Abstract
Nodal marginal zone B-cell lymphoma (MZL) is a rare and not extensively studied entity that accounts for approximately 2% of all non-Hodgkin lymphomas. Complementarity-determining regions 2 and 3 (CDR2, CDR3) of the immunoglobulin heavy-chain variable region (VH) genes were amplified by polymerase chain reaction (PCR), cloned, and sequenced in 8 patients with nodal MZL. All showed a potentially functional VH rearrangement. The use of VH gene families was unbiased and without overrepresentation of any particular VH gene or gene family. The presence of somatic VH mutations was detected, with a deviation from the closest germ line sequence ranging from 4% to 17% in 6 of 8 patients. In 3 mutations, the replacement-to-silent mutation ratio suggested the presence of an antigen-selected process. Sequencing different subclones of the same cloned PCR products allowed the detection of intraclonal variability in 4 analyzed patients. The observed pattern of VH mutations suggested that nodal MZL, formerly deemed a malignancy of memory B cells, may arise from different subsets of marginal zone B cells—the naive B cells that express unmutated VH genes—from memory B cells showing somatic mutations without intraclonal variation, and from germinal center B cells defined by their capacity to undergo the somatic hypermutation process.
Introduction
The entity traditionally designated as monocytoid B-cell lymphoma is classified in the Revised European American Classification of Lymphoid Neoplasms (REAL) and in World Health Organization (WHO) Classification of Hematological Malignancies as nodal marginal zone B-cell lymphoma (MZL).1,2 By definition, in the WHO classification the term nodal is used to design cases primarily involving lymph nodes, and it excludes any case with prior or concurrent localization in an extranodal site other than bone marrow, liver, or spleen.3 Although nodal MZL shares many histologic and immunologic features with extranodal MZL of MALT type,1,2,4,5 clinical characteristics, natural history, and prognosis suggest that nodal MZL should be considered a distinct disease entity.6,7 Based on molecular findings, the relation between the 2 entities is still controversial.8 9
Generation of antigen receptors by somatic recombination of variable, diversity, and joining segments is a unique feature of B and T cells, occurring in an early phase of development. In B-cell ontogenesis, after the successful construction of a functional, nonautoreactive antigen receptor, naive B cells move to the germinal centers of secondary lymphoid organs. In these structures, upon antigen encounter, during T-cell–dependent B-cell development, the antibody expressed by a B cell may be modified by class-switch recombination, somatic hypermutation,10 and, according to some suggestions, receptor editing.11 Somatic hypermutation, which introduces predominantly point mutations into the immunoglobulin variable region genes, increases immunoglobulin diversity and can modify the immunoglobulin affinity for antigen.12 Because somatic hypermutation appears to be restricted to B cells proliferating within the microenvironment of the germinal center,13somatically mutated V-region genes are a hallmark of germinal center B cells and their descendants. Thus, sequence analysis of the immunoglobulin variable region genes of normal B cells and B-cell lymphomas can be applied to determine either the ancestral derivation from follicle center cells or the continued influence of the follicle center microenvironment.14
The pattern of somatic mutations in the VH genes of most B-cell non-Hodgkin lymphomas, with clustering of replacement mutations in complementarity-determining regions (CDRs), indicates that the tumor cells or their precursors were selected for antigen-receptor expression during the process of mutation.15 Furthermore, intraclonal nucleic acid sequence variation, suggestive of ongoing mutations, indicates that tumor B cells are still under the influence of a mutation mechanism.15 Extranodal MZLs of MALT type are thought to arise from post–germinal center B cells. In gastric MZL,Helicobacter pylori–specific T cells drive the proliferation of the neoplastic B cells.16 Immunoglobulin VH genes show a pattern of somatic mutations consistent with a selection by antigen, suggesting that continued exposure to antigen may play a role in the persistence of lymphoma.17,18 The specificity of extranodal MZL B cells for autoantigens and the characteristic growth of extranodal MZLs in the background of autoimmune diseases represent important elements in the comprehension of the pathogenesis of this lymphoma entity.19 20
Analyses of immunoglobulin heavy-chain variable region genes in nodal MZLs have been limited to a few series of patients.21-23Usually the sequences analyzed showed the presence of somatic mutations, suggesting the derivation of the tumor population from post-follicular, antigen-experienced B cells. Based on these results and according to the immunophenotypical characteristics of the tumor cells, nodal marginal zone B-cell lymphomas have been suggested to represent malignancies of memory B cells.15 Nevertheless, in all these studies, the technical approach of amplifying rearranged variable region genes, followed by direct sequencing of the PCR products prevented the reliable detection of potential ongoing mutations within the tumor cells.
Our study was aimed at acquiring insight into the nature of the immunoglobulin heavy-chain variable region sequences in nodal MZLs to confirm the presence of somatic mutations and identify mutation patterns reminiscent of antigen selection processes. In addition, the study was devised to explore the presence of ongoing mutations by cloning and sequencing the VH genes of 8 patients with histologically reviewed nodal MZLs.
Materials and methods
All molecular analyses were performed on tumor tissues collected at diagnosis from patients with nodal MZL.
Pathology review
DNA extraction
Genomic DNA was extracted from lymph nodes, bone marrow, and peripheral blood specimens using phenol/chloroform extraction after 48-hour proteinase K digestion at 56°C. In patients 1, 2, 3, and 8, DNA was obtained from routinely processed formalin-fixed and paraffin-embedded bioptic specimens; in patients 4 to 7, it was obtained from frozen tissue.
PCR amplification and cloning
A seminested strategy was used for the PCR amplification of the VH genes using the FR2A consensus primer, complementary to the conserved framework-2 segment of the variable region, and the LJH and VLJH consensus primers for the J region.24Patient samples were analyzed together with DNA from the Raji cell line as positive control and with a negative reagent control containing all PCR reagents without any template DNA. PCR products were visualized on 7% nondenaturing polyacrylamide gels stained with ethidium bromide. Patients apparently lacking PCR products were assessed for the presence of amplifiable DNA: a 268-bp segment of the β-globin gene was amplified by PCR using specific primers (PCO4: 5′ CAACTTCATCCACGTTCACC 3′; GH20: 5′ GAAGAGCCAAGGACAGGTAC 3′).
Cloning of monoclonal cases was performed with the pGEM T-easy vector (Promega, Madison, WI) using DNA excised and purified with Sephacryl MicroSpin S-400 HR columns (Amersham Pharmacia Biotech, Uppsala, Sweden) from monoclonal bands in the polyacrylamide gels. Randomly picked recombinant clones showing the expected insert size were analyzed using fluorescence sequencing technology on a 377 automatic DNA sequencer (Applied Biosystems, Foster City, CA) using the ABI Prism Big Dye Terminator Kit (PerkinElmer, Foster City, CA) as recommended by the manufacturer. All clones were sequenced in both directions.
Sequence analysis
Tumor-related VH genes were identified based on the presence of analogous CDR3 sequences. DNA sequences were analyzed for homologies in the GenBank database using the Basic Local Alignment Search Tool (BLAST) software, available at the National Center for Biotechnology Information (NCBI). Homology with germ line VH, DH, and JH genes was identified using the V-Base database44 and the IgBlast software of the NCBI.45 The ability to code for functional heavy chains was determined by translation of DNA sequences into amino acids.
Analysis of intraclonal heterogeneity
To evaluate the presence of ongoing mutations in nodal MZLs, at least 7 clones from each specimen were sequenced. For evaluation of intraclonal heterogeneity, the following definitions were used:unconfirmed mutation—a substitution mutation observed in only one of the VH gene molecular clones from the same tumor specimen; confirmed mutation—a mutation observed in more than one of the VH gene molecular clones from the same tumor specimen. Only the confirmed mutations were considered as evidence of intraclonal heterogeneity; the unconfirmed mutations were instead ascribed to Taq polymerase error.25
Antigen selection
To determine the pattern of somatic mutations compatible with antigen selection, 2 different methods were applied.26First, the ratio of replacement-to-silent mutations (R/S) in the CDR2 and FW3 regions was studied. A sequence was considered to be antigen selected when the R/S ratio in the CDR2 was higher than 2.9 and the R/S ratio in the FW3 region was lower than 1.5. Second, only the R/S ratio of the somatic mutations in the FW3 region was considered. A sequence was regarded as antigen selected when the R/S ratio was less than 1.6.
Results
Assessment of clonality
CDR2 and CDR3 of the immunoglobulin heavy-chain variable region (VH) genes were amplified in 10 patients with nodal MZL. Eight patients, characterized by a single band on polyacrylamide gel electrophoresis indicating a monoclonal rearrangement, underwent cloning and sequencing procedures and are the subjects of the present study.
Patients' clinical characteristics
Most patients showed advanced stage at presentation. In all of them prominent and disseminated nodal involvement was present; peripheral blood involvement was not detected in any of them. Moderate spleen enlargement, not pathologically investigated, was reported in 3 patients, none of whom had regional lymphadenopathy. No patient had a history of previous or concurrent autoimmune disease. The main clinical characteristics at diagnosis are summarized in Table1.
Patients' main clinical characteristics at diagnosis
| Case no. . | Age (y) . | Sex . | ECOG PS . | Ann Arbor stage . | Nodal sites of involvement . | Extramodal sites of involvement . | Site of diagnostic biopsy . | LDH . | β-2 microglobulin . | AbHCVs . | Serum monoclonal immunoglobulins . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | F | 0 | IV A | Cervical, axillary, mediastinal, para-aortic, iliac | Bone marrow | Inguinal node | NA | NA | NA | Absent |
| 2 | 50 | M | 0 | IV A | Mediastinal, coeliac, para-aortic, axillary | Bone marrow | Axillary node | Normal | Elevated | − | Absent |
| 3 | 69 | M | 0 | IV A | Cervical, supraclavicular, mediastinal | Bone marrow | Supraclavicular node | Normal | NA | NA | Absent |
| 4 | 64 | M | 0 | IV A | Inguinal, axillary, mediastinal | Spleen, bone marrow | Inguinal node | Elevated | Elevated | + | IgMκ = 0.5 g/dL |
| 5 | 42 | F | 0 | III A | Inguinal, iliac, lombo-aortic | None | Axillary node | Normal | Normal | − | Absent |
| 6 | 68 | F | 0 | III A | Mediastinal, axillary, cervical | Spleen | Axillary node | Normal | NA | − | Absent |
| 7 | 68 | M | 0 | IV A | Supraclavicular, cervical, axillary | Spleen, bone marrow | Supraclavicular node | Normal | Normal | − | IgGλ = 1.1 g/dL |
| 8 | 50 | M | 0 | II A | Retroperitoneal | None | Retroperitoneal node | Normal | NA | − | IgMλ = 0.4 g/dL |
| Case no. . | Age (y) . | Sex . | ECOG PS . | Ann Arbor stage . | Nodal sites of involvement . | Extramodal sites of involvement . | Site of diagnostic biopsy . | LDH . | β-2 microglobulin . | AbHCVs . | Serum monoclonal immunoglobulins . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | F | 0 | IV A | Cervical, axillary, mediastinal, para-aortic, iliac | Bone marrow | Inguinal node | NA | NA | NA | Absent |
| 2 | 50 | M | 0 | IV A | Mediastinal, coeliac, para-aortic, axillary | Bone marrow | Axillary node | Normal | Elevated | − | Absent |
| 3 | 69 | M | 0 | IV A | Cervical, supraclavicular, mediastinal | Bone marrow | Supraclavicular node | Normal | NA | NA | Absent |
| 4 | 64 | M | 0 | IV A | Inguinal, axillary, mediastinal | Spleen, bone marrow | Inguinal node | Elevated | Elevated | + | IgMκ = 0.5 g/dL |
| 5 | 42 | F | 0 | III A | Inguinal, iliac, lombo-aortic | None | Axillary node | Normal | Normal | − | Absent |
| 6 | 68 | F | 0 | III A | Mediastinal, axillary, cervical | Spleen | Axillary node | Normal | NA | − | Absent |
| 7 | 68 | M | 0 | IV A | Supraclavicular, cervical, axillary | Spleen, bone marrow | Supraclavicular node | Normal | Normal | − | IgGλ = 1.1 g/dL |
| 8 | 50 | M | 0 | II A | Retroperitoneal | None | Retroperitoneal node | Normal | NA | − | IgMλ = 0.4 g/dL |
ECOG PS indicates Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; AbHCVs, anti–hepatitis C virus antibodies; NA, not assessed; Ig, immunoglobulin.
Immunohistochemistry
Table 2 shows immunohistochemical features of the patients analyzed.
Immunohistochemical characteristics of the specimens analyzed
| Case no. . | CD20 . | CD3 . | CD5 . | CD10 . | sigM . | sigD . | CD23 . | Light chain . |
|---|---|---|---|---|---|---|---|---|
| 1 | + | NA | − | − | + | − | − | κ |
| 2 | + | NA | − | − | (+) | − | − | NA |
| 3 | + | NA | − | − | − | − | − | κ |
| 4 | + | − | − | − | + | NA | NA | κ |
| 5 | + | − | − | − | + | NA | NA | λ |
| 6 | + | − | −/+ | − | + | + | NA | κ |
| 7 | + | − | − | − | − | − | NA | λ |
| 8 | + | NA | − | − | + | − | − | NA |
| Case no. . | CD20 . | CD3 . | CD5 . | CD10 . | sigM . | sigD . | CD23 . | Light chain . |
|---|---|---|---|---|---|---|---|---|
| 1 | + | NA | − | − | + | − | − | κ |
| 2 | + | NA | − | − | (+) | − | − | NA |
| 3 | + | NA | − | − | − | − | − | κ |
| 4 | + | − | − | − | + | NA | NA | κ |
| 5 | + | − | − | − | + | NA | NA | λ |
| 6 | + | − | −/+ | − | + | + | NA | κ |
| 7 | + | − | − | − | − | − | NA | λ |
| 8 | + | NA | − | − | + | − | − | NA |
For abbreviations, see Table 1.
Usage of VH, DH, and JH genes
Table 3 shows the number of tumor-derived clones identified among the total number of clones sequenced for each sample. Sequences not related differed individually and were likely derived from contaminating normal B cells. In Table 3, the VH gene used by each tumor and its homology with the germ line counterpart are also reported. In 4 patients, VHgene segments were derived from the VH3 family, in 3 patients they were derived from the VH4 family, and in one patient they were derived from the VH2 family. No bias with respect to the previously reported27 preferential use of the segments VH 4-34 and VH 1-69 was observed. Assignment of D gene segments was based on the homology between CDR3 sequences and the germ line D genes. Adopting the rules proposed by Corbett et al,28 which require at least 10 consecutive nucleotides of identity to assign a D segment, a D gene was identified in only 3 patients (patients 2, 3, 7). In 4 patients (patients 4-6, 8), the assignment was based on less stringent rules29: minimal homology of 6 matches in a row or 7 matches interrupted by one mismatch. Priority was given to the homology with a D segment when both D and JH homologous regions overlapped (only in patients 7 and 8). In patient 1, no rule could support D-segment assignment. JH4 was used in 2 patients, whereas in 2 patients sequence homology was found with the JH6 segment. JH4 is the most commonly used JH gene segment at all stages of ontogeny,30 but the small number of patients with an assignable germ line JH segment did not allow us to make any conclusion about the relative frequency of this usage. No JH segment could be assigned to patients 2 and 4. CDR3s did not show remarkable similarities in lengths or sequence.
Analysis of tumor-derived VH genes
| Case no. . | Patient . | Clone . | VH family . | Gem line V gene . | Homology (%) . | R/S CDR2 . | Mutation FW3 . | D segment . | JH segment . | Tumor-derived clones/clones sequenced . | Frame . | CDR2 R:S ratio . | FW3 R:S ratio . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PE | M4 | VH3 | 3 -30 | 90 | 4/1 | 6/3 | — | JH6 | 10/14 | In | 4 | 2 |
| 2 | GM | M5 | VH2 | 2 -26 | 91 | 4/0 | 5/3 | 3 -10 | — | 11/13 | In | 4 | 1.6 |
| 3 | BA | M7 | VH4 | 4-59/4-61 | 100 | — | — | 3 -3 | JH6c | 12/13 | In | — | — |
| 4 | ML | M14-27 | VH4 | 4 -39 | 94 | 1/1 | 4/3 | (1 -26) | — | 26/28 | In | 1 | 1.3 |
| 5 | FMC | M15 | VH4 | 4 -34 | 83 | 7/1 | 10/7 | (1 -14) | JH4a/b | 11/11 | In | 7 | 1.4 |
| 6 | CG | M19 | VH3 | 3 -23 | 100 | — | — | (6 -25) | JH4d | 7/7 | In | — | — |
| 7 | JS | M20-28 | VH3 | 3 -15 | 93 | 2/3 | 4/2 | 6 -19 | — | 25/26 | In | 0.7 | 2 |
| 8 | ON | M22 | VH3 | 3 -48 | 96 | 4/0 | 2/0 | (1-1/1-20) | — | 15/15 | In | 4 | 2 |
| Case no. . | Patient . | Clone . | VH family . | Gem line V gene . | Homology (%) . | R/S CDR2 . | Mutation FW3 . | D segment . | JH segment . | Tumor-derived clones/clones sequenced . | Frame . | CDR2 R:S ratio . | FW3 R:S ratio . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PE | M4 | VH3 | 3 -30 | 90 | 4/1 | 6/3 | — | JH6 | 10/14 | In | 4 | 2 |
| 2 | GM | M5 | VH2 | 2 -26 | 91 | 4/0 | 5/3 | 3 -10 | — | 11/13 | In | 4 | 1.6 |
| 3 | BA | M7 | VH4 | 4-59/4-61 | 100 | — | — | 3 -3 | JH6c | 12/13 | In | — | — |
| 4 | ML | M14-27 | VH4 | 4 -39 | 94 | 1/1 | 4/3 | (1 -26) | — | 26/28 | In | 1 | 1.3 |
| 5 | FMC | M15 | VH4 | 4 -34 | 83 | 7/1 | 10/7 | (1 -14) | JH4a/b | 11/11 | In | 7 | 1.4 |
| 6 | CG | M19 | VH3 | 3 -23 | 100 | — | — | (6 -25) | JH4d | 7/7 | In | — | — |
| 7 | JS | M20-28 | VH3 | 3 -15 | 93 | 2/3 | 4/2 | 6 -19 | — | 25/26 | In | 0.7 | 2 |
| 8 | ON | M22 | VH3 | 3 -48 | 96 | 4/0 | 2/0 | (1-1/1-20) | — | 15/15 | In | 4 | 2 |
NA indicates not assessed.
Somatic hypermutations in tumor-related VHgenes
Table 3 summarizes mutation patterns of the VH region. In all but 2 patients, the sequences analyzed contained somatic mutations with respect to the closest germ line sequences. The percentage of sequence homology ranged from 83% to 96% (Figure1). Most hypermutation events consisted of single base changes, but double and triple substitutions also frequently occurred, mostly in the same codon. In addition to point mutations, patient 5 displayed a multiple-base segment insertion (Figure 1) in the presence of a maintained open-reading frame, an event believed to be linked to the hypermutation process.31 The demonstration of this finding in our small sample, according to the value found in normal germinal center B cells,30 further suggests the relevance of the hypermutation process in this patient. Two patients (patients 3 and 6), as stated before, showed no somatic mutations with respect to the germ line VHsegment.
Nucleotide and deduced amino acid sequences of VH genes derived from nodal MZLs.
Comparisons were made with the most homologous germ line VHgenes. Dashes represent identity with the representative germ line sequence. Nucleotide differences are shown in lowercase letters whenever a replacement mutation is concerned. Silent mutations are shown in capital letters. Replacement amino acids are also indicated at the bottom of each scheme. One insertion (case no. 5) is shown in FW2 as extra nucleotides.
Nucleotide and deduced amino acid sequences of VH genes derived from nodal MZLs.
Comparisons were made with the most homologous germ line VHgenes. Dashes represent identity with the representative germ line sequence. Nucleotide differences are shown in lowercase letters whenever a replacement mutation is concerned. Silent mutations are shown in capital letters. Replacement amino acids are also indicated at the bottom of each scheme. One insertion (case no. 5) is shown in FW2 as extra nucleotides.
Antigen selection and coding capacity
As already stated, 2 methods were applied for the estimation of antigen selection in the mutated B cells. The 2 methods agreed in identifying antigen selection criteria in one patient (patient 5). Two other patients (patients 2 and 4) only satisfied the second method criteria. On the contrary, the 3 remaining patients with mutations lacked signs of antigen selection considering both methods. All patients analyzed showed a functional VH rearrangement, devoid of stop codons.
Intraclonal nucleotide sequence variation
Extensive molecular cloning was applied to search for intraclonal heterogeneity. In 2 patients (patients 2 and 7), the extensively mutated clonal VH gene isolates did not show intraclonal heterogeneity. In patient 7, the analyses were applied to DNA extracted from both lymph node and peripheral blood of the patient, but no confirmed mutation was assessed.
In all 4 remaining patients bearing somatic mutations (patients 1, 4, 5, 8), intraclonal variability was documented according to the stringent criteria applied. In patient 4, the investigations were extended to genomic DNA extracted from lymph node and bone marrow samples. This approach allowed us to detect intraclonal variations.
Discussion
Analyses of antigen-receptor genes in human lymphoma may represent a useful tool in understanding their pathogenesis and clonal history, and it may also produce clinically relevant information.32 33 The present study was undertaken to better define the somatic mutation pattern of immunoglobulin VH genes in nodal MZLs to identify the possible progenitor cells of this tumor.
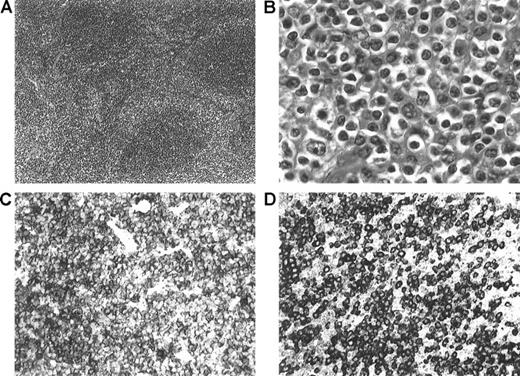
The rare and controversial nature of this entity suggests the need for stringent diagnostic criteria. In our patients, diagnoses were made according to the REAL/WHO classification, taking into account (Figure2) the morphologic (a typical marginal zone pattern assessed in all patients), immunophenotypical, and clinical features.
Morphologic and immunophenotypic features of nodal MZL.
(A) Low-power view showing a parafollicular/perisinusoidal distribution of the neoplastic cells that surround some residual B-cell follicles (hematoxylin and eosin staining (H&E), × 100). (B) High-power view showing marginal zone/monocytoid B cells with medium-sized round (to irregular) nuclei and abundant pale cytoplasm (H&E, × 100). (C) CD20 staining showing strong positivity of the neoplastic cells (× 400). (D) Immunostaining for IgM, showing strong surface staining of the neoplastic lymphocytes (× 400).
Morphologic and immunophenotypic features of nodal MZL.
(A) Low-power view showing a parafollicular/perisinusoidal distribution of the neoplastic cells that surround some residual B-cell follicles (hematoxylin and eosin staining (H&E), × 100). (B) High-power view showing marginal zone/monocytoid B cells with medium-sized round (to irregular) nuclei and abundant pale cytoplasm (H&E, × 100). (C) CD20 staining showing strong positivity of the neoplastic cells (× 400). (D) Immunostaining for IgM, showing strong surface staining of the neoplastic lymphocytes (× 400).
Seven of 8 patients had an immunophenotype clearly consistent with the diagnosis of nodal MZL. The remaining patient (patient 6) exhibited an atypical pattern with concomitant slight CD5 and immunoglobulin D (IgD) expression. However, CD5+ MZLs have already been described,7,34 and the concurrent IgD expression has also been reported in the nodal MZL, although it is more frequent in splenic MZL.35 The main clinical characteristics at diagnosis, including the rate of bone marrow involvement, were consistent with those reported in the literature.36-39
Based on the analysis of the VH gene rearrangements in our series, nodal MZLs appear as a heterogeneous entity with respect to the histogenetic derivation. Indeed, MZL seems to arise from different subsets of marginal zone B cells—virgin B cells that express unmutated VH genes, memory B cells showing somatic mutations, and germinal center B cells defined by their capacity to undergo the somatic hypermutation process. Analysis of the VH genes in nonneoplastic marginal zone B cells has also produced contradictory results. Indeed, the possible presence of both virgin B cells and hypermutated B cells has been reported in nodal marginal zone B cells40 and in monocytoid B cells,26suggesting the presence of different modalities for the recruitment of B cells in the marginal zone. It is of interest that 3 distinct pathways have been shown in animal models: (1) maturation of virgin recirculating B cells, a process that occurs in the absence of T cells and does not require B-cell proliferation; (2) colonization by memory B cells generated in germinal centers; (3) recruitment of both virgin and memory marginal zone B cells into antibody responses.41
Previous studies devoted to the genetic analysis of the antigen receptor in nodal MZL have failed to show any intraclonal variability, probably because the approach of amplifying the VH genes from cell populations, followed by direct sequencing of the PCR products, likely prevented the detection of potential ongoing mutations within the tumor cells.21-23
Whether the subsets of nodal MZLs showing intraclonal heterogeneity or homogeneous somatic mutations originate from germinal center cells and post–germinal center cells, respectively, or whether the transforming events may render the cell independent of the influence of germinal center microenvironment is unclear. Because neither nodal MZLs nor extranodal MZLs of MALT type display immunophenotypical characteristics of germinal center cells, it could be speculated that the tumor cells located in the direct vicinity of germinal centers might be affected by the mutation machinery active in these neighboring germinal centers.
Analysis of mutations in VH genes can provide insight concerning the role of antigen before or during lymphoma clonal outgrowth.10 All our patients showed a potentially functional VH rearrangement. Six patients had characteristics of the presence of somatic mutations. In one patient, as previously described in normal B cells and in follicular lymphoma,42 insertion events have been observed at the coding region of the VH genes. In one patient bearing somatic mutations, there was evidence of positive selection as demonstrated by analysis of R/S ratios in CDR2 and FW3; the low rate of R mutations in FRW3 also suggested the negative selection of nonfunctional immunoglobulin in this tumor. In 2 other cases the pattern of somatic mutation in FW3 is consistent with the structural conservation of the antigen receptor. These findings represent the prerequisites for the identification of an antigen-driven process in that prolonged exposure to an antigen may play a role in the pathogenesis of the lymphoma. A major question is, what is the responsible antigen. Together with follicular center, diffuse, large B-cell lymphoma and extranodal MZL, monocytoid B-cell lymphoma was most frequently associated with hepatitis C virus (HCV) infection.27 43 Our small series of patients, characterized by low prevalence of HCV infection, cannot give biologic insights into this subject.
Several lines of evidence suggest the role of an autoimmune process in the pathogenesis of extranodal MZL,19,20 but no significant data support the role of autoantigens in the growth and persistence of the nodal MZL. In our series, no homologies with known autoantibody sequences were detected, nor was there a preferential usage of the specific VH segments previously involved in autoimmune processes and described in HCV-negative nodal MZLs.27
The heterogeneous pattern of VH gene somatic mutations observed in our series seems to reflect the heterogeneity described in the immunophenotypic profile of primary nodal MZLs, with some cases resembling splenic MZL.35 Interestingly, patient 6, one of the few to lack evidence of somatic mutations, expressed surface IgD. The identification of different subsets of nodal MZLs with respect to the VH mutational status seems to indicate that heterogeneous entities may fall under the definition of nodal MZLs, as already suggested on the basis of the immunophenotypic findings.35 The possible clinical implications of these different geno-phenotypic patterns are still not ascertained and warrant further investigation.
We thank Miss Michela Gisi for her expert technical assistance, Dr Claudio Realini for helpful discussion, Dr Andrea Rossi for help with clinical data, and Dr Lewis Rowett for reviewing the manuscript.
Supported in part by the Fondazione Ticinese per la Ricerca sul Cancro and by the Fondazione “San Salvatore.” A.C. is supported by an ESMO Fellowship grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Emanuele Zucca, Division of Medical Oncology, Oncology Institute of Southern Switzerland, Ospedale San Giovanni, 6500 Bellinzona, Switzerland; e-mail: ielsg@ticino.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal