Abstract
Internal tandem duplication (ITD) mutations of the receptor tyrosine kinase FLT3 have been found in 20% to 30% of patients with acute myeloid leukemia (AML). These mutations constitutively activate the receptor and appear to be associated with a poor prognosis. Recent evidence that this constitutive activation is leukemogenic renders this receptor a potential target for specific therapy. In this study, dose-response cytotoxic assays were performed with AG1295, a tyrosine kinase inhibitor active against FLT3, on primary blasts from patients with AML. For each patient sample, the degree of cytotoxicity induced by AG1295 was compared to the response to cytosine arabinoside (Ara C) and correlated with the presence or absence of a FLT3/ITD mutation. AG1295 was specifically cytotoxic to AML blasts harboring FLT3/ITD mutations. The results suggest that these mutations contribute to the leukemic process and that the FLT3 receptor represents a therapeutic target in AML.
Introduction
The receptor tyrosine kinase FLT3 is expressed on the blasts in most patients with acute myeloid leukemia (AML).1,2 Activation of FLT3 on leukemic cells by FLT3 ligand (FL) leads to receptor dimerization and signal transduction along pathways that promote cell growth and inhibit apoptosis.3-7
In 17% to 27% of patients with AML, the leukemic blasts have small internal tandem duplications (ITDs) in the juxtamembrane region of the cytoplasmic domain of FLT3.8-10 These mutations cause constitutive activation of the receptor and their presence appears to influence clinical outcome.9,11-13 Expression of a constitutively activated FLT3 receptor in Ba/F3 or 32D cells results in leukemic transformation.14-16 Thus the FLT3 receptor, activated either by coexpression of FL or by an ITD mutation, may contribute to leukemogenesis.
Transformation through FLT3 requires an activated kinase domain.14 If FLT3 contributes to the leukemic process in AML, then a drug that inhibits its kinase activity might be an effective therapeutic agent. AG1295 is a quinoxaline derivative that inhibits the tyrosine kinase activity of platelet-derived growth factor receptor (PDGF-R) and KIT, receptors closely related to FLT3.17 18 To help validate FLT3 as a therapeutic target in AML, we characterized the effect of AG1295 on primary leukemic blasts from AML patients using a dimethylthiazol diphenyltetrazolium bromide (MTT) colorimetric assay. We then correlated the response with the ITD status of the sample and compared it to the response to cytosine arabinoside (Ara C), the most widely used drug in AML therapy.
Study design
Reagents
AG1295 was obtained from Aviv Gazit (Hebrew University, Jerusalem, Israel) and was dissolved in DMSO. Ara C was obtained from Sigma (St Louis, MO). Antibodies and cytokines were obtained as described.14
Patient samples
Specimens were procured as part of a protocol approved by the Institutional Review Board. We used consecutive diagnostic samples from adult patients with de novo AML procured between May 1997 and August 1999.
Cell culture
Bone marrow mononuclear cells were recovered by Ficoll-Hypaque (Amersham, Piscataway, NJ) density centrifugation and stored frozen in complete medium (RPMI/10% fetal calf serum plus penicillin/streptomycin plus glutamine) plus 10% DMSO at −140°C. Cells were thawed rapidly into complete medium with more than 90% viability as assayed by trypan blue exclusion and were incubated at 37°C and 5% CO2. We encountered a variable but significant loss of viability after thawing, which is a typical feature of leukemia cells.19 To eliminate cells that were dead or dying as a result of the freeze-thaw process, we incubated samples in complete medium for 12 hours after thawing followed by treatment with DNAse I (Boehringer-Mannheim, Indianapolis, IN) to eliminate cell clumps and reisolation over Ficoll-Hypaque.
Reverse transcription–polymerase chain reaction for FLT3/ITD mutations
Total RNA was isolated using RNeasy columns (Qiagen, Valencia, CA) and reverse transcribed. The resulting complementary DNA (or control lacking reverse transcriptase) was used as a template for polymerase chain reaction (PCR) with primers R5 (5′-TGTCGAGCAGTACTCTAAACA-3′) and R6 (5′-ATCCTAGTACCTTCCCAAACTC-3′).8 Products were resolved on 2.5% agarose gels. FLT3/ITD mutations were identified as bands migrating above the expected 365-bp size of the wild-type FLT3 fragment. Dideoxy sequencing was performed to confirm the FLT3/ITD status on the first 4 samples identified in this manner.
Immunoprecipitation and immunoblotting
The AML blasts were incubated in complete medium with increasing concentrations of AG1295 for 4 hours. Lysis and immunoprecipitation were performed as described.14
MTT assay
The MTT assay kit (Roche, Indianapolis, IN) was used according to the manufacturer's instructions. Each drug concentration was tested in duplicate or triplicate. Linear regression analysis was used to calculate a P value for the difference of the means of FLT3/ITD mutant versus nonmutant samples.
Annexin V assay
Blasts (200 000 in 0.5 mL medium) were assayed with annexin V (Pharmingen, San Diego, CA) by flow cytometry according to the manufacturer's instructions.
Results and discussion
Twenty-three consecutive patient samples of de novo AML procured and stored in our leukemia bank were studied. The FAB classifications were M1 (n = 4), M2 (n = 5), M3 (n = 3), M4 (n = 7), and M5 (n = 4). All 23 samples had FLT3 messenger RNA (mRNA) detectable by reverse transcription (RT)-PCR. Eight samples (34.8%) expressed FLT3/ITD mutations (with FAB classifications M1 [n = 1], M2 [n = 1], M3 [n = 3], M4 [n = 1], M5 [n = 2]). One FLT3/ITD patient had trisomy 8, 3 had 15:17 translocations, and the remaining 4 had normal cytogenetics. Of the FLT3/ITD patients, the trisomy 8 patient and the 3 promyelocytic patients are alive and disease-free at more than 30 months. The remaining 4 failed to achieve a complete remission and have died.
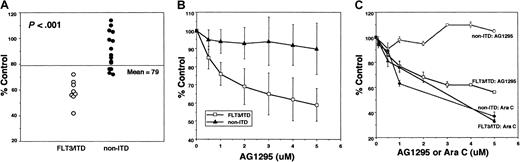
Metabolic activity as determined in the MTT assay was used to quantitate drug-mediated cytotoxicity. The 5-μM dose of AG1295 was used as the maximum dose because nonspecific inhibitory effects can be seen in cell lines at higher doses.20 The cytotoxic responses to 5 μM AG1295, expressed as percent of control optical density (OD), are shown in Figure1A. There was a wide range of responses to AG1295, with the 8 FLT3/ITD specimens being the 8 strongest responders (P < .001). The group of 15 non-ITD specimens display either a more intermediate or no response.
MTT assay results.
Assays on AML blasts were carried out in 96-well plates. Drugs were prepared at 2 × concentration in complete medium and 50-μL aliquots were added to wells. Control wells contained DMSO in medium (final 0.125%) for AG1295 experiments, or phosphate-buffered saline (PBS) alone for Ara C experiments. Blasts were resuspended at 4 × 106/mL and 50-μL aliquots were added to the wells (final 200 000 cells/well in 100 μL volume). After 72 hours of incubation at 37°C in 5% CO2, the plates were assayed for MTT uptake. Optical densities (OD) were determined by spectrophotometry at 562 nm. The y-axis of each graph displays the mean OD of the drug treatments expressed as a percentage of the untreated (DMSO or PBS) controls. (A) Response of AML samples to 5 μM AG1295. Blasts were incubated as described with 5 μM AG1295 for 72 hours in duplicate or triplicate wells of 96-well plates before subjecting them to the MTT assay. All 23 samples (8 FLT3/ITD and 15 non-ITD) are shown in this scatterplot, grouped according to their FLT3/ITD status. TheP value shown refers to the difference between these 2 groups and is based on a 2 sample t test. (B) Dose-response curve of FLT3/ITD and non-ITD AML samples treated with AG1295. Each of the 23 samples shown in panel A was incubated with increasing concentrations of AG1295 for 72 hours prior to the MTT assay. The data are grouped according to FLT3/ITD status and a mean response for each AG1295 concentration was calculated. Errors bars represent the SD of the mean OD of the treatment points expressed as a percentage of the mean OD of the control wells. Each point on the FLT3/ITD curve represents the mean of 8 samples; those on the non-ITD curve represent that of 15 samples. (C) Dose-response curves for AG1295 versus Ara C. A FLT3/ITD AML sample and a non-ITD sample were each exposed to increasing concentrations of either AG1295 or Ara C for 72 hours prior to the MTT assay. Error bars represent the SD of the OD of the triplicate samples at each concentration, expressed as a percentage of the mean OD of the control wells. The x-axis displays the micromolar concentration of either AG1295 or Ara C.
MTT assay results.
Assays on AML blasts were carried out in 96-well plates. Drugs were prepared at 2 × concentration in complete medium and 50-μL aliquots were added to wells. Control wells contained DMSO in medium (final 0.125%) for AG1295 experiments, or phosphate-buffered saline (PBS) alone for Ara C experiments. Blasts were resuspended at 4 × 106/mL and 50-μL aliquots were added to the wells (final 200 000 cells/well in 100 μL volume). After 72 hours of incubation at 37°C in 5% CO2, the plates were assayed for MTT uptake. Optical densities (OD) were determined by spectrophotometry at 562 nm. The y-axis of each graph displays the mean OD of the drug treatments expressed as a percentage of the untreated (DMSO or PBS) controls. (A) Response of AML samples to 5 μM AG1295. Blasts were incubated as described with 5 μM AG1295 for 72 hours in duplicate or triplicate wells of 96-well plates before subjecting them to the MTT assay. All 23 samples (8 FLT3/ITD and 15 non-ITD) are shown in this scatterplot, grouped according to their FLT3/ITD status. TheP value shown refers to the difference between these 2 groups and is based on a 2 sample t test. (B) Dose-response curve of FLT3/ITD and non-ITD AML samples treated with AG1295. Each of the 23 samples shown in panel A was incubated with increasing concentrations of AG1295 for 72 hours prior to the MTT assay. The data are grouped according to FLT3/ITD status and a mean response for each AG1295 concentration was calculated. Errors bars represent the SD of the mean OD of the treatment points expressed as a percentage of the mean OD of the control wells. Each point on the FLT3/ITD curve represents the mean of 8 samples; those on the non-ITD curve represent that of 15 samples. (C) Dose-response curves for AG1295 versus Ara C. A FLT3/ITD AML sample and a non-ITD sample were each exposed to increasing concentrations of either AG1295 or Ara C for 72 hours prior to the MTT assay. Error bars represent the SD of the OD of the triplicate samples at each concentration, expressed as a percentage of the mean OD of the control wells. The x-axis displays the micromolar concentration of either AG1295 or Ara C.
We performed dose-response experiments on all of the samples (8 FLT3/ITDs and 15 non-ITDs). Despite the wide variability of cytotoxicity observed among patient samples, there was very little variability seen when an experiment was repeated on a given patient sample. The data points from each group (FLT3/ITD versus non-ITD) were averaged and a composite dose-response curve of FLT3/ITD specimens versus non-ITD specimens was generated (Figure 1B). The FLT3/ITD specimens respond in a dose-dependent manner to AG1295. On comparing the 2 curves displayed in Figure 1B, there are statistically significant differences between responses (ie, no overlap between the 95% confidence intervals) in the FLT3/ITD versus non-ITD groups at each concentration point above 0.5 μM.
Twelve of 23 specimens demonstrated moderate or bright KIT expression by flow cytometry in our institution's clinical laboratory. We did not observe any correlation between response to AG1295 and KIT expression in these samples.
Panels A and B in Figure 1 demonstrate that AG1295 is selectively cytotoxic to blasts harboring a FLT3/ITD mutation. To compare the magnitude of the AG1295 response with that of a standard chemotherapeutic drug, we carried out dose-response studies using Ara C. The variation and degree of response to Ara C was similar to what has been previously published for AML blast samples.21,22Figure 1, panel C, shows a representative experiment on a FLT3/ITD sample and a non-ITD sample, comparing the dose-response of AG1295 to that of Ara C. The response of the FLT3/ITD specimen to AG1295 was similar to the Ara C response. For comparison, Ara C continuous infusion regimens (eg, “7 + 3”) achieve sustained levels of approximately 1 μM.23
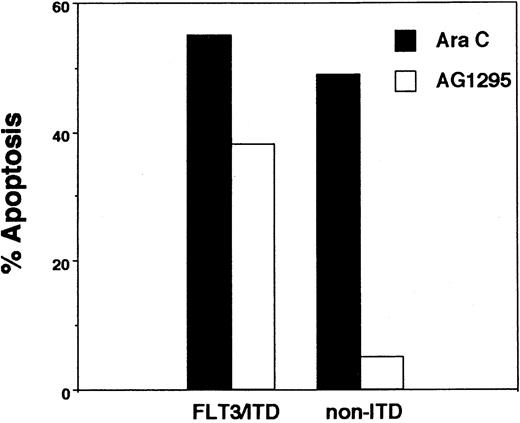
The MTT assay is a convenient and sensitive measure of cytotoxicity, but it does not directly measure cell death. To confirm that the cytotoxic effects of AG1295 are due to induction of apoptosis, we carried out annexin V binding assays on a limited number of samples. We were unable to perform this assay on the majority of samples due to inadequate cell numbers and the high background of apoptosis seen in many of the samples after thawing. This is the reason the MTT assay was used for the bulk of this study. Nonetheless, AG1295 induced apoptosis to a similar degree in the 3 FLT3/ITD specimens tested with annexin V and had minimal effect on the non-ITD samples. Figure2 shows a representative result, where the percent of apoptosis induced by 5 μM AG1295 is compared with that caused by 5 μM Ara C.
Apoptosis assay.
Blasts from a FLT3/ITD sample and a non-ITD sample were incubated for 48 hours with either 5 μM AG1295 or 5 μM Ara C. They were then assayed for annexin V binding as described. Cell populations from frozen samples have a fraction of blasts that die from the freeze-thaw cycle. Results are displayed as the percent of cells induced by drug to bind annexin V after first subtracting the baseline necrotic population from both the treatment and control (DMSO) groups.
Apoptosis assay.
Blasts from a FLT3/ITD sample and a non-ITD sample were incubated for 48 hours with either 5 μM AG1295 or 5 μM Ara C. They were then assayed for annexin V binding as described. Cell populations from frozen samples have a fraction of blasts that die from the freeze-thaw cycle. Results are displayed as the percent of cells induced by drug to bind annexin V after first subtracting the baseline necrotic population from both the treatment and control (DMSO) groups.
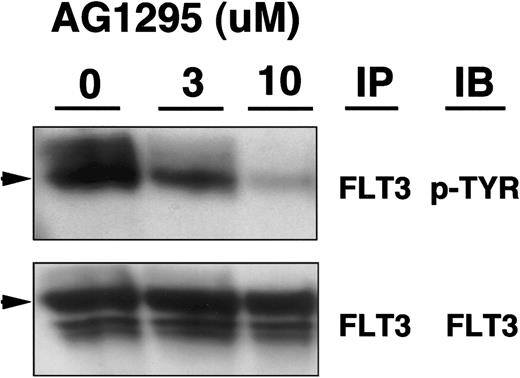
Previously, AG1295 was shown to inhibit the activities of the related receptors PDGF-R and KIT.17 18 For direct biochemical evidence that this drug inhibits FLT3 activity, we incubated AML blasts showing constitutive activation of FLT3 with increasing concentrations of AG1295 and examined the phosphorylation status of the receptor. Figure 3 shows decreasing phosphorylation of the receptor in the presence of increasing concentrations of AG1295, as would be predicted for inhibition of FLT3 tyrosine kinase activity.
Inhibition of FLT3 phosphorylation by AG1295.
Blasts from an AML sample with constitutively activated FLT3 were incubated at 37°C and 5% CO2 for 4 hours with increasing concentrations of AG1295. Clarified lysates (500 μg) were immunoprecipitated (IP) with anti-FLT3 antibody, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to membranes, immunoblotted (IB) with antiphosphotyrosine (p-tyr) or anti-FLT3 antibodies, and visualized with chemiluminescence. Arrowheads identify FLT3.
Inhibition of FLT3 phosphorylation by AG1295.
Blasts from an AML sample with constitutively activated FLT3 were incubated at 37°C and 5% CO2 for 4 hours with increasing concentrations of AG1295. Clarified lysates (500 μg) were immunoprecipitated (IP) with anti-FLT3 antibody, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to membranes, immunoblotted (IB) with antiphosphotyrosine (p-tyr) or anti-FLT3 antibodies, and visualized with chemiluminescence. Arrowheads identify FLT3.
AG1295 is not completely restricted to activity against FLT3 and could well inhibit the activity of other tyrosine kinases in these blast samples. However, the fact that the greatest cytotoxic responses are observed in FLT3/ITD specimens provides strong evidence that this effect is mediated through direct inhibition of FLT3. The finding that inhibition of this receptor induces apoptosis in the FLT3/ITD samples implies that these mutations are important to the development or maintenance of the leukemic phenotype. Perhaps more importantly, these data provide the proof of concept that a FLT3 tyrosine kinase inhibitor represents a new therapeutic strategy in AML. Similar attempts to target the ABL tyrosine kinase in chronic myelogenous leukemia are proving successful.24 Although AG1295 itself is not clinically useful due to problems with solubility and bioavailability, other FLT3 inhibitors can be developed that will overcome these limitations.
Supported by grants NCI CA70970 (D.S.), Leukemia and Lymphoma Society (D.S.), Children's Cancer Foundation (D.S.), Alexander and Margaret Stewart Trust (D.S.), National Institutes of Health Training Grant (5T32CA09071-201A) (M.L.), Oncology Center Core Grant (5P30CA06973-36) (E.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donald Small, Johns Hopkins University School of Medicine, Bunting Blaustein Cancer Research Building, Rm 253, 1650 Orleans St, Baltimore, MD 21231-1000; e-mail: donsmall@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal