mice always occur at the same time as in controls, but they were also consistently greater than the peak levels in controls. This apparent paradox of a greater-than-normal response of splenic CFCs in c-mpl−/− mice, despite data indicating the important role of TPO in supporting primitive hematopoietic progenitors,46 is striking. Most primitive HSCs, defined by their ability to repopulate the immune and hematopoietic systems and to differentiate into both myeloid and lymphoid lineages during many months, are stimulated to cycle rapidly after 5-FU treatment.47,48 However, c-mpl−/− mice are profoundly deficient in such cells, and their bone marrow hematopoietic repopulating activity is dramatically impaired.23,24 In addition, stem cell populations expressing c-mpl were shown to have a much better in vivo hematopoietic reconstituting ability.24 Our results strongly suggest that at least a component of the population of primitive HSCs in normal mice that is spared by 5-FU treatment is Mpl negative.
Initial observations suggested that the response of young and old c-mpl−/− mice to 5-FU differed. Therefore, we injected groups of young (6-12 weeks) and old (33-46 weeks) c-mpl−/− mice with 5-FU. Older c-mpl−/−mice had a much more marked response, with a mean platelet level of 1700 × 109/L at the peak of the response (day 20), whereas the mean platelet level in young mice was only 600 × 109/L. This corresponded to a 7.6-fold and a 4.7-fold increase, respectively, in platelet counts (control C57/Bl6 mice had a 2.1-fold increase). Correspondingly, the increase in splenic CFU-MKs was greater in older (373 fold) than in younger (90 fold) c-mpl−/− mice. Comparison of the platelet response to administration of 5-FU in young and older wild-type C57/Bl6 mice revealed that the difference in response between old and young mice was not a unique characteristic of c-mpl−/− mice but a general phenomenon linked to age. These results are in agreement with those of de Haan et al,49 who reported that relative and absolute numbers of the most primitive stem cells were 3- to 4-fold higher in old (12 months) than in young (6 weeks) mice and that in C57/Bl6 mice the stem cell population increased at a constant rate from late gestation to 20 months of age.50 In addition, another study found that the number of spleen colonies detected after lethal irradiation and bone marrow transplantation increased with the age of the recipient.51 It is pertinent that the age-related increase in spleen colonies present in RFM/Un mice older than 30 weeks resulted almost entirely from increased numbers of MK colonies in the older mice. Normal, untreated RFM/Un mice also had an increase in baseline platelet counts as they aged, with almost all the increase occurring during the first 25 weeks of life.51 These results exactly parallel our observations.
We propose the following hypothesis to explain our results. In control C57/Bl6 mice as well as in c-mpl−/− mice, the pool of HSCs spared by 5-FU enters into cycle, and subsequently, all myeloid progenitors are amplified. The observations that peak levels of splenic myeloid colonies in c-mpl−/− mice occurred at the same time as in normal mice and were consistently greater than levels in normal controls indicate that this step of amplification is independent of Mpl. In c-mpl−/− mice, the MKs produced are negative for Mpl and, because of their lack of ability to respond to TPO, they may require a longer period to produce platelets. TPO was shown to be essential for the full development of ploidy levels of MKs,34 possibly by means of the extracellular signal-regulated protein kinase–mitogen-activated protein kinase pathway.52 Nevertheless, our results show that the ploidy of MKs from c-mpl−/− mice increased after 5-FU injection (7 days after injection, as with MKs from control mice). Cyclin D3, which is expressed prominently in MKs,53 also seems to be important for MK differentiation and ploidy levels.54Therefore, it is possible that cyclins assume increased importance in c-mpl−/− mice after 5-FU treatment. In C57/Bl6 mice, the marked increase in levels of MKs and circulating platelets (c-mpl positive) is subsequently down-regulated by TPO clearing, which allows a return to normal platelet levels. However, because the platelets produced in c-mpl−/− mice after 5-FU administration are negative for Mpl (data not shown), normal down-regulation cannot take place and the return to baseline values takes longer than in C57/Bl6 mice.
In conclusion, our findings indicate that the mechanism by which 5-FU produces thrombocytosis does not require TPO-Mpl signaling. A single injection of 150 mg/kg into c-mpl−/− mice produced a marked increase in platelet production. As postulated previously, thrombocytosis is not a direct result of the mild thrombocytopenia that follows 5-FU administration6 but is due to perturbation of a currently unidentified population of MK progenitors, the response of which is at least partly independent of TPO. In addition, the huge expansion of all splenic myeloid progenitors in c-mpl−/−mice observed after 5-FU injection indicates the existence of an Mpl-negative population of HSCs spared by 5-FU.
We thank Dr J. P. Rosa and S. Osdoit for help in performing platelet function assays.
Supported by the Institut National de la Santé et de la Recherche Médicale, Institut des Vaisseaux et du Sang, and the US Department of Veterans Affairs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michèle Souyri, Institut National de la Santé et de la Recherche Médicale, U506, Hôpital Paul Brousse, 14, Avenue Paul-Vaillant Couturier, 94807 Villejuif Cedex, France; e-mail: msouyri@infobiogen.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal