Abstract
Both type I interferons (IFNs) as well as lipopolysaccharide (LPS) individually compromise selected monocytic or dendritic cell (DC) functions. This study investigates the influence of these agents on the differentiation and the regulation of cell death of monocyte-derived DCs generated in the presence of granulocyte-macrophage colony-stimulating factor plus interleukin-4 (IL-4). It is reported that excessive apoptosis occurred rapidly in monocyte-derived DC cultures, if IFN-α or IFN-β was added in combination with LPS or lipoteichoic acid (LTA). The small fraction of cells surviving in such cultures displayed a mature DC phenotype with expression of CD83, CD80, and CD86. IL-10 was found in the supernatants of monocyte-derived DC cultures, if supplemented with LPS or IFN-α plus LPS but not in control cultures. When monocyte-derived DCs were generated in the presence of IFN-α without LPS, these cells displayed an immature DC phenotype with a reduction of cell recovery but no overt apoptosis. However, the addition of LPS, LTA, LPS plus IFN-γ, or tumor necrosis factor α (TNF-α) plus prostaglandin E2 to such cells again resulted in the rapid induction of apoptosis in the majority of cells, together with a reduced production of IL-12 p70 and TNF-α. Together, these data indicate an exquisite sensitivity of monocyte-derived DCs to activation-induced cell death if generated in the presence of IFN-α, indicating the existence of an important mechanism of immunosuppression caused by IFN-α–inducing agents, such as viral or bacterial stimuli.
Introduction
Evidence accumulating during many years has shown that viral and bacterial infections can impair the function of many cells of the immune system. Dendritic cells (DCs) are of central relevance within the immune system because only these cells can activate naive T cells and thereby initiate an adaptive immune response. Thus, understanding their function during infections might give insight into important immunopathologic mechanisms. For in vitro studies, DCs are commonly derived either from CD34-expressing precursors or from monocytes (reviewed by Banchereau and Steinman1). With regard to monocyte-derived DCs several effects of bacterial and viral stimuli in vitro and in vivo have already been described.
For instance, interferon α (IFN-α), which is transiently released during the first wave of innate immunity in response to bacteria and viruses,2-10 has been reported to suppress interleukin-12 (IL-12) production of monocytes and DCs.11-14 The superfamily of type I IFNs comprises at least 12 IFN-α species and a single IFN-β that differentially bind to a common receptor.15 Alpha IFNs are produced in modest amounts by monocytes, macrophages, and B lymphocytes9 and in much larger quantities by the precursors of lymphoid DCs (pDC2),9,16,17 whereas IFN-β is mainly produced by fibroblasts.18 Despite their established protective role in innate immunity because of their antibacterial and antiviral activity,2,10,19-21 there is increasing evidence that type I IFNs might also act negatively on immunity not only by abrogating IL-12 production but also by impairing DC differentiation22 and reducing the phagocytic and oxidative activity of monocytes.23
Other studies showed that lipopolysaccharide (LPS) has suppressive effects on monocytes and monocyte-derived DCs,4,10possibly in part mediated by LPS-triggered type I IFN production. A transient presence of endotoxin during the differentiation of monocyte-derived DCs abrogates IL-12 production of the resulting immature DCs on a second stimulation with LPS.24 If permanently present during DC differentiation, it completely desensitizes the cells to all further LPS-mediated maturation signals.25 Monocytes from septic patients were found to be hyporesponsive to LPS stimulation ex vivo, and this hyporesponsiveness was reversed during IFN-γ treatment that also lead to clearance of sepsis,26 suggesting that monocyte deactivation might be a major mechanism of immunosuppression.
Despite these data it is unclear how DCs respond to a combination of type I IFNs and bacterial products. With the use of the culture of monocyte-derived DCs, our study revealed a strong synergistic effect between type I IFNs and products from gram-negative as well as gram-positive bacteria for the induction of apoptosis. This effect occurs if both stimuli are present together during the whole culture but also when the bacterial stimulus is added later to immature DCs generated in the presence of type I IFNs.
Materials and methods
Monocyte isolation and DC culture
Human mononuclear cells were obtained from blood containing citrate as anticoagulant from healthy donors by density gradient centrifugation using endotoxin-free Ficoll-Paque-PLUS (Pharmacia, Uppsala, Sweden). Monocytes were isolated from these mononuclear cells by a second density gradient centrifugation (M.L. and W.H., manuscript submitted, March 2001) using Ficoll-Paque-PLUS adjusted to 1.068 g/mL by dilution with phosphate-buffered saline (BioWhittaker, Walkersville, MD). The remaining cells, containing 80% to 90% monocytes as assessed by counting on a Sysmex F820 instrument, were frozen in RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 10% dimethyl sulfoxide (Sigma, St Louis, MO) and 20% fetal calf serum containing less than 100 pg/mL endotoxin (PAA Laboratories, Linz, Austria). For DC differentiation monocytes were cultured in 24-well culture plates (Iwaki, Japan) at 0.5 × 106/mL in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM L-glutamine (Life Technologies), 1000 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Leucomax; Roche, Basel, Switzerland), and 1000 U/mL recombinant human IL-4 (Strathmann Biotec, Hamburg, Germany). Half of the medium (including all supplements) was exchanged every 2 days. IFN-α2c (Berofor; Boehringer Ingelheim, Vienna, Austria), recombinant human IFN-β (Sigma), LPS fromEscherichia coli 055:B5 (Sigma) and lipoteichoic acid (LTA) from Bacillus subtilis (Sigma) were added in different concentrations and combinations at the indicated time points. Further, some cultures were additionally supplemented with 1000 U/mL recombinant human tumor necrosing factor α (TNF-α; Bender MedSystems, Vienna, Austria), 1 μg/mL prostaglandin E2 (PGE2; Sigma), 1000 U/mL IFN-γ (Bender MedSystems), or 40 000/24-well irradiated (6000 rad) SNJB7 neuroblastoma cells transfected with CD40 ligand (CD40L).46
Flow cytometric analysis
For immunophenotypic analysis the following monoclonal antibodies were used: anti-CD80–phycoerythrin (PE; clone MAB104; Immunotech, Marseille, France), anti-CD83–PE (clone HB15A; Immunotech), anti-CD86–fluorescein isothiocyanate (FITC; clone 2331; Pharmingen, San Diego, CA), anti-CD1a–FITC (clone HI149; Pharmingen), and anti-CD14–allophycocyanin (clone MoP9; Becton Dickinson, Erembodegem, Belgium). Apoptosis was detected by staining with Annexin V–FITC (Alexis, San Diego, CA) according to the manufacturer's protocol. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA), and data analysis was performed with CellQuest software (Becton Dickinson).
Cytokine measurements
On the basis of standard sandwich enzyme-linked immunosorbent assay methodology, commercially available pairs of monoclonal antibodies (Pharmingen) were used to quantify human IL-12 p70, IL-10, and TNF-α.
Results
Combination of type I IFNs with LPS or LTA induces extensive apoptosis in cultures of monocyte-derived DCs
IFN-α and LPS each are described to affect the differentiation of monocyte-derived DCs. To investigate potential synergistic effects between type I IFNs and bacterial stimuli on monocyte differentiation toward DCs, we first analyzed the effect of IFN-α and LPS present during the whole culture period. IFN-α (1000 U/mL) as well as 1 ng/mL LPS were added on day 0 alone or in combination to monocytes in medium containing IL-4 and GM-CSF. On day 6, light microscopy indicated extensive cell death in the cultures containing both LPS and IFN-α (not shown).
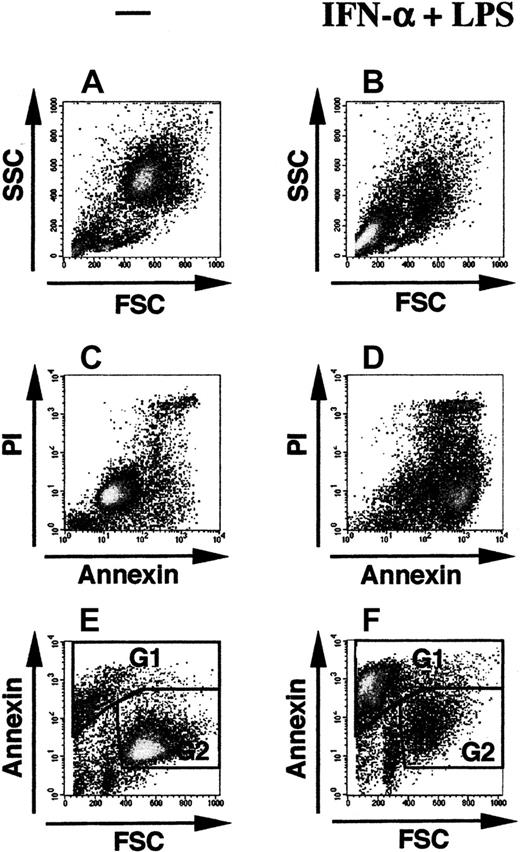
Apoptotic cell death is characterized by the binding of Annexin V and by cell shrinkage (in contrast to necrosis). Figure1 shows the flow-activated cell sorter (FACS) analysis of a DC culture treated with IFN-α plus LPS compared with an untreated culture after staining with Annexin V–FITC and propidium iodide. Figure 1A illustrates that in the control cultures the majority of cells are contained within a relatively homogenous DC population with regard to cell size (estimated by forward scatter [FSC]) and granularity (estimated by the side scatter [SSC]), both parameters are decreased if IFN-α plus LPS had been added to the culture (Figure 1B). Most of the IFN-α–plus–LPS–treated cells acquired Annexin V positivity, indicating the presence of extensive apoptosis, with a subpopulation being also positive for propidium iodide (Figure 1D). Double-positive cells represent cells that have already lost integrity of their membranes during the late apoptotic process. In this representation, however, a clear demarcation of the apoptotic cell population was not possible. For the quantification of apoptosis we, therefore, used the observed reduction in the forward scatter together with increased binding of Annexin V to achieve a sufficient discrimination of apoptotic cells (Figure 1E,F). The same mode of representation was used for the quantification of apoptosis in all further experiments. We could also detect the typical condensation of apoptotic nuclei in cytospins of cells stained with Annexin V and 4,6-diamidino-2-phenylindole (not shown).
Quantification of apoptosis by FACS analysis after staining with Annexin V–FITC and propidium iodide.
Density gradient-purified monocytes were cultured for 6 days with GM-CSF plus IL-4 in the absence (A,C,E) or presence (B,D,F) of 1000 U/mL IFN-α plus 1 ng/mL LPS. Shown are the results of a single experiment by 3 alternative modes of representations displaying the forward/side scatter profile (A-B), Annexin V–FITC versus propidium iodide (PI) (C-D), and forward scatter versus Annexin V–FITC (E-F). Gates G1 (apoptotic cells) and G2 (viable cells) were drawn arbitrarily in the representation forward scatter versus Annexin (E-F), because in this representation both populations could best be discriminated. In this experiment cells in gate G1 (apoptotic cells) amounted to 12.2% of total cells in panel E (control culture) and 64.8% in panel F (culture with IFN-α plus LPS), whereas the cells in gate G2 were considered as viable cells (75% of total cells in panel E and 22% in panel F). The small population of lymphocytes (best visible in panel A) amounted to 8.3% of total events in this experiment.
Quantification of apoptosis by FACS analysis after staining with Annexin V–FITC and propidium iodide.
Density gradient-purified monocytes were cultured for 6 days with GM-CSF plus IL-4 in the absence (A,C,E) or presence (B,D,F) of 1000 U/mL IFN-α plus 1 ng/mL LPS. Shown are the results of a single experiment by 3 alternative modes of representations displaying the forward/side scatter profile (A-B), Annexin V–FITC versus propidium iodide (PI) (C-D), and forward scatter versus Annexin V–FITC (E-F). Gates G1 (apoptotic cells) and G2 (viable cells) were drawn arbitrarily in the representation forward scatter versus Annexin (E-F), because in this representation both populations could best be discriminated. In this experiment cells in gate G1 (apoptotic cells) amounted to 12.2% of total cells in panel E (control culture) and 64.8% in panel F (culture with IFN-α plus LPS), whereas the cells in gate G2 were considered as viable cells (75% of total cells in panel E and 22% in panel F). The small population of lymphocytes (best visible in panel A) amounted to 8.3% of total events in this experiment.
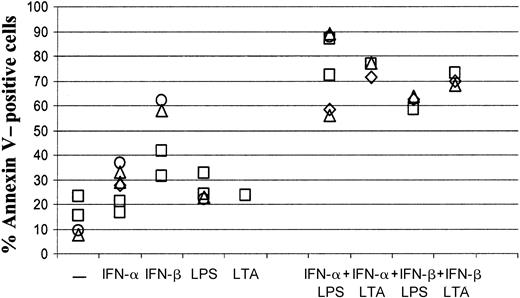
In the following experiments we investigated if the observed synergy in induction of apoptosis also exists between IFN-β as an alternative to type I IFN and LTA, a cell wall component of gram-positive bacteria. Figure 2 compares the fraction of apoptotic cells on day 6 in cultures containing IFN-α or IFN-β and LPS or LTA alone or in combination. In contrast to the effect of the single factors, all combinations of a type I IFN with a bacterial stimulus induced strong apoptosis.
Type I IFNs in combination with LPS or LTA induce apoptosis.
Different combinations of 1000 U/mL IFN-α, 1000 U/mL IFN-β, 1 ng/mL LPS, and 1 μg/mL LTA were added on day 0 to cultures supplemented with IL-4 and GM-CSF. The percentage of Annexin-positive cells was determined by FACS analysis on day 6 as detailed in Figure 1. The data were obtained from 4 different donors, each represented by an individual symbol. Two donors (■, ▵) were tested twice in independent experiments.
Type I IFNs in combination with LPS or LTA induce apoptosis.
Different combinations of 1000 U/mL IFN-α, 1000 U/mL IFN-β, 1 ng/mL LPS, and 1 μg/mL LTA were added on day 0 to cultures supplemented with IL-4 and GM-CSF. The percentage of Annexin-positive cells was determined by FACS analysis on day 6 as detailed in Figure 1. The data were obtained from 4 different donors, each represented by an individual symbol. Two donors (■, ▵) were tested twice in independent experiments.
Titration experiments with IFN-α plus LPS (Figure3A) and IFN-α plus LTA (Figure 3B) revealed strong synergistic effects of 100 to 1000 U/mL IFN-α with both bacterial components at low concentrations, and in kinetic studies it was evident that apoptosis occurred rapidly following the addition of IFN-α plus LPS (Figure 4).
Dose dependency of apoptosis in response to IFN-α plus LPS or LTA.
IFN-α and LPS (A) or LTA (B) was added at the indicated concentrations alone (▴) or together with 10 U/mL (●), 100 U/mL (♦), or 1000 U/mL IFN-α (▪) on day 0 to cultures of monocytes supplemented with IL-4 and GM-CSF. The induction of apoptosis was quantified by FACS analysis on day 6 as detailed in Figure1.
Dose dependency of apoptosis in response to IFN-α plus LPS or LTA.
IFN-α and LPS (A) or LTA (B) was added at the indicated concentrations alone (▴) or together with 10 U/mL (●), 100 U/mL (♦), or 1000 U/mL IFN-α (▪) on day 0 to cultures of monocytes supplemented with IL-4 and GM-CSF. The induction of apoptosis was quantified by FACS analysis on day 6 as detailed in Figure1.
Rapid induction of apoptosis in cultures containing IFN-α and LPS.
On day 0, 1000 U/mL IFN-α ± 1 ng/mL LPS was added to monocyte cultures supplemented with GM-CSF plus IL-4. Annexin V positivity was quantified on days 2, 4, and 6. ■, control; ░, IFN-α; ▪, IFN-α plus LPS. Shown are the data obtained with monocytes from 3 different donors (mean ± SD).
Rapid induction of apoptosis in cultures containing IFN-α and LPS.
On day 0, 1000 U/mL IFN-α ± 1 ng/mL LPS was added to monocyte cultures supplemented with GM-CSF plus IL-4. Annexin V positivity was quantified on days 2, 4, and 6. ■, control; ░, IFN-α; ▪, IFN-α plus LPS. Shown are the data obtained with monocytes from 3 different donors (mean ± SD).
Surviving cells in cultures containing IFN-α plus LPS are phenotypically mature DCs
The extensive apoptosis in the presence of IFN-α and LPS strongly reduced the yield of viable cells. The phenotype of the remaining cells was compared with that of control cultures of immature DCs differentiated in the absence of IFN-α plus LPS and with cells derived from such control cultures but matured by the addition of LPS for the last 3 days of the culture. FACS analysis for selected DC markers for all cultures was done on day 8 (Figure5A). The permanent presence of IFN-α plus LPS resulted in an increased expression of CD83, CD80, and CD86 in the surviving cells, indicating the presence of mature DCs. These increases were not seen in the presence of IFN-α or LPS alone (not shown). When these cells were additionally exposed to the strong maturation stimulus IFN-γ plus CD40L from day 5 on, there was almost no further response (Figure 5B).
Surviving DCs generated in the presence of IFN-α plus LPS have a mature phenotype.
Monocyte-derived DCs were generated with GM-CSF plus IL-4 in the absence (control cultures, dotted lines, A) or presence of IFN-α plus LPS (bold lines, A-B). During the last 3 days, a part of both cultures was subjected to a maturation step employing 100 ng/mL LPS (control cultures, thin line, A) or CD40L plus IFN-γ (cultures containing IFN-α plus LPS, thin line, B). Cells were stained on day 8 of total culture for the presence of CD83, CD80, and CD86; only large viable cells were analyzed.
Surviving DCs generated in the presence of IFN-α plus LPS have a mature phenotype.
Monocyte-derived DCs were generated with GM-CSF plus IL-4 in the absence (control cultures, dotted lines, A) or presence of IFN-α plus LPS (bold lines, A-B). During the last 3 days, a part of both cultures was subjected to a maturation step employing 100 ng/mL LPS (control cultures, thin line, A) or CD40L plus IFN-γ (cultures containing IFN-α plus LPS, thin line, B). Cells were stained on day 8 of total culture for the presence of CD83, CD80, and CD86; only large viable cells were analyzed.
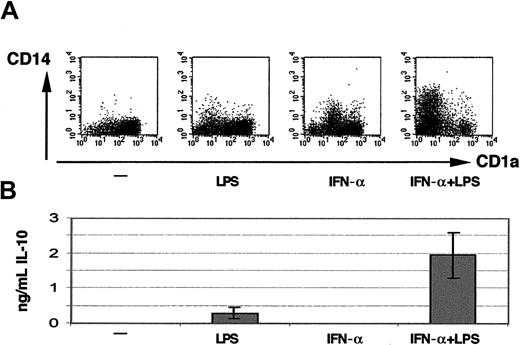
Although the remaining viable cells of the cultures containing IFN-α and LPS thus resembled mature DCs, there were peculiarities. Despite a homogeneous expression of CD83, CD80, and CD86 (Figure 5A), further analysis revealed CD14 expression in a subpopulation of cells (Figure6A). To some extent, varying between individuals, some CD14 expression was also seen with IFN-α alone. Because IL-10 was described to induce differentiation of immature monocyte-derived DCs into CD14+ macrophagelike cells,27 we tested if the observed CD14 expression was correlated with elevated IL-10 production. In the cultures containing both IFN-α and LPS approximately 2 ng/mL IL-10 was found on day 4 (Figure 6B). Although there was no CD14 expression at all with LPS alone, it also induced some IL-10 (300 pg/mL) as previously reported,28 whereas IFN-α alone induced some CD14 but not any IL-10.
CD14 expression and IL-10 production of cells differentiated in presence of IFN-α plus LPS.
The dot plots in panel A were obtained by FACS analysis on day 6 from cultures containing 1000 U/mL IFN-α and/or 1 ng/mL LPS as indicated. Despite homogeneous expression of the DC maturation markers CD83, CD80, and CD86 (not displayed), the expression of CD14 is retained in a subpopulation of cells cultured in the presence of IFN-α plus LPS. Shown are the data from one representative experiment; similar results were obtained in 3 further experiments. Panel B displays the amount of IL-10 found in the supernatants on day 4 (mean ± SD of 3 independent experiments).
CD14 expression and IL-10 production of cells differentiated in presence of IFN-α plus LPS.
The dot plots in panel A were obtained by FACS analysis on day 6 from cultures containing 1000 U/mL IFN-α and/or 1 ng/mL LPS as indicated. Despite homogeneous expression of the DC maturation markers CD83, CD80, and CD86 (not displayed), the expression of CD14 is retained in a subpopulation of cells cultured in the presence of IFN-α plus LPS. Shown are the data from one representative experiment; similar results were obtained in 3 further experiments. Panel B displays the amount of IL-10 found in the supernatants on day 4 (mean ± SD of 3 independent experiments).
Addition of IFN-α plus LPS to immature DCs induces maturation without apoptosis
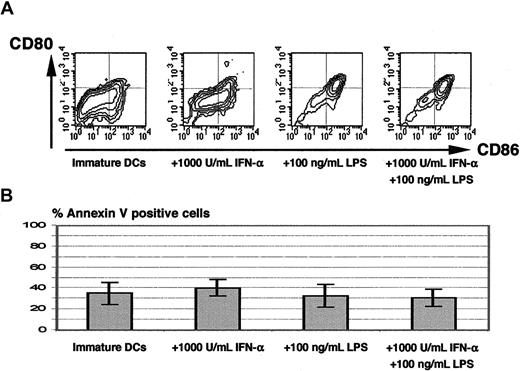
In the experiments described above, type I IFNs and bacterial stimuli were added to monocytes on day 0. To investigate the response of immature DCs to a combination of these stimuli, immature DCs were challenged with LPS or IFN-α alone or with a combination of both. LPS plus IFN-α as well as LPS alone triggered maturation with induction of CD83 (not shown) and a very high expression of CD80 and CD86 3 days after stimulation (Figure 7A). IFN-α alone did not induce maturation. Importantly, no increased apoptosis was observed in the cultures containing IFN-α plus LPS (Figure 7B).
The addition of IFN-α plus LPS to immature DCs on day 7 triggers maturation without apoptosis.
Panel A shows the phenotype of monocyte-derived DCs on day 10 (ie, 3 days after addition of the indicated stimuli). The percentage of apoptotic cells on day 10 is shown in panel B (mean ± SD of 3 independent experiments).
The addition of IFN-α plus LPS to immature DCs on day 7 triggers maturation without apoptosis.
Panel A shows the phenotype of monocyte-derived DCs on day 10 (ie, 3 days after addition of the indicated stimuli). The percentage of apoptotic cells on day 10 is shown in panel B (mean ± SD of 3 independent experiments).
Immature DCs generated in the presence of IFN-α undergo apoptosis in response to a variety of activation stimuli
The above experiments had investigated the effects of type I IFNs and bacterial stimuli when added together at the start of the culture to monocytes or to monocyte-derived immature DCs. Next we asked whether bacterial products or other activating signals would induce apoptosis if added later to immature DCs, which had been differentiated in the presence of IFN-α. Therefore, immature DCs generated in the presence or absence of 1000 U/mL IFN-α were stimulated with LTA, LPS, LPS plus IFN-γ, and TNF-α plus PGE2 on day 5. Three days later, extensive apoptosis was present in all cultures that had been differentiated in the presence of IFN-α (Figure 8A). Importantly, apoptosis was induced to the same extent by activation of the IFN-α precultured DCs with TNF-α plus PGE2 as with LPS or LTA, suggesting that IFN-α had sensitized the immature DCs to activation-induced cell death in general. To some extent 1000 U/mL IFN-α alone also increased apoptosis on day 8, but addition of the maturation stimuli dramatically raised the percentage of Annexin V–binding cells. The expression of CD80, CD86, and CD83 of the surviving cells in these cultures was completely identical to that of the respective activated DCs differentiated without IFN-α (data not shown). Although quantitatively reduced, IFN-α primed immature DCs were also competent for production of IL-12 p70 and TNF-α in response to LPS plus IFN-γ, whereas IL-10 was not produced (Figure9).
Immature DCs generated by GM-CSF plus IL-4 in the presence of IFN-α undergo apoptosis in response to a variety of activation stimuli.
The response of immature DCs generated in the permanent presence of 1000 U/mL IFN-α (▪) to the indicated stimuli was compared with that of immature DCs generated in the absence of IFN-α (░). Apoptosis was quantified on day 8 (ie, 3 days after activation with 1 μg/mL LTA, 100 ng/mL LPS, 100 ng/mL LPS plus 1000 U/mL IFN-γ, and 1000 U/mL TNF-α plus 1 μg/mL PGE2 as indicated [mean ± SD of 3 independent experiments]).
Immature DCs generated by GM-CSF plus IL-4 in the presence of IFN-α undergo apoptosis in response to a variety of activation stimuli.
The response of immature DCs generated in the permanent presence of 1000 U/mL IFN-α (▪) to the indicated stimuli was compared with that of immature DCs generated in the absence of IFN-α (░). Apoptosis was quantified on day 8 (ie, 3 days after activation with 1 μg/mL LTA, 100 ng/mL LPS, 100 ng/mL LPS plus 1000 U/mL IFN-γ, and 1000 U/mL TNF-α plus 1 μg/mL PGE2 as indicated [mean ± SD of 3 independent experiments]).
Cytokine production of DCs generated in the presence of IFN-α in response to LPS plus IFN-γ.
The production of IL-12 p70, TNF-α, and IL-10 was determined on day 8 (ie, 3 days after activation with 100 ng/mL LPS plus 1000 U/mL IFN-γ). The response of immature DCs generated in the permanent presence of 1000 U/mL IFN-α (▪) was compared with that of immature DCs generated in the absence of IFN-α (░) (mean ± SD of 3 independent experiments).
Cytokine production of DCs generated in the presence of IFN-α in response to LPS plus IFN-γ.
The production of IL-12 p70, TNF-α, and IL-10 was determined on day 8 (ie, 3 days after activation with 100 ng/mL LPS plus 1000 U/mL IFN-γ). The response of immature DCs generated in the permanent presence of 1000 U/mL IFN-α (▪) was compared with that of immature DCs generated in the absence of IFN-α (░) (mean ± SD of 3 independent experiments).
Discussion
In this study we describe the effects of the combined action of type I IFNs and microbial products on the differentiation and survival of monocyte-derived DCs. We found that IL-4/GM-CSF–stimulated cultures of monocytes differentiating toward DCs in the presence of IFN-α or IFN-β underwent strong apoptosis if microbial products such as LPS or LTA were additionally added. Provided that type I IFNs were present from the initiation of the cultures, bacterial products also induced apoptosis when added later to immature DCs. Immature DCs differentiated in the presence of IFN-α died also in response to TNF-α plus PGE2. No apoptosis was seen, however, when type I IFNs alone or together with a bacterial stimulus were added to already differentiated immature DCs. Cells surviving IFN-α plus LPS treatment developed into cells displaying a mature DC phenotype with an expression of CD83, CD80, and CD86 identical to that displayed by normal mature DCs, grown in the absence of IFN-α and further matured by an appropriate stimulus.
Our results fit well with a recent report,22 in which a reduced yield of monocyte-derived DCs grown in the presence of type I IFNs was described. We show that this reduction is due to apoptosis and is dramatically enhanced by the addition of a bacterial stimulus even at a low dose (30-100 pg/mL LPS). The kinetics of the apoptosis induction through the combined action of IFN-α and LPS was very rapid. Only 2 days after culture initiation a predominant fraction of cells underwent apoptosis. A similar synergy has been reported for the bovine system, in which macrophages rapidly undergo LPS-induced apoptosis after priming with IFN-α.29 Interestingly, however, murine macrophages go in apoptosis in response to LPS plus IFN-γ but are protected by pretreatment with IFN-α or β.30
Somehow contrasting the detrimental effects of type I IFNs on DC differentiation seen in our study, 2 recent reports suggested IFN-α as an adjuvant supporting DC development with GM-CSF in the absence of IL-4.31,32 Interestingly, Santini et al31described also an increase of Annexin binding cells in their cultures containing IFN-α plus GM-CSF, although in their study a beneficial effect of IFN-α on the generation and maturation of DCs prevailed. In our system it was mainly the addition of a second stimulus, such as LPS, LTA, or TNF-α plus PGE2, that resulted in the apoptosis of the majority of newly generated DCs. Our data thus imply that such IFN-α–pretreated immature DCs in general are sensitized to activation-induced cell death.
The phenotype of the surviving cells differed depending on the time point of the addition of the bacterial stimulus. In the presence of IFN-α, GM-CSF, and IL-4, the cells developed into viable CD14− and CD1a+ immature DCs (Figure 6A). Addition of bacterial stimuli to the immature DCs precultured with IFN-α induced strong apoptosis, but the surviving cells expressed CD83, CD80, and CD86 completely identically to mature control DCs (Figure 8 and not shown). In the cultures in which both stimuli were present from the initiation of the culture, the few viable cells also displayed a mature DC phenotype without further activation (Figure 5A) and hardly responded to further stimulation with IFN-γ plus CD40L (Figure 5B). These cells also stimulated allogeneic T cells in a mixed lymphocyte reaction in accordance with their mature DC phenotype (not shown).
The mature population resulting from the early presence of IFN-α plus LPS further contained a varying but substantial fraction of CD14+ cells (Figure 6A). Because IL-10 was described to shift differentiation of DC cultures toward macrophagelike cells by raising the expression of the receptor for the endogenously produced macrophage colony-stimulating factor,27 we analyzed the culture supernatants for the presence of IL-10. In accordance with earlier reports,28 33 we found little amounts of IL-10 in cultures containing a low dose of LPS and markedly raised amounts in cultures supplemented with LPS plus IFN-α (Figure 6B). Because the addition of IL-10 to cultures treated with IFN-α alone strongly increased CD14 expression and a neutralizing anti–IL-10 monoclonal antibody added to IFN-α plus LPS-stimulated cultures decreased CD14 expression (data not shown), endogenously produced IL-10 indeed appeared to influence the phenotype of the cells in our system.
With regard to the underlying mechanism of apoptosis induction, endogenously produced nitric oxide (NO) could be a possible candidate mediating cell death. In murine macrophages NO is induced by LPS in synergy with a type I IFN or IFN-γ,34 and in murine dendritic cells NO production in response to LPS plus IFN-γ was reported to cause apoptosis.35 In bovine macrophages IFN-α could prime for LPS-induced apoptosis, but this effect did not correlate with enhanced NO production, instead LPS-induced NO production was strongly reduced by IFN-α.29 In the human system controversy exists as to whether and under which conditions monocytic cells can be activated for increased NO production.36-39 Sharara et al38 found a modestly increased NO production of human monocytes treated with IFN-α, and Weinberg et al39 reported that a basal NO production by human monocytes could not be increased by treatment with LPS plus IFN-γ. Together these published data suggest that significant NO production by human DCs is unlikely to occur. In accordance with such an interpretation of the published literature, we found no inhibition of IFN-α plus LPS induced apoptosis by the NO synthase inhibitor L-NMMA (NG-monomethyl-L-arginine, monoacetate) (data not shown).
Apart from being induced directly in the DCs, alternatively, apoptosis might be mediated indirectly, for instance, through the action of T cells expressing Fas ligand. Both sensitivity as well as resistance of human monocyte-derived DCs to Fas-mediated apoptosis has been described.40-42 In preliminary experiments, cross-linked Fas ligand neither induced nor a blocking CD95 Fas antibody reduced apoptosis in our system (not shown). Most of our DC cell preparations based on density gradient centrifugation purified monocytes contained between 5% and 15% T cells. However, further purification and depletion of these T cells by FACS sorting did not have any influence on the apoptosis induction in response to IFN-α plus LPS (not shown), making an involvement of contaminating T cells unlikely.
Regardless of the intracellular mechanism responsible for the observed apoptosis, what is the biological relevance of our finding? It is conceivable that bacterial products and sufficiently high concentrations of type I IFNs could coincide during bacterial infections, because bacteria and their products have been shown to induce type I IFNs in monocyte and macrophage cells at least under certain circumstances.2-4,10 Although LPS directly induces IFN-α in GM-CSF–primed human monocytes4 and human DCs,10 we have not seen marked apoptosis in response to LPS alone nor has such a phenomenon been described to our knowledge. This phenomenon could be due to a requirement of IFN priming before LPS can subsequently induce apoptosis, which would not occur if the type I IFN accumulation is delayed during culture. Indeed, such a critical timing of both stimuli was described with bovine macrophages.29 However, in vivo LPS-induced IFN-α could make other monocytic cells or DCs not yet in contact with the LPS susceptible to subsequent activation-induced cell death. Monocytes from septic patients might be primed by type I IFNs by such a mechanism and indeed have been reported to display a predilection to undergo apoptosis in response to LPS.43
Type I IFNs are produced in large amounts by the rare type 2 DCs9,16,17 and at much lower levels by other more frequent cells such as monocytes or B cells.9 Usually, this synthesis of type I IFN by activated cells appears to be only transient,44 resulting in elevated type I IFN serum levels in acute but not in chronic viral infections.5-7 Thus, cell death by coincidence of IFN-α priming and bacterial activation seems less likely in chronic viral infections but still could occur, for instance in bacterially colonized tissues (ie, the upper respiratory and the gastrointestinal tract during an acute viral illness).
Apart from infectious situations, a prolonged and more systemic presence of type I IFNs might also be achieved during treatment with type I IFNs, as currently employed in hepatitis C, multiple sclerosis, or selected malignancies. This treatment could result in the permanent priming of monocytes and immature DCs for activation-induced apoptosis in response to any infection or other activating stimuli. Interestingly, a transient decrease in monocytes has been reported after IFN-α application to healthy humans.45
IFN-α and endotoxin have already been individually known to influence the function of monocytes and derived DCs. Our study reveals that both agents in combination are potent inducers of apoptosis in these cells, pointing to a new and potentially relevant mechanism of immunosuppression in infectious disease.
We thank Dr G. Mehes (CCRI, Vienna, Austria) for technical assistance in fluorescence microscopy and Dr H. Kovar (CCRI) for expert advice in detection of apoptosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wolfgang Holter, Children's Cancer Research Institute, Kinderspitalgasse 6, A-1090, Vienna, Austria; e-mail:holter@ccri.univie.ac.at.




![Fig. 8. Immature DCs generated by GM-CSF plus IL-4 in the presence of IFN-α undergo apoptosis in response to a variety of activation stimuli. / The response of immature DCs generated in the permanent presence of 1000 U/mL IFN-α (▪) to the indicated stimuli was compared with that of immature DCs generated in the absence of IFN-α (░). Apoptosis was quantified on day 8 (ie, 3 days after activation with 1 μg/mL LTA, 100 ng/mL LPS, 100 ng/mL LPS plus 1000 U/mL IFN-γ, and 1000 U/mL TNF-α plus 1 μg/mL PGE2 as indicated [mean ± SD of 3 independent experiments]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/3/10.1182_blood.v98.3.736/5/m_h81511329008.jpeg?Expires=1767738219&Signature=ch7zHJLmV3~EhKe85J53ultSdGBzhxHCAg-Vyph8IWRLlGbce747RLPoWGddAkGEJzaeDh-96sifx7lpdqs~9BoFgP~OhLjw7FgjtZtGw2TRekSJz~LIs-HGUxM30tdwY5o8nXRIDDf88X15v5SF0s5rdP6fQdp9ZT5Zb-eVc22wRpoNRELcrVktsUK-tlkkGvaXJh9b3ImeIz9iC5nqOOMs3I6TNdotppLvnUS72ovsuWOlmlRLmebOi4V~~t~Jlv0AJUr0d57yjgDvnnGwkSSH3dSzC6LKPr44~Fr~jjrxYSQhJETaXSwP51DDbgH6KWPaJjG~LfMrvcy1PJX6mA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal