Abstract
During immunosuppression, cytomegalovirus (CMV) can reactivate and cause serious clinical problems. Normally, abundant virus replication is suppressed by immune effector mechanisms. To study the interaction between CD8+ T cells and persisting viruses, frequencies and phenotypes of CMV-specific CD8+ T cells were determined in healthy individuals and compared to those in renal transplant recipients. In healthy donors, function of circulating virus-specific CD8+ T cells, as measured by peptide-induced interferon γ (IFN-γ) production, but not the number of virus-specific T cells enumerated by binding of specific tetrameric peptide/HLA complexes, correlated with the number of CMV-specific IFN-γ–secreting CD4+ helper T cells. Circulating CMV- specific CD8+ T cells did not express CCR7 and may therefore not be able to recirculate through peripheral lymph nodes. Based on coexpression of CD27 and CD45R0 most CMV-specific T cells in healthy donors appeared to be memory-type cells. Remarkably, frequencies of CMV-specific CD8+ T cells were significantly higher in immunosuppressed individuals than in healthy donors. In these patients CMV-specific cells predominantly had an effector phenotype, that is, CD45R0+CD27−CCR7− or CD45RA+CD27−CCR7− and contained both granzyme B and perforin. Our data show that in response to immunosuppressive medication quantitative and qualitative changes occur in the CD8+ T-cell compartment. These adaptations may be instrumental to maintain CMV latency.
Introduction
The immunologic control of persistent viral infections, like infections with the family of herpes viruses, requires the coordinated actions of many cell types.1CD4+ T cells appear to play a key role in this process because they orchestrate the various effector arms of the immune system. In mice it has been shown that the production of neutralizing antibodies to many viruses critically depends on the availability of specific CD4+ helper T cells.2,3 In line with this, we recently demonstrated that in primary human cytomegalovirus (CMV) infection the emergence of helper T cells precedes the appearance of virus-specific antibodies.4 CD8+ T cells eliminate virus-infected cells and are thought to be the major effector cells in controlling persistent infection. In many instances, differentiation of CD8+ T cells into competent effector cells depends on the presence of helper CD4+ T cells.5 6
On encountering viruses, CD8+ T cells can differentiate from naive T cells into effector T cells that through cytolysis and secretion of specific cytokines control virus replication and into memory cells that provide enhanced immunity after renewed contact with the same pathogen. To study the development of these cells in clinically relevant situations in humans, various phenotypic markers have been used that allow separation of the aforementioned subsets. Naive CD8+ T cells express CD45RA and CD62L and also the costimulatory receptors CD27 and CD28, whereas memory-type cells express CD45R0, CD27, and CD28 molecules and low levels of CD62L. Effector-type cells have a CD45RA+CD27−CD28− phenotype and contain perforin, granzyme B, and CD95 ligand to directly execute cytolysis.7,8 More recently, Sallusto and coworkers9 separated subsets of peripheral blood CD4+ and CD8+ T cells based on the expression of CCR7, which binds secondary lymphoid chemokine (SLC) and macrophage inflammatory protein 3γ (MIP-3γ), chemokines that direct migration of lymphocytes to secondary lymphoid organs. CD8+CCR7+CD45RA− T cells, designated central memory cells (TCM), have the potential to migrate to peripheral lymph nodes and do not directly produce cytokines after in vitro stimulation. Effector memory cells (TEM) do not express CCR7 and have perforin-containing granules and may directly exert effector functions in peripheral tissues. It thus appears that the regulation of CD27 and CCR7 is concordant in CD8+ T cells, but this has not yet been directly addressed.
Human CMV is a persistent β-herpesvirus that is present in approximately 50% of the adult population. Latent infection is asymptomatic in healthy individuals but can cause serious disease in immunocompromised individuals. Severely impaired T-cell function leads to viral reactivation and morbidity due to cytopathic effects of uncontrolled viral replication, and CMV reactivation coincides with higher levels of immunosuppressive therapy. Interestingly, however, most renal transplant recipients who receive medication for adequate suppression of the allogeneic immune response do not suffer from episodes of clinically apparent CMV reactivation and therefore appear to efficiently control overt virus replication. Recent technologic developments such as the generation of tetrameric major histocompatibility complex (MHC)–peptide complexes and peptide-induced interferon γ (IFN-γ) production allow direct measurement of frequencies and functions of virus-specific T cells. We used these advancements to characterize the dynamic interaction between cellular immune responses and persisting CMV in healthy individuals and in asymptomatic renal transplant recipients on basic immunosuppressive drug therapy.
Materials and methods
Subjects
For transsectional studies, HLA-A2+, CMV-seropositive individuals without immunosuppressive therapy (n = 13) were selected from healthy personnel members and the Academic Medical Center nephrology outpatient clinic, as well as HLA-A2+, CMV-seropositive renal transplant recipients on immunosuppressive drug therapy (n = 7) from the Academic Medical Center transplantation outpatient clinic (Table1). Immunosuppressive therapy in renal transplant recipients consisted of cyclosporin A, blood trough levels aimed at 150 ng/mL, and prednisone 10 mg daily.
Characteristics of subjects
| Group . | No. . | Age (mean) . | Time after Tx (mean, months) . | Rejections (no. patients) . | Rejection treatment . | CMV-reactivation* (no. patients) . | Positive urine cultures (no. patients) . |
|---|---|---|---|---|---|---|---|
| Controls | 13 | 42 | na | na | na | na | na |
| Transsectional RTx patients | 7 | 58 | 36, 5 | 2 | corticosteroids | 0 | 4 |
| Longitudinal RTx patients | 6 | 47 | 12 | 1 | corticosteroids | 0 | 2 |
| Group . | No. . | Age (mean) . | Time after Tx (mean, months) . | Rejections (no. patients) . | Rejection treatment . | CMV-reactivation* (no. patients) . | Positive urine cultures (no. patients) . |
|---|---|---|---|---|---|---|---|
| Controls | 13 | 42 | na | na | na | na | na |
| Transsectional RTx patients | 7 | 58 | 36, 5 | 2 | corticosteroids | 0 | 4 |
| Longitudinal RTx patients | 6 | 47 | 12 | 1 | corticosteroids | 0 | 2 |
CMV indicates cytomegalovirus; na, not applicable; RTx, renal transplantation; PCR, polymerase chain reaction.
As defined by positive PCR or 4-fold rise in specific antibody titer.
For the longitudinal study, 6 HLA-A2+, CMV-seropositive renal transplant recipients were selected. Heparinized peripheral blood samples were collected before transplantation and 12 months after transplantation. Peripheral blood mononuclear cells (PBMCs) were isolated using standard density gradient centrifugation techniques as described by Rentenaar and colleagues.10 All patients gave written informed consent and the study was approved by the local medical ethical committee.
Generation of HLA-A2.1/CMVpp65(nlvpmvatv)tetrameric complexes
Tetrameric complexes were generated essentially as described by Altman and coworkers.11 In brief, purified HLA-A2.1 heavy chain and β2-microglobulin was synthesized using a prokaryotic expression system (pET; Novagen, Milwaukee, WI). The heavy chain was modified by deletion of the transmembrane/cytosolic tail and COOH-terminal addition of a sequence containing the BirA enzymatic biotinylation site. The HLA-A2.1–binding CMVpp65-derived peptide NLVPMVATV (single-letter amino acid codes) was used for refolding. The refolded product was isolated using high-performance liquid chromatography (HPLC), biotinylated by BirA (expressed using the pET expression system, purified using Clontech cobalt beads, Palo Alto, CA) in the presence of biotin (Molecular Probes, Eugene, OR), adenosine triphosphate (ATP; Sigma Chemical, St Louis, MO), and MgCl. The biotinylated product was separated from free biotin by gel filtration using HPLC.12 Streptavidin-phycoerythrin (PE) or streptavidin-allophycocyanin (APC) conjugate (Molecular Probes) was added in a 1:4 molar ratio.
Immunofluorescentstaining and flow cytometry
Freshly isolated or thawed PBMCs were resuspended in RPMI, containing 10% fetal calf serum (FCS) and antibiotics. Half a million PBMCs were incubated with fluorescent-labeled conjugated monoclonal antibodies (mAbs; concentrations according to manufacturer's instructions) and an appropriate concentration of tetrameric complexes in a small volume for 30 minutes at 4°C, protected from light. To validate specificity of the HLA-A2.1/CMVpp65(nlvpmvatv)a control HLA-A2.1 tetramer containing the HIV-specificSLYNTVATL peptide (a gift from Stefan Kostense, Central Laboratory for Blood Transfusion (CLB), Amsterdam, The Netherlands) was used as negative control. Additional negative controls consisted of HLA-A2.1− CMV-seropositive or HLA-A2.1+ CMV-seronegative healthy individuals and renal transplant recipients. Negative controls always showed tetramer staining of less than 0.01% of total lymphocytes (data not shown). Cells were washed in phosphate-buffered saline (PBS) containing 0.01% (w/v) NaN3 and 0.5% (w/v) bovine serum albumin (PBA). For staining with the mouse antihuman CCR7 mAb, a 3-step staining protocol was performed consisting of incubation with the anti-CCR7 antibody (a gift from Lijun Wu, Leukosite, Cambridge, MA) for 30 minutes at 4°C protected from light, washing, incubation with PE-conjugated goat anti–mouse immunoglobulin (Ig; Southern Biotechnology, Birmingham AL) for 30 minutes at 4°C protected from light, incubation with 10% (v/v) normal mouse serum (CLB, Amsterdam, The Netherlands), followed by incubation with directly conjugated mAbs and tetrameric complexes for 30 minutes at 4°C protected from light. Analyses consisted of APC-conjugated tetramers and CD8-PerCP (Becton Dickinson, San Jose, CA) in combinations with either CD45RA–fluorescein isothiocyanate (FITC; Becton Dickinson) and CD27-PE (Becton Dickinson), anti-CCR7 and CD45RA-FITC (Becton Dickinson), CD94-PE (Pharmingen, San Diego, CA), NKB-1–PE (Becton Dickinson), CD158a-PE, or CD158b-PE (both Immunotech, Marseilles, France). Analysis of cells for the expression of cell surface markers was performed using a FACS Calibur flow cytometer and Cellquest software (Becton Dickinson). In addition, CCR7 expression on CD8+T cells was analyzed by flow cytometry, using goat antimouse Ig-FITC (CLB) in conjunction with either CD8-PerCP (both Becton Dickinson), CD45RA-PE, CD27-biotin (both CLB), and streptavidin-APC (Pharmingen) or CD28-PE and CD45R0-APC (both Becton Dickinson).
Determination of CMV-specific CD4+and CD8+T cells by intracellular cytokine staining
The CMV-specific CD4+ and CD8+ T-cell frequencies were determined essentially according to the method described by Waldrop and coworkers13 and Kern and colleagues,14respectively. Briefly, 106 freshly isolated PBMCs were incubated for 6 hours in the presence of either CMV antigen (Biowhittaker, Wokingham, United Kingdom, 60 μL/mL), control antigen (Biowhittaker, 60 μL/mL, negative control) (determination of CMV-specific CD4+ T cells), Staphylococcus aureus enterotoxin B (SEB, ICN/Fluka, 2 μg/mL, positive control), the HLA-A2–binding CMV peptide or the HLA-B7–binding CMV peptide (negative control), or the HLA-A2–binding HIV peptide (negative control) (see below, final concentration of 10 μg/mL, determination of CMV-specific CD8+ T cells). CD28 mAb (clone 15E8 CLB) was added as 3 μg/mL (final concentration) in a final volume of 2 mL/tube RPMI 1640 (Gibco, Paisley, United Kingdom) containing 10% heat inactivated FCS (Integro, Zaandam, The Netherlands), penicillin, and streptomycin. For the final 5 hours of culture, brefeldin A (Sigma) was added to the culture in a final concentration of 10 μg/mL. Cells were transferred to FACS tubes, fixed in 2 mL/tube FACS lysing solution (Becton Dickinson), permeabilized in 0.5 mL/tube FACS permeabilizing solution followed by (intracellular) staining with IFN-γ–FITC (Becton Dickinson) and CD69-PE (Becton Dickinson) and CD4-APC (Becton Dickinson) or CD8-APC (21C008AX, clone UCHT-4, Imgen, ITK, Uithoorn, The Netherlands). Cells were washed in PBA and refixed in Cellfix (Becton Dickinson) until flow cytometric analysis the following day. Flow cytometric analysis was performed using a FACS Calibur equipped with a 488-nm argon ion laser and a 635-nm red diode laser. Data files containing 50 000 events positive for CD4-APC or CD8-APC fluorescence within a lymphocyte gate were saved. Frequencies of CD69+IFN-γ+ cells within the CD4+ or CD8+lymphocyte gate were determined using Cellquest software (Becton Dickinson) and designated CMV-specific CD4+ or CD8+ T-cell frequencies, respectively. Negative controls showed less than 0.05% of CD69+IFN-γ+ cells (data not shown).
Peptides
The HLA-A2 binding CMVpp65-derived peptide NLVPMVATV and the HLA-B7 binding CMVpp65-derived peptide TPRVTGGA were purchased from the IHB-LUMC peptide synthesis library facility (Leiden, The Netherlands). The HIV Gag p17-derived peptide SLYNTVATL was kindly provided by Stefan Kostense (CLB, Amsterdam, The Netherlands). The peptides were generated by standard Fmoc techniques and purified by ether precipitation and HPLC techniques. The peptides were dissolved in dimethylsulfoxide (DMSO; Merck, Darmstadt, Germany) in a concentration of 5 mg/mL.
Intracellular granzyme B and perforin staining
Intracellular granzyme B and perforin staining was performed as described previously.16 In short, half a million PBMCs were stained with fluorescent-labeled conjugated mAbs to CD8 (Becton Dickinson), CD27 (Becton Dickinson), and CMV-tetrameric complexes, washed once with PBA, then fixed with 50 μL buffered formaldehyde acetone solution and subsequently permeabilized by washing with 0.1% saponine 50 mM D-glucose. Cells were then incubated with antigranzyme B (CLB) and antiperforin antibodies (Hölzel Diagnostika, Köln, Germany) according to manufacturers' instructions. Flow cytometric analysis was performed immediately.
CMV–polymerase chain reaction
Quantitative polymerase chain reaction (PCR) was performed in EDTA whole blood samples as described for plasma or serum.4
Viralculture
Viral culture was done by cocultivation of urine and human diploid fibroblasts. Microscopic examination for the appearance of CMV-specific cytopathologic effects was performed.
Anti-CMV IgG
Anti-CMV IgG was determined in serum using the AxSYM microparticle enzyme immunoassay (Abbott, Abbott Park, IL) according to the manufacturer's instructions. Measurements were calibrated relative to a standard serum. Results are expressed as a ratio of the measurement to a standard serum (IgM).
Statisticalanalysis
Between-group analysis was performed using the nonparametric Mann-Whitney test. Within-group analysis was performed using the Wilcoxon signed rank test. Two-sided testing was done; Pvalues lower than .05 were considered statistically significant.
Results
Correlations of CMV-specific CD4+and CD8+T-cell frequencies in healthy individuals
As previously demonstrated by us and others, CMV-specific CD4+ T cells can readily be detected in the circulation of healthy virus carriers using CMV antigen-induced IFN-γ production as read-out (Figure 1A). Likewise, CD8+ T cells could be visualized using the immunodominant CMV peptide in HLA-A2+ donors (Figure 1B). Frequencies of IFN-γ–producing cells within the CD8+ subset ranged between 0.18% and 0.80%. (Figure 2A). To directly visualize CMV-specific T cells, tetrameric HLA-A2.1/NLVPMVATV complexes were generated. These complexes bound 0.54% to 3.77% of CD8+ T cells (Figures 1C, 2B). Accordingly, only on average 20% of the peptide-specific T cells in these chronic virus carriers were able to secrete IFN-γ in this short-term activation assay. Interestingly, in line with studies in mice, CD4 helper T-cell frequencies correlated with the percentage of CD8+ T cells secreting IFN-γ after peptide stimulation (Figure 1D, r = 0.7982, P < .05) but not with the amount of specific tetramer binding cells (data not shown).
CMV-specific T-cell frequencies in healthy individuals.
(A) Dot plot of anti–IFN-γ–FITC fluorescence (x-axis, arbitrary units, log scale) versus CD69-PE fluorescence (y-axis, arbitrary units, log scale) of lymphocytes gated on positive CD4-APC fluorescence after incubation with CMV antigen. (B) Dot plot of anti–IFN-γ–FITC fluorescence (x-axis, arbitrary units, log scale) versus CD69-PE fluorescence (y-axis, arbitrary units, log scale) of lymphocytes gated on bright positive CD8-APC fluorescence after incubation with the HLA-A2–binding CMV-pp65 peptide NLVPMVATV. (C) NLVPMVATV-HLA-A2.1/tetramer-APC fluorescence (“tetramer,” x-axis, arbitrary units, log scale) versus CD8-PerCP fluorescence (y-axis, arbitrary units, log scale) of lymphocytes gated on forward scatter and side scatter parameters. (D) Correlation of the frequency of IFN-γ–producing CMV-specific CD4+ T cells (x-axis, percentage of CD4+ T cells) and IFN-γ–producing HLA-*0201 restricted CMVpp65(NLVPMVATV)-specific CD8+ T cells (y-axis, percentage of CD8+ T cells) in HLA-A2+CMV-seropositive healthy individuals (n = 7). IFN-γ–producing CMV-specific CD4+ T-cell frequencies correlate with IFN-γ–producing CMV-specific CD8+ T cells (r = 0.7928, P = .0480).
CMV-specific T-cell frequencies in healthy individuals.
(A) Dot plot of anti–IFN-γ–FITC fluorescence (x-axis, arbitrary units, log scale) versus CD69-PE fluorescence (y-axis, arbitrary units, log scale) of lymphocytes gated on positive CD4-APC fluorescence after incubation with CMV antigen. (B) Dot plot of anti–IFN-γ–FITC fluorescence (x-axis, arbitrary units, log scale) versus CD69-PE fluorescence (y-axis, arbitrary units, log scale) of lymphocytes gated on bright positive CD8-APC fluorescence after incubation with the HLA-A2–binding CMV-pp65 peptide NLVPMVATV. (C) NLVPMVATV-HLA-A2.1/tetramer-APC fluorescence (“tetramer,” x-axis, arbitrary units, log scale) versus CD8-PerCP fluorescence (y-axis, arbitrary units, log scale) of lymphocytes gated on forward scatter and side scatter parameters. (D) Correlation of the frequency of IFN-γ–producing CMV-specific CD4+ T cells (x-axis, percentage of CD4+ T cells) and IFN-γ–producing HLA-*0201 restricted CMVpp65(NLVPMVATV)-specific CD8+ T cells (y-axis, percentage of CD8+ T cells) in HLA-A2+CMV-seropositive healthy individuals (n = 7). IFN-γ–producing CMV-specific CD4+ T-cell frequencies correlate with IFN-γ–producing CMV-specific CD8+ T cells (r = 0.7928, P = .0480).
Renal transplant recipients display higher frequencies of CMV-specific T cells.
(A) Frequencies of IFN-γ–producing CMV-specific CD8+ T cells (y-axis, IFN-γ+CD69+ cells as percentage of CD8+ T cells) in CMV-seropositive HLA-A2+ healthy individuals (○) and CMV-seropositive HLA-A2+ renal transplant recipients (●). Frequencies of these CMV-specific CD8+ T cells were higher in renal transplant recipients (Mann-Whitney, P = .0082). (B) Frequencies of NLVPMVATV–HLA-A2.1 tetramer binding CD8+ T cells (y-axis, percentage of CD8+ T cells) in CMV-seropositive HLA-A2+ control individuals without immunosuppression (○) and in CMV-seropositive HLA-A2+patients after renal transplantation (●). Frequencies of these CMV-specific CD8+ T cells were higher in renal transplant recipients (Mann-Whitney, P = .0056). (C) Frequencies of CD27− cells among CMV-specific CD8+ T cells (y-axis, percentage of tetramer-positive CD8+ T cells) in CMV-seropositive HLA-A2+ control individuals (○) and renal transplant recipients (●). Frequencies of CD27−cells among tetramer-positive CD8+ T cells were statistically significantly different in renal transplant recipients compared to control individuals (Mann-Whitney,P = .0216).
Renal transplant recipients display higher frequencies of CMV-specific T cells.
(A) Frequencies of IFN-γ–producing CMV-specific CD8+ T cells (y-axis, IFN-γ+CD69+ cells as percentage of CD8+ T cells) in CMV-seropositive HLA-A2+ healthy individuals (○) and CMV-seropositive HLA-A2+ renal transplant recipients (●). Frequencies of these CMV-specific CD8+ T cells were higher in renal transplant recipients (Mann-Whitney, P = .0082). (B) Frequencies of NLVPMVATV–HLA-A2.1 tetramer binding CD8+ T cells (y-axis, percentage of CD8+ T cells) in CMV-seropositive HLA-A2+ control individuals without immunosuppression (○) and in CMV-seropositive HLA-A2+patients after renal transplantation (●). Frequencies of these CMV-specific CD8+ T cells were higher in renal transplant recipients (Mann-Whitney, P = .0056). (C) Frequencies of CD27− cells among CMV-specific CD8+ T cells (y-axis, percentage of tetramer-positive CD8+ T cells) in CMV-seropositive HLA-A2+ control individuals (○) and renal transplant recipients (●). Frequencies of CD27−cells among tetramer-positive CD8+ T cells were statistically significantly different in renal transplant recipients compared to control individuals (Mann-Whitney,P = .0216).
Cell surface phenotype of CMV-specific CD8+T cells in healthy individuals
On the basis of functional similarities of the subsets defined by CD27 and CCR7,7,9 one would expect that these molecules are being expressed in a concordant way. The localization of CCR7− cells in CD8+ T-cell subsets defined by CD27 and CD45RA expression was determined (Figure3C). Confirming recently published data,17 most (mean 97.0%, n = 4 healthy donors) effector-type CD45RA+CD27− T cells7 lacked CCR7. Additional analysis showed that most CCR7− T cells also lacked CD28 and CD45R0 molecules (Figure 3D). However, an appreciable number of CCR7− T cells appeared to be contained within the CD27+CD28+CD45R0+ population (mean 33.1%). CCR7− T cells were virtually absent from the naı̈ve T-cell subset (mean 1.5%).
CCR7− CD8+ T cells are among CD27+ and CD27− cells.
(A) Histogram of CD8 expression (x-axis, arbitrary units, log scale) versus cell number (y-axis) in PBMCs from one representative healthy individual. The horizontal line designates the gate for CD8bright lymphocytes. (B) Histogram of CCR7 expression (x-axis, arbitrary units, log scale) versus cell number (y-axis) in gated CD8bright T cells. The horizontal line designates the gate for CCR7− cells (black). (C) Dot plot of CD27 expression (x-axis, arbitrary units, log scale) versus CD45RA expression (y-axis, arbitrary units, log scale) in gated CD8bright T cells. CCR7− cells are represented in black, whereas CCR7+ cells are represented in gray. (D) Dot plot of CD28 expression (x-axis, arbitrary units) versus CD45R0 expression (y-axis, arbitrary units) in gated CD8bright T cells. CCR7− cells are represented in black, whereas CCR7+ cells are represented in gray.
CCR7− CD8+ T cells are among CD27+ and CD27− cells.
(A) Histogram of CD8 expression (x-axis, arbitrary units, log scale) versus cell number (y-axis) in PBMCs from one representative healthy individual. The horizontal line designates the gate for CD8bright lymphocytes. (B) Histogram of CCR7 expression (x-axis, arbitrary units, log scale) versus cell number (y-axis) in gated CD8bright T cells. The horizontal line designates the gate for CCR7− cells (black). (C) Dot plot of CD27 expression (x-axis, arbitrary units, log scale) versus CD45RA expression (y-axis, arbitrary units, log scale) in gated CD8bright T cells. CCR7− cells are represented in black, whereas CCR7+ cells are represented in gray. (D) Dot plot of CD28 expression (x-axis, arbitrary units) versus CD45R0 expression (y-axis, arbitrary units) in gated CD8bright T cells. CCR7− cells are represented in black, whereas CCR7+ cells are represented in gray.
To test whether in antigen-specific T cells expression of the various markers is comparable to that in the total population, CMV-specific T cells (Figure 4A) were analyzed for CD27, CCR7, and CD45RA coexpression. In all donors, CMV-specific CD8+ T cells were of the TEMtype,9 that is, lacked expression of CCR7 (Figure 4B). Remarkably, however, with respect to the expression of CD27 and CD45RA molecules heterogeneous phenotypes were found. In most healthy donors, CMV-specific T cells appeared to be predominantly memory cells, that is, CD27+CD45RA− (Figure 4C, right panel). However, in 3 donors mostly CD27−CD45RA+effector cells were found (Figure 4C, left panel). In all donors presence or absence of CD27 strictly correlated with the expression of CD28 (data not shown). The phenotypes of CMV-specific T cells in individual donors were stable in time (data not shown).
CMV-specific CD8+ T cells in healthy CMV carriers are CCR7− but can be either CD27+CD45RA− memory cells or CD27−CD45RA+ effector cells.
Dot plots and photographs are from 2 healthy CMV-seropositive, HLA-A2+ individuals; left, donor L; right, donor B. (A) NLVPMVATV–HLA-A2.1 tetramer-APC fluorescence (“tetramer,” x-axis, arbitrary units, log scale) versus CD8-PerCP fluorescence (y-axis, arbitrary units) of lymphocytes gated on forward-scatter and side-scatter parameters. The quadrangles represent the gates for the analyses in panels B and C. Similar gates were used for the experiment represented in panel D. (B) Mouse anti-CCR7/goat anti–mouse-PE fluorescence (x-axis, arbitrary units, log scale) versus CD45RA-FITC fluorescence (y-axis, arbitrary units, log scale) within either gated CD8+ T cells (CD8+) or gated tetramer-positive CD8+ T cells (tetramer+). (C) CD27-PE fluorescence (x-axis, arbitrary units, log scale) versus CD45RA-FITC fluorescence (y-axis, arbitrary units, log scale) within either gated CD8+ T cells (CD8+) or gated tetramer-positive CD8+ T cells (tetramer+). (D) Dot plots gated on tetramer-positive CD8+ T cells from one donor with a predominant effector phenotype (left) and one donor with a memory phenotype (right). CD27-FITC fluorescence (y-axis) versus granzyme B–PE fluorescence (x-axis). (E) Frequencies of granzyme B–positive cells (▪, y-axis, percentage of tetramer-positive CD8+ T cells) from donors with an effector (left bar) or memory (right bar) phenotype.
CMV-specific CD8+ T cells in healthy CMV carriers are CCR7− but can be either CD27+CD45RA− memory cells or CD27−CD45RA+ effector cells.
Dot plots and photographs are from 2 healthy CMV-seropositive, HLA-A2+ individuals; left, donor L; right, donor B. (A) NLVPMVATV–HLA-A2.1 tetramer-APC fluorescence (“tetramer,” x-axis, arbitrary units, log scale) versus CD8-PerCP fluorescence (y-axis, arbitrary units) of lymphocytes gated on forward-scatter and side-scatter parameters. The quadrangles represent the gates for the analyses in panels B and C. Similar gates were used for the experiment represented in panel D. (B) Mouse anti-CCR7/goat anti–mouse-PE fluorescence (x-axis, arbitrary units, log scale) versus CD45RA-FITC fluorescence (y-axis, arbitrary units, log scale) within either gated CD8+ T cells (CD8+) or gated tetramer-positive CD8+ T cells (tetramer+). (C) CD27-PE fluorescence (x-axis, arbitrary units, log scale) versus CD45RA-FITC fluorescence (y-axis, arbitrary units, log scale) within either gated CD8+ T cells (CD8+) or gated tetramer-positive CD8+ T cells (tetramer+). (D) Dot plots gated on tetramer-positive CD8+ T cells from one donor with a predominant effector phenotype (left) and one donor with a memory phenotype (right). CD27-FITC fluorescence (y-axis) versus granzyme B–PE fluorescence (x-axis). (E) Frequencies of granzyme B–positive cells (▪, y-axis, percentage of tetramer-positive CD8+ T cells) from donors with an effector (left bar) or memory (right bar) phenotype.
Cytolytic mediators and natural killer cell receptors in CMV-specific T cells
The absence of CD27 corresponds within the expression of cytolytic mediators such as perforin and granzyme B.7 To establish if this phenomenon holds for virus-specific T cells, CMV-specific CD8+ T cells of a healthy donor with a predominant effector phenotype, and cells of one healthy donor with a predominant memory phenotype were analyzed for granzyme B expression by flow cytometry. Indeed, a high percentage of antigen-specific effector cells, that is, CD27−CCR7−CD45RA+, contained granzyme B, whereas only low numbers of CD27+CCR7−CD45RA− cells contained the molecule (Figure 4D-E).
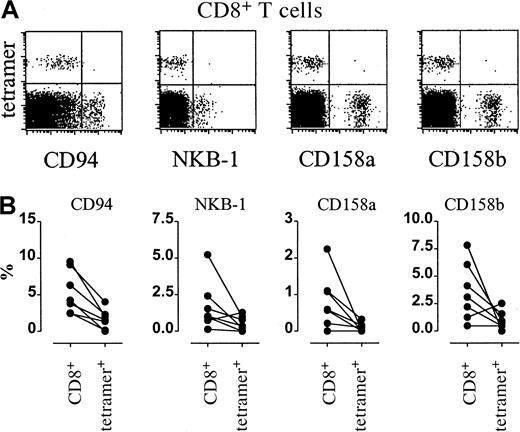
In analogy with findings on CD28− T cells,18we previously demonstrated19 that when CD8+ T cells differentiate toward the CD27− effector type, T-cell expression of natural killer cell receptors (NKRs), both of the KIR and C-type lectin type, is acquired. Huard and Karlsson20postulated that repeated stimulation by antigen in vivo would up-regulate NKR expression. We therefore analyzed expression of CD94, NKB-1, CD158a, and CD158b on CD8+ T cells of healthy individuals who are persistently exposed to virus. Remarkably, irrespective of the CD27 phenotype of the CMV-specific T cells of the donors analyzed, the expression levels of the various NKRs were invariably low compared with the total CD8 population (Figure5A-B).
CMV-specific CD8+ T cells do not express killer inhibitory receptors.
(A) Dot plots of killer inhibitory receptor–PE fluorescence (x-axis, arbitrary units, log scale) versus NLVPMVATV–HLA-A2.1 tetramer-APC fluorescence (“tetramer,” y-axis, arbitrary units, log scale) within gated CD8bright T cells from CMV-seropositive individuals. From left to right, expressions of CD94, NKB-1, CD158a, and CD158b are shown. (B) Frequencies of inhibitory receptor–expressing cells within CD8+ T cells (CD8+, left columns, percentage of CD8+ T cells) or within tetramer-positive CD8+ T cells (tetramer+, right columns, percentage of tetramer-positive CD8+ T cells).
CMV-specific CD8+ T cells do not express killer inhibitory receptors.
(A) Dot plots of killer inhibitory receptor–PE fluorescence (x-axis, arbitrary units, log scale) versus NLVPMVATV–HLA-A2.1 tetramer-APC fluorescence (“tetramer,” y-axis, arbitrary units, log scale) within gated CD8bright T cells from CMV-seropositive individuals. From left to right, expressions of CD94, NKB-1, CD158a, and CD158b are shown. (B) Frequencies of inhibitory receptor–expressing cells within CD8+ T cells (CD8+, left columns, percentage of CD8+ T cells) or within tetramer-positive CD8+ T cells (tetramer+, right columns, percentage of tetramer-positive CD8+ T cells).
Frequencies and properties of CMV-specific CD8+ T cells in renal transplant recipients
Frequencies of CMV-specific helper CD4+ T cells as measured by antigen-induced IFN-γ secretion did not differ significantly between healthy donors and recipients of renal transplants who were taking immunosuppressive drugs for on average 36.5 months (median 0.20 versus 0.47%, P = .53, data not shown and Rentenaar et al4). However, the frequencies of virus-specific CD8+ T cells analyzed either by peptide-induced IFN-γ secretion or by direct visualization with HLA-A2.1–NLVPMVATV tetramers were significantly higher in the transplant recipients (P = .0082 respectively .0056, Figure 2A-B). Similar to CD8+ T cells in healthy donors approximately 20% of the antigen-specific CD8+ T cells were able to secrete IFN-γ in 6-hour stimulation assays (P = .558 between control individuals and transplant recipients, data not shown). In renal transplant recipients no significant correlation was found between helper cell frequencies and either IFN-γ–producing or number of CMV-specific CD8+ T cells (data not shown).
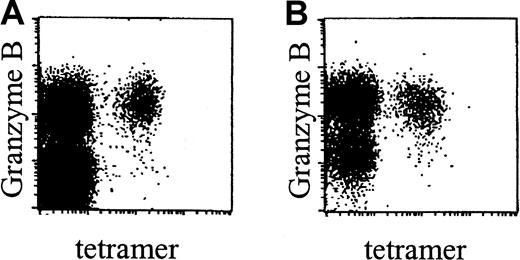
Phenotypic analyses showed that, in accordance with data obtained in healthy donors, CMV-specific CD8+ T cells in renal transplant recipients were CCR7− and did not express NKRs (data not shown). Interestingly however, these cells were predominantly of the effector phenotype, that is, CD45RA+CD27− (Figure 2C). In line with this surface phenotype, these cells expressed perforin (data not shown) and granzyme B (Figure 6A-B).
CMV-specific CD8+ T cells in renal transplant recipients contain granzyme B.
Dot plots of NLVPMVATV–HLA-A2.1 tetramer-APC fluorescence (“tetramer,” x-axis, arbitrary units, log scale) versus granzyme B–PE fluorescence (y-axis, arbitrary units, log scale) within gated CD8bright T cells, in a healthy control individual (A) and an immunosuppressed renal transplant recipient (B). Subjects were matched for their CD27−CD45RA+ phenotype of CMV-specific CD8+ T cells.
CMV-specific CD8+ T cells in renal transplant recipients contain granzyme B.
Dot plots of NLVPMVATV–HLA-A2.1 tetramer-APC fluorescence (“tetramer,” x-axis, arbitrary units, log scale) versus granzyme B–PE fluorescence (y-axis, arbitrary units, log scale) within gated CD8bright T cells, in a healthy control individual (A) and an immunosuppressed renal transplant recipient (B). Subjects were matched for their CD27−CD45RA+ phenotype of CMV-specific CD8+ T cells.
Repetitive antigenic stimulation induces loss of CD27
The transsectional findings and the data of Gillespie21 suggested that immunosuppressive therapy altered the CMV-specific CD8+ T-cell compartment in a quantitative and qualitative fashion. To test this directly, we investigated the effect of immunosuppressive therapy on the phenotype of CMV-specific CD8+ T cells in patients before and 12 months after transplantation. Mean frequencies of CMV-specific CD8+ T cells rose from 2.08% to 3.31% during this period (P = .313, not significant). Moreover, in 5 of 6 patients the percentage of CD27− effector cells increased at the expense of the CD27+ memory cells. Interestingly, this change was most dramatic in a patient who had continuous positive urine cultures for CMV after transplantation (Figure7A-B).
Viral antigen drives CD27 down-modulation on antigen-specific CD8+ T cells.
(A) Dot plots of CD27 expression (x-axis, arbitrary units, log scale) versus CD45RA expression (y-axis, arbitrary units, log scale) within gated tetramer-positive CD8+ T cells before transplantation (0M) and 12 months after transplantation (12M) from one patient without positive urine cultures (left panel) and one patient with positive urine cultures (right panel). (B) Frequencies of CD27−cells among CMV-specific CD8+ T cells (y-axis, percentage of tetramer-positive CD8+ T cells) in CMV-seropositive HLA-A2+ renal transplant recipients (designated by capitals) before and 12 months after transplantation (x-axis, months).
Viral antigen drives CD27 down-modulation on antigen-specific CD8+ T cells.
(A) Dot plots of CD27 expression (x-axis, arbitrary units, log scale) versus CD45RA expression (y-axis, arbitrary units, log scale) within gated tetramer-positive CD8+ T cells before transplantation (0M) and 12 months after transplantation (12M) from one patient without positive urine cultures (left panel) and one patient with positive urine cultures (right panel). (B) Frequencies of CD27−cells among CMV-specific CD8+ T cells (y-axis, percentage of tetramer-positive CD8+ T cells) in CMV-seropositive HLA-A2+ renal transplant recipients (designated by capitals) before and 12 months after transplantation (x-axis, months).
Discussion
The data presented in this paper establish a number of key features of CD8+ T cells involved in the control of persistent viruses, specifically CMV. CD4+ T cells are believed to be instrumental in the initiation of CD8+T-cell expansion via the stimulation of dendritic cells,22,23 but their role in maintaining adequate numbers and function of specific CD8+ T cells is less well understood. Zajac and colleagues5 observed that in CD4−/− mice, function rather than number of lymphocytic choriomeningitis virus–specific CD8+ T cells is impaired. In human immunodeficiency virus (HIV) infection it has been shown that strong CD4+ T-cell responses are essential to maintain effective cytotoxic T-cell responses and for control of viremia.24-26 Also in our panel of healthy individuals, the frequency of functional (determined by peptide-induced IFN-γ secretion) but not the total number of CMV-specific CD8+ T cells correlated with the percentage of functional CMV-specific CD4+ T cells. In renal transplant recipients receiving standard immunosuppressive therapy relatively high percentages of functional CD4+ and CD8+ CMV-specific T cells were found, but a statistical relation between the subsets could not be demonstrated. The reason for this is unclear but possible effects of immunosuppressants on the read-out system cannot be excluded. Further analysis awaits the availability of class II/CMV-peptide complexes that allow direct visualization of the helper cell compartment.
The CMV-specific CD8+ T cells can be typified as TEM cells, but based on expression of CD27 a further subdivision in functional subpopulations can be made. We would prefer to reserve the annotation “effector” for the CD27−population because only within this population a very high frequency of cells with granzyme B, perforin, and CD95 ligand expression is found (this report and Hamann et al7). In agreement with the fact that most CD27− T cells lack CCR7, CD27−cells in humans are not found within secondary lymphoid tissue, such as tonsil, but rather can be found in the skin, lung, and gut.27-29 Also in mice CD27− T cells are absent from peripheral and mesenteric lymph nodes but relatively high numbers are localized in the spleen (Baars and coworkers, manuscript in preparation). In view of the similarities in expression of CCR7 and CD27 and because of the notion that tumor necrosis factor receptor (TNF-R) family members might be involved in the regulation of chemokine receptors,30 we tested whether CD27 ligation would have a direct effect on CCR7 expression. However, although cellular activation influenced CCR7 expression,31 no specific effect of CD27 triggering was observed (P.A.B., unpublished observation, 2000). Our data show that although CD27 and CCR7 are expressed on overlapping sets of CD8+ T cells, populations can be found that do express one marker only. This may not be surprising because the molecules serve distinct purposes and are likely being regulated in an independent fashion.
When CD8+ T cells progress through the proposed naive, memory, and effector stages,32 expression of NKRs, both of the Ig and C-type lectin type, coordinately increases.19Inhibitory NKRs may set thresholds for activation precluding that any type of cell with low antigen expression would be targeted by circulating effector cytotoxic T lymphocytes (CTLs). Huard and Karlsson20 suggested that repeated exposure to specific antigens would increase expression of NKRs on specific T cells. Our data, however, unequivocally demonstrate that NKRs are barely if all expressed on T cells specific for CMV. The reason for this apparent paradox is unclear at this moment. Probably, antigens that persistently stimulate the immune system differ in their way of being presented and the context of presentation may have both direct and indirect effects on the expression of activation-regulating receptors. Elegant work from several groups has shown that CMV contains several genes that influence immune recognition.33-36 In this perspective it will be of interest to determine if other persisting viruses recruit memory and effector cells that also lack NKRs. In any case the data infer that control of CMV replication might be efficient because of lack of interference by NKRs in conjunction with high frequencies of CMV-specific effector cells.
The CMV-specific T cells have been detected in CD45R0high, CD45RAhigh, and CD28− T-cell subsets by clonotypic oligonucleotide probes.37 More direct studies recently performed by Appay and coworkers38 using tetramer staining showed that the vast majority of CMV-reactive T cells in HIV-infected individuals had an effector, CD27− phenotype. Also in those renal transplantation patients who were on immunosuppressive therapy for more than 30 months, nearly all CMV-specific T cells were CD27−. In the case of the immunosuppressed individuals studied here there is an actual increase in effector cells as the percentage of tetramer-positive cells rises over time and CD8 numbers are at a constant level (data not shown). However, most healthy donors and patients prior to renal transplantation had relatively low percentages of CMV-specific CD8+ T cells and these cells had mostly a memory phenotype. How to account for these differences? Immunosuppression, caused by either HIV infection or immunosuppressive medication, is likely to render surveillance against persisting viruses less efficient in vivo. Concerning cyclosporin A, this may be in fact on a per cell basis because of direct interference with activation signals that are generated via the T-cell receptor (TCR).39 Consequently, cycles of virus replication may occur that, despite suppression, stimulate expansion of CD4+ and CD8+ T cells. In the presence of a competent helper cell compartment CD8+T cells may differentiate into effector cells, which, due to repeated rounds of subclinical virus reactivation, may accumulate over time. This notion would be in agreement with recent analyses by Appay and colleagues38 in HIV-infected persons. Their data strongly suggest that terminal differentiation of CD8+ T cells is dependent on adequate T-cell help because CMV-specific CD8+T cells do differentiate into competent effector cells, whereas HIV-specific cells do not.13 In short, chronic active virus infection may favor the differentiation of antigen-specific CD8+ T cells toward CD27− effector cells.40 It is unclear what specific signals drive differentiation of naive T cells into effector vis-a-vis memory CD8+ T cells. As discussed above, antigen may not be the sole factor in determining the CD8+ phenotype. Costimulatory signals delivered via cytokines or membrane-bound ligands may specifically regulate this process. In this respect it is interesting to note that both CD28 and CD27 costimulatory receptors augment effector functions and are down-modulated from the cell surface after interaction with their specific ligands.
Our data infer that despite effective immunosuppression of allospecific T-cell–mediated graft rejection the immune system is able to mount an adaptive response to persistent viruses such as CMV. In most healthy individuals, an equilibrium is achieved with a predominance of virus-controlling memory-type CD8+ T cells. However, in response to viral and host factors, CD8 responses may be shifted toward effector-type cells. Indeed, in a number of our normal donors effector-type cells predominated. These individuals were healthy and did not have signs of viral replication in vivo. Interestingly, in elderly individuals the proportion of CD27−41 (and Rep and coworkers, unpublished observation, 1984), CD28−,42 CD11bright43CD8+ T cells increases, suggesting that effector-type CD8+ T cells accumulate with age. This natural process may be accelerated in immunocompromised individuals who, due to pharmacotherapeutic immunosuppression, may be less fit to sufficiently repress viral replication. However, with adequate help from virus-specific CD4+ T cells these individuals may both quantitatively and qualitatively increase their effector CD8+ T-cell compartment thereby achieving a new equilibrium with the persisting virus. It follows that when this balance fails to establish, clinical disease may develop.
The authors thank the patients and volunteers for their blood donations, Dr F. Bemelman for assistance in collecting patient material, Frank van Diepen for excellent technical assistance, Dr R. Boom for development of the CMV-PCR, technicians from the Department of Clinical Virology for performing CMV-PCRs and cultures, and Drs Frank Miedema, Debbie van Baarle, and Michiel Betjes for critical reading of the manuscript. Dr Lijun Wu is thanked for providing the anti-CCR7 antibody and Dr Stefan Kostense for providing the HLA-A2/HIV-SLYNTVATL tetramers and peptide.
Supported by grants from the Dutch Kidney Foundation, C98-1724 (L.E.G.) and C95-1455 (R.J.R.).
L.E.G. and R.J.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laila E. Gamadia, Clinical Immunology Laboratory G1-109, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: l.e.gamadia@amc.uva.nl.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal