Arsenic trioxide has been shown to be effective in treating acute promyelocytic leukemia (APL), with minimal overall toxicity reported to date. A phase I/II study was initiated in June 1998 using arsenic trioxide for relapsed APL to determine the maximum tolerated or minimal effective dose and to determine the efficacy of treatment at that dose. Ten patients received 1 to 4 monthly cycles of treatment with 0.1 mg/kg per day intravenous arsenic trioxide. Six of 7 patients evaluable for response achieved cytogenetic or molecular complete remission. However, 3 patients died suddenly during the first cycle of treatment. Autopsies obtained on 2 of these failed to identify a cause of sudden death, despite evidence of pulmonary hemorrhage in one. A third patient, for whom an autopsy was not performed, became asystolic and died while on continuous cardiac telemetry. These observations suggest that arsenic trioxide may be significantly or even fatally toxic at doses currently used and that caution is warranted in its use.
Introduction
The prognosis for patients with newly diagnosed acute promyelocytic leukemia (APL) has been significantly improved during the past decade with the incorporation of all-transretinoic acid (ATRA) into conventional remission induction regimens and its use in postremission maintenance therapy. Disease-free survival rates of 67% to 84% at 2 to 4 years have been reported in several large series in which chemotherapy and ATRA were used in combination.1-4 However, subsets of patients at high risk have been identified (based on elevated white blood cell count at presentation, increased age, time to remission) for whom outcomes remain considerably less favorable,3 4 and, until recently, potentially curative treatment options for patients with refractory and relapsed APL were limited to allogeneic stem cell transplantation.
Several recent reports have documented the efficacy of arsenic trioxide for treating APL, including relapsed or refractory disease.5-9 Hematologic remission rates of 85% to 90% have been reported using doses of approximately 0.10 to 0.15 mg/kg per day for variable durations, with most patients eventually achieving cytogenetic or molecular remission without additional chemotherapy. Most toxicities reported have been surprisingly mild and transient. These have included skin rashes, musculoskeletal pain, nausea, headaches, dizziness, and elevated creatinine and liver transaminase levels. A retinoic acid–like syndrome, characterized by hypoxia, pulmonary infiltrates, and transient leukocytosis, was observed in up to one third of patients during early treatment and was successfully treated with corticosteroids.9 Peripheral sensorimotor neuropathy, with symptoms ranging from paresthesias to paresis, has been reported by our group and others.8-12Electrocardiographic changes, including QTc prolongation, T-wave abnormalities, and second-degree heart block, have been observed, but there are no prior reports in the peer-reviewed literature of serious ventricular arrhythmias or treatment-related deaths among patients with APL treated with arsenic trioxide.
To determine the maximum tolerated dose (MTD) or minimal effective dose (MED) of arsenic trioxide for treating relapsed or refractory APL, to further define the efficacy of treatment at that dose, and to determine the acute and chronic toxicities of arsenic trioxide, we initiated a phase I/II study in June 1998. Our data from the initial phase of the study indicate that though active against APL, arsenic trioxide may be significantly, perhaps fatally, toxic at doses currently in use.
Patients, materials, and methods
Eligibility
Patients with relapsed APL or those who did not enter remission after initial ATRA and chemotherapy were eligible for study entry with signed informed consent. One patient was treated exactly according to the protocol as an institutional review board–approved single patient exception 1 week before full institutional review board approval of the study at Washington University.
Participating institutions
Participating institutions were Washington University Medical Center, St Louis, MO; Oregon Health Sciences Center, Portland, OR; University of Pennsylvania Medical Center, Philadelphia, PA; and Wake Forest University Baptist Medical Center, Winston-Salem, NC. This study was reviewed and approved by the respective institutional review boards at each participating institution.
Study drug
Arsenic trioxide in ACS reagent grade was purchased from Sigma Chemical (A5081; St Louis, MO). A 10-mg/mL stock solution was prepared by dissolving the compound in 0.5 N sodium hydroxide solution and then adding concentrated hydrochloric acid to achieve a final pH of 7.4. For patients 1 to 4, the pharmacy performed the dissolution step for each arsenic dose on the day of treatment and subsequently diluted the dose into 500 mL normal saline immediately before administration. Subsequently, to ensure greater uniformity and quality control, a method was developed for filter sterilization of individual lots of drug into multiuse 50-mL glass vials (35; Baxa, Engelwood, CA). Samples from each lot were subjected to and passed analyses for sterility, the absence of endotoxin, particulate matter, and heavy metal contamination, and quantitative analysis for arsenic III and arsenic V (performed by Sigma Chemical on a contract basis). Drug preparation procedures were reviewed and approved by the Food and Drug Administration under IND52597.
Treatment
Arsenic trioxide was administered as a 2-hour intravenous infusion through a central venous catheter for 28 consecutive days during each cycle, followed by a 2-week treatment break. Up to 3 induction cycles were administered until cytogenetic remission was achieved, after which one additional consolidation cycle was given. The trial was designed as a dose-escalation study in its initial phase, beginning at a dose of 0.10 mg/kg per day and increasing in 0.05 mg/kg per day increments with successive 3-patient cohorts until dose-limiting toxicity was observed or until a response rate of 2 of 3 patients was observed and confirmed in a second 3-patient cohort, to establish a minimum effective dose. As the result of 2 unexpected deaths, the weight used to calculate dosage in obese patients was capped at 150% of height-based ideal body weight in the last patient treated. The phase II portion of the study, in which 20 additional patients were to be enrolled and treated, has not been undertaken. Treatment was initially permitted on an outpatient basis after the first 3 days. Because of the early deaths of 2 patients, the protocol was modified for the treatment of patient 10 to require hospitalization with continuous cardiac monitoring for the first 21 days of treatment.
Evaluation
Bone marrow evaluations were obtained after each 28-day treatment cycle and included routine microscopic analysis, cytogenetics, and reverse transcription–polymerase chain reaction (RT-PCR) analysis for detection of the PML/RAR transcript. Patients who died before day 28 of cycle 1 were considered inevaluable for response. Toxicity was formally evaluated on a weekly basis. Biweekly serum chemistries, blood counts, and coagulation parameters were obtained. Electrocardiograms (ECGs) initially were obtained before and after the first dose of each cycle and thereafter as clinically indicated. After 2 early deaths were observed, ECGs were obtained weekly on the last patient treated to monitor more closely for evidence of progressive QTc prolongation. Audiograms were obtained before each cycle and after the completion of therapy.
Pharmacokinetics
Whole blood samples were obtained 1, 2, 4, 6, and 12 hours after arsenic infusion on days 1 to 3 and day 28 of cycle 1. Twenty-four–hour urine collections were obtained after the first and last treatments of cycle 1. Quantitative arsenic determinations were performed by Mayo Medical Laboratories on a contract basis. Because urine arsenic measurements were based on the amount of elemental arsenic present, and whereas arsenic trioxide consists of only 76% arsenic by weight, the percentage of the daily arsenic dose excreted was calculated as [mg arsenic in urine/0.76 × mg arsenic trioxide administered].
Results
Responses
Nine patients at 4 centers were enrolled (Table1), in addition to a patient treated according to protocol as a single patient exception, whose data are also presented (identified where applicable with an asterisk). All patients had relapsed APL after they previously achieved remissions with ATRA and chemotherapy, and all carried the t(15;17) translocation. Two patients had relapses in the central nervous system (CNS) and received biweekly intrathecal methotrexate through an Ommaya reservoir, given concomitantly with intravenous arsenic trioxide until the cerebrospinal fluid was rendered free of leukemia cells. Both patients with CNS involvement also had either cytogenetic or molecular evidence of APL in the bone marrow. All patients were treated with 0.10 mg/kg per day arsenic trioxide, with the exception of patient 10, who received 0.055 mg/kg per day based on actual body weight (285% of ideal body weight). After 1 to 3 induction cycles, 6 of 7 evaluable patients entered cytogenetic remission. Five of the 6 responders completed an additional consolidation cycle, and 5 of 6 were in molecular remission after completing treatment. Relapses occurred in 5 of 6 responders, at intervals of 1, 2, 11, 13, and 19 months after completing treatment and subsequently died within 1 to 2 months. Two of these patients were retreated with arsenic but failed to respond. Two patients underwent allogeneic stem cell transplantation but subsequently died of recurrent leukemia. Two patients underwent unrelated donor bone marrow transplantation after completing arsenic treatment. One patient remains alive and disease-free 8 months after completing arsenic treatment, whereas one patient died with persistent APL 9 months after completing arsenic treatment. Other than the patients who subsequently had allogeneic or unrelated donor transplantation, no other therapy was administered after the completion of arsenic trioxide therapy. The Kaplan-Meier survival probability at 1 year was 50% (Figure 1).
Patient characteristics and outcomes
| Patient . | Age (y) . | Sex . | Disease status at study entry . | No. arsenic cycles . | Best response . | Outcome (posttreatment follow-up) . |
|---|---|---|---|---|---|---|
| 1* | 35 | M | Fourth relapse | 4 | Cyto, PCR | Dead, relapsed disease (3 mo) |
| 2 | 71 | M | First relapse (CNS) | 3 | Cyto, PCR | Dead, relapsed disease (21 mo) |
| 3 | 34 | M | First relapse | <1 | N/E | Sudden death, d15, cycle 1 |
| 4 | 23 | M | First relapse | 1 | Cyto, PCR | Allogeneic transplant Dead, relapsed disease (13 mo) |
| 5 | 55 | M | First relapse | 3 | Cyto | Dead, relapsed disease (3 mo) |
| 6 | 49 | M | First relapse (CNS) | 1 | Cyto, PCR | Allogeneic transplant Dead, relapsed disease (12 mo) |
| 7 | 11 | M | First relapse | 3 | Persistent disease | MUD transplant Dead, VOD (9 mo) |
| 8 | 19 | F | First relapse | 3 | Cyto, PCR | MUD transplant Alive, disease free (8 mo) |
| 9 | 26 | F | First relapse | <1 | N/E | Sudden death, d11, cycle 1 |
| 10 | 37 | F | Third relapse | <1 | N/E | Sudden death, d9, cycle 1 |
| Patient . | Age (y) . | Sex . | Disease status at study entry . | No. arsenic cycles . | Best response . | Outcome (posttreatment follow-up) . |
|---|---|---|---|---|---|---|
| 1* | 35 | M | Fourth relapse | 4 | Cyto, PCR | Dead, relapsed disease (3 mo) |
| 2 | 71 | M | First relapse (CNS) | 3 | Cyto, PCR | Dead, relapsed disease (21 mo) |
| 3 | 34 | M | First relapse | <1 | N/E | Sudden death, d15, cycle 1 |
| 4 | 23 | M | First relapse | 1 | Cyto, PCR | Allogeneic transplant Dead, relapsed disease (13 mo) |
| 5 | 55 | M | First relapse | 3 | Cyto | Dead, relapsed disease (3 mo) |
| 6 | 49 | M | First relapse (CNS) | 1 | Cyto, PCR | Allogeneic transplant Dead, relapsed disease (12 mo) |
| 7 | 11 | M | First relapse | 3 | Persistent disease | MUD transplant Dead, VOD (9 mo) |
| 8 | 19 | F | First relapse | 3 | Cyto, PCR | MUD transplant Alive, disease free (8 mo) |
| 9 | 26 | F | First relapse | <1 | N/E | Sudden death, d11, cycle 1 |
| 10 | 37 | F | Third relapse | <1 | N/E | Sudden death, d9, cycle 1 |
Cyto indicates cytogenetic complete response; PCR, molecular complete response; CNS, central nervous system; N/E, not evaluable; MUD, matched unrelated donor transplant; VOD, veno-occlusive disease.
Single patient exception.
Kaplan-Meier probability.
The Kaplan-Meier probability of survival after study entry is plotted against time.
Kaplan-Meier probability.
The Kaplan-Meier probability of survival after study entry is plotted against time.
Sudden deaths
Three patients (patients 3, 9, and 10) died suddenly during the first cycle of treatment, on days 15, 11, and 9, respectively.
Patient 3 had APL in first relapse with the following: WBC, 7100 cells/μL; mild disseminated intravascular coagulation (DIC); prothrombin time (PT), 15.3 seconds (normal range, 11.0-13.0 seconds); FDP, 64 μg/mL (normal range, 1-8 μg/mL). DIC parameters normalized within the first 4 days of treatment. On day 11, he was admitted to the hospital with fever, neutropenia, thrombocytopenia, and radiographic patchy pulmonary infiltrates. His WBC was 9400 cells/μL with 21% promyelocytes. He remained clinically stable, however, and arsenic treatment was continued. On day 15, he was found to be thrombocytopenic and was transfused with platelets. Thirty minutes after completion of his platelet transfusion, he was found apneic and unresponsive. Before that, he had been in no acute distress, vital signs were stable, and he had not called for assistance. There was no cardiac electrical activity documented at the time he was found, and resuscitation attempts to restore a meaningful cardiac rhythm were unsuccessful. A magnesium level of 2.3 mEq/L and a potassium level of 3.8 mM, respectively, were documented at the time of death. Autopsy revealed alveolar hemorrhage but no evidence of pulmonary embolism, myocardial infarction, or stroke. A blood bank workup to exclude the possibility of a serious platelet transfusion reaction demonstrated no evidence of bacterial contamination or cross-reactive alloantibody in the product. Blood cultures obtained on admission and 48 hours before death were negative. The infusion of arsenic dose 14 had been completed approximately 22 hours earlier.
Patient 9 had APL in first relapse with leukopenia and thrombocytopenia but without circulating blasts or promyelocytes and without evidence of DIC. She was clinically stable and receiving arsenic trioxide on an outpatient basis when she was found dead in her bed at home on day 11, approximately 16 hours after arsenic dose 10 had been completed. Hypomagnesemia (1.1 mEq/L) was documented 3 days before death and was treated with magnesium supplementation. Two days before her death her potassium level was documented as 3.3. Autopsy revealed no anatomic cause of death.
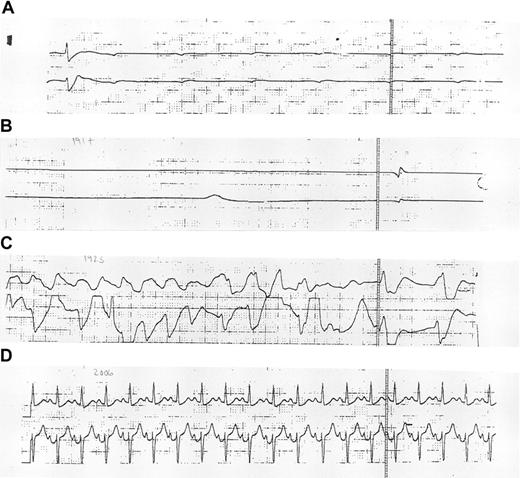
Patient 10 had a WBC of 35 000 cells/μL before starting arsenic treatment. During the course of treatment, her leukocytosis progressively worsened, for which she was treated with low-dose Ara-C; on day 7, with a WBC of 91 000 cells/μL, she underwent leukopheresis. Mild DIC, based on PT elevation (13.1 seconds) and D-dimer elevation (4000-8000 U; normal, less than 500 U), with normal fibrinogen levels, was present before arsenic treatment. DIC persisted throughout the course of her disease and was treated with low-dose heparin. On day 5 of treatment, respiratory distress developed secondary to volume overload or retinoic acid–like syndrome, for which she required endotracheal intubation and mechanical ventilation. Chest radiography showed pulmonary edema. Corticosteroids and fluid diuresis were initiated and resulted in improved oxygenation; despite this, she continued to require mechanical ventilation. Arsenic trioxide was resumed on day 6. On day 9, 7 hours after the completion of arsenic treatment and while she was on continuous cardiac monitoring, she became asystolic. Available monitoring strips demonstrated a single wide QRS complex and several nonconducted P-waves at a rate of 50 bpm, without the emergence of an escape rhythm (Figure2A), and the subsequent disappearance of all electrical activity (Figure 2B). Atropine was administered, after which pulseless, wide, complex tachycardia and subsequent ventricular fibrillation developed (Figure 2C). Epinephrine and unsynchronized cardioversion administration restored her pulse and blood pressure, which were transiently maintained with escalating doses of phenylephrine HCl. ECG demonstrated a narrow complex tachycardia with a QTc interval of approximately 441 millisecond (ms) (normal QTc, less than 440 ms) (Figure 2D). Two hours after the initial asystolic event, in the face of progressive hypotension, she again became asystolic, and further resuscitation events were unsuccessful. Her potassium level at the time of the initial asystolic event was 4.2 mM; her magnesium level was 2.3 mEq/L. An autopsy was not performed.
Electrocardiographic monitoring.
Electrocardiographic monitoring strips obtained during (A-C) and 2 hours after (D) the initial resuscitation of patient 10 are shown.
Electrocardiographic monitoring.
Electrocardiographic monitoring strips obtained during (A-C) and 2 hours after (D) the initial resuscitation of patient 10 are shown.
ECGs were obtained daily on patient 10 preceding her death and showed QTc intervals in the 439 to 508 ms range (482 ms 12 hours before the initial asystolic event). ECGs obtained 10 and 14 days before the deaths of patients 3 and 9 were unremarkable. Each patient was treated with a different lot of the study drug. In patient 3, arsenic trioxide was prepared daily from powdered form in the pharmacy, as described above. In patients 9 and 10, arsenic trioxide was administered from different preparation lots. The 3 deaths occurred at 2 separate institutions. All 3 patients were obese (greater than 150% of ideal body weight), young (26-37 years of age), and of African American descent. Ciprofloxacin had been used to treat infection in all 3 patients but was discontinued 4 to 5 days before death in patients 3 and 9. Fluconazole was used for patient 10 at the time of death and had been discontinued 3 days earlier in patient 9. There were no other significant overlapping medications, and no medications with known arrhythmogenic activity were documented at the time of or preceding these deaths. Phenylephrine HCl was used in patient 10 only after her initial asystolic event. None of these patients had a personal or a family history of cardiac disease.
Other toxicities
Other transient toxicities were observed (Table2), including musculoskeletal pain, creatinine, transaminase, and amylase elevations, diarrhea, constipation, anxiety/depression, and high-frequency hearing loss. Chronic atrial fibrillation/flutter of unclear etiology developed in one patient 3 months after the completion of arsenic therapy; he subsequently underwent allogeneic transplantation. His arrhythmia was controlled with amiodarone, but he subsequently died with relapsed APL.
Acute toxicities observed during treatment
| Symptom/sign . | Grade . | Incidence . |
|---|---|---|
| Anxiety/depression | 1-2 | 1 of 10 |
| Constipation | 1-2 | 4 of 10 |
| Creatinine elevation | 1-2 | 1 of 10 |
| Diarrhea | 1-2 | 4 of 10 |
| Dyspnea | 1-2 | 2 of 10 |
| 3-4 | 1 of 10 | |
| Edema/fluid retention | 1-2 | 3 of 10 |
| 3-4 | 1 of 10 | |
| Fever | 1-2 | 6 of 10 |
| Hearing loss | 1-2 | 2 of 10 |
| LDH elevation | 3-4 | 1 of 10 |
| Musculoskeletal pain | 1-2 | 5 of 10 |
| 3-4 | 2 of 10 | |
| Pancreatitis | 3-4 | 1 of 10 |
| Paresthesias | 1-2 | 1 of 10 |
| Pneumonia | 1-2 | 2 of 10 |
| QTc prolongation | 441-480 ms | 6 of 10 |
| 481-520 ms | 3 of 10 | |
| Tinnitus | 1-2 | 2 of 10 |
| Transaminase elevation | 1-2 | 2 of 10 |
| Zoster outbreak | — | 1 of 10 |
| Symptom/sign . | Grade . | Incidence . |
|---|---|---|
| Anxiety/depression | 1-2 | 1 of 10 |
| Constipation | 1-2 | 4 of 10 |
| Creatinine elevation | 1-2 | 1 of 10 |
| Diarrhea | 1-2 | 4 of 10 |
| Dyspnea | 1-2 | 2 of 10 |
| 3-4 | 1 of 10 | |
| Edema/fluid retention | 1-2 | 3 of 10 |
| 3-4 | 1 of 10 | |
| Fever | 1-2 | 6 of 10 |
| Hearing loss | 1-2 | 2 of 10 |
| LDH elevation | 3-4 | 1 of 10 |
| Musculoskeletal pain | 1-2 | 5 of 10 |
| 3-4 | 2 of 10 | |
| Pancreatitis | 3-4 | 1 of 10 |
| Paresthesias | 1-2 | 1 of 10 |
| Pneumonia | 1-2 | 2 of 10 |
| QTc prolongation | 441-480 ms | 6 of 10 |
| 481-520 ms | 3 of 10 | |
| Tinnitus | 1-2 | 2 of 10 |
| Transaminase elevation | 1-2 | 2 of 10 |
| Zoster outbreak | — | 1 of 10 |
LDH indicates lactate dehydrogenase; QTc, QT interval.
Pharmacokinetic data
Blood and 24-hour urine arsenic levels were obtained in all patients (Table 3). Peak arsenic levels in blood were barely detectable during days 1 to 3 in most patients (range, less than 0.05-0.13 μg/mL), whereas by day 28, mean peak levels of 0.18 μg/mL (range, 0.13-0.31 μg/mL) were observed in evaluable patients. Day 3 arsenic levels in patients 3 and 9 were undetectable; the day 7 arsenic level in patient 10 was 0.078 μg/mL. Mean urinary excretion of arsenic during the first 24 hours of treatment was 18.7% of the daily dose (range, 6.1%-31.0%). Urinary excretion in the patients who died was 12.8%, 10.1%, and 21.3%, respectively, of the daily dose.
Pharmacokinetic data
| Patient . | Peak blood arsenic . | 24-hour urine excretion (day 1, % of dose) . | Day 1 . | ||
|---|---|---|---|---|---|
| (day 3, μg/mL) . | (day 28, μg/mL) . | Vd(L/kg) . | AUC (μg/mL · h) . | ||
| 13-150 | <0.05 | 0.15 | NA | NA | NA |
| 2 | 0.13 | 0.31 | 22.9 | 0.91 | 1.37 |
| 33-151 | <0.05 | NA | 12.8 | ND | ND |
| 4 | 0.07 | 0.14 | 6.1 | ND | ND |
| 5 | 0.09 | 0.13 | 25.2 | 1.22 | 1.10 |
| 6 | 0.08 | 0.15 | 14.5 | 2.46 | 0.91 |
| 7 | <0.05 | NA | 24.4 | ND | ND |
| 8 | 0.065 | NA | 31.0 | ND | ND |
| 93-151 | <0.05 | NA | 10.1 | ND | ND |
| 103-151 | 0.0783-152 | NA | 21.3 | ND | ND |
| Patient . | Peak blood arsenic . | 24-hour urine excretion (day 1, % of dose) . | Day 1 . | ||
|---|---|---|---|---|---|
| (day 3, μg/mL) . | (day 28, μg/mL) . | Vd(L/kg) . | AUC (μg/mL · h) . | ||
| 13-150 | <0.05 | 0.15 | NA | NA | NA |
| 2 | 0.13 | 0.31 | 22.9 | 0.91 | 1.37 |
| 33-151 | <0.05 | NA | 12.8 | ND | ND |
| 4 | 0.07 | 0.14 | 6.1 | ND | ND |
| 5 | 0.09 | 0.13 | 25.2 | 1.22 | 1.10 |
| 6 | 0.08 | 0.15 | 14.5 | 2.46 | 0.91 |
| 7 | <0.05 | NA | 24.4 | ND | ND |
| 8 | 0.065 | NA | 31.0 | ND | ND |
| 93-151 | <0.05 | NA | 10.1 | ND | ND |
| 103-151 | 0.0783-152 | NA | 21.3 | ND | ND |
NA indicates not available; ND, not detectable.
Single patient exception.
Sudden deaths.
Collected on day 7.
The arsenic concentration-time curve was best described by a 2-compartment model.13 However, compartmental analysis could only be achieved in 3 of 11 patients because of undetectable blood concentrations in most samples on days 1 to 3. The volume of distribution (VD steady-state) in these 3 patients ranged from 0.91 to 2.46 L/kg. Area under the concentration-time curve for the first dose (24 hours) ranged from 0.91 to 1.37 μg/mL per hour. Mean trough arsenic concentration was evaluable in 9 patients (Figure3). Blood arsenic was undetectable during days 1 to 28 in 4 of 9 patients, despite daily dosing. In 2 of 9 patients, arsenic concentrations were undetectable for the first 3 days and accumulated to 0.10 and 0.11 μg/mL, respectively, on day 28. Three patients demonstrated a steady accumulation of blood arsenic over time. At the end of the first cycle (day 28), the mean trough concentrations over 8 hours in these 3 patients were 0.16, 0.11, and 0.10 μg/mL, respectively.
Arsenic trough concentrations.
Mean blood arsenic trough concentrations over the course of cycle 1 are plotted against time for each evaluable patient.
Arsenic trough concentrations.
Mean blood arsenic trough concentrations over the course of cycle 1 are plotted against time for each evaluable patient.
Discussion
In this study, we observed impressive activity of arsenic trioxide in relapsed APL, consistent with earlier reports.5-9However, despite rapid molecular remissions in 6 of 7 patients, the durability of remissions in our series was short-lived in most cases, and no patients remained in remission without further therapy. The number of patients in this study is small, but our experience nonetheless suggests that more prolonged postremission treatment or a higher daily dose of arsenic may be required for optimal disease control. However, the potential for increased toxicity with more aggressive arsenic dosing may ultimately limit the usefulness of either approach.
The incidence of unexplained sudden death among APL patients treated with this agent was striking. Potential explanations for these deaths include both treatment-related arsenic toxicity and leukemia-related complications, including sepsis and bleeding. All 3 early deaths in our study occurred in patients with persistent APL, which is often associated with life-threatening DIC. Two of these patients were unstable enough to require hospitalization, whereas one required mechanical ventilation and had evidence of moderate DIC preceding her death. One of the patients, however, was clinically stable and was receiving treatment as an outpatient at the time of her death. Autopsies performed on 2 of the patients failed to identify a definite anatomic cause of sudden death, such as pulmonary embolism, stroke, or coronary artery thrombosis, increasing the likelihood that cardiac arrhythmia might have played a causative role in their deaths. Moreover, the sudden onset of asystole, without other preceding arrhythmia, was documented in one of these patients. The presence of mild to borderline electrolyte abnormalities in one patient before death might have played a contributory role in lowering the arrhythmia threshold, highlighting the importance of aggressive electrolyte management in these patients.
The incidence of cardiac arrhythmias after arsenic poisoning has been well documented.14-16 Two cases of torsades des pointes ventricular tachycardia and one case of ventricular fibrillation (preceded by torsades) have been reported after accidental arsenic ingestion. In all 3 cases, QTc prolongation and T-wave inversions, indicative of widespread repolarization abnormalities, were noted to precede the onset of ventricular tachycardia. Moreover, in each case, there was evidence of profound peripheral neuropathy before the arrhythmias, which occurred between 4 days and 4 weeks after arsenic exposure. It has been speculated that autonomic dysregulation, as opposed to a direct cardiotoxic effect of arsenic, might have played a role in the development of these arrhythmias.15 16
Frequent electrocardiographic prolongation of the QTc interval has been reported in patients with APL receiving arsenic trioxide, as well as cases of second- and third-degree heart block requiring permanent pacemaker placement.8,9,17 In one recently published series of 19 patients with non-APL hematologic malignancies treated with 10 to 20 mg/d intravenous arsenic trioxide, ventricular tachycardia (torsades des pointes) developed after 16 to 42 days of treatment in 3 patients (2 of whom died as a result). Antecedent mild QTc prolongation was noted in all 3 patients, whereas ventricular ejection fractions were normal.18 In another series of 40 APL patients treated with 0.15 mg/kg per day arsenic trioxide for variable periods, no life-threatening arrhythmias or treatment-related deaths were reported, despite the frequent observation of QTc prolongation.9 Likewise, in series of 15 and 32 patients, respectively, with relapsed APL treated with 10 mg/d arsenic trioxide in China, no arrhythmias or treatment-related deaths were reported.2 3
The incidence of unexplained deaths in 3 of 10 patients reported herein is in contrast to the initial Sloan-Kettering8 and subsequent US Multicenter studies,9 in which 52 patients were treated and no treatment-related deaths were reported. If the deaths in our study were in fact treatment-related, this discrepancy may reflect a difference in the formulation of the arsenic trioxide drug product used in our study. In the former studies8,9and in individual patients treated on a compassionate-use basis through the Cancer Trials Evaluation Program, arsenic trioxide was provided by a then privately held pharmaceutical company (PolarX, New York, NY), whose manufacturing processes are considered proprietary. This product (Trisenox, Cell Therapeutics, Seattle, WA) has subsequently been approved by the Food and Drug Administration for use in relapsed APL. There is no information available on the drug product used in the Chinese studies.5-7 However, the assayed purity of the starting compound used in our study, the minimal extent of processing involved in its preparation, and the rigorous postpreparation assays for purity, sterility, endotoxin, and concentration to which the drug product was subjected and passed make it unlikely that formulation issues accounted for the potential toxicity observed in our series.
The fact that all 3 patients who died early in our study were of African American descent raises the possibility of a genetic basis for differing susceptibilities to arsenic trioxide toxicity between individual patients. Inorganic arsenic undergoes enzymatic covalent modification, primarily in the liver, to generate monomethylated and dimethylated forms.19 Methylated arsenic is considerably less toxic than trivalent inorganic arsenic and is eliminated more readily by renal excretion. Extensive variation in the extent of arsenic methylation in urine has been described within human populations.19 It has been speculated that differences in the capacity to methylate arsenic may underlie different susceptibilities to arsenic toxicity between persons,19 a phenomenon that could account for the fatal outcomes observed in our series. However, there is no evidence in the literature to suggest that arsenic metabolism among African Americans differs significantly from that among other racial groups. Although we observed no obvious differences in arsenic pharmacokinetics and elimination between patients who died early and those who did not, we cannot rule out potentially important differences in arsenic methylation in these patients because fractionation into organic and inorganic forms was not performed.
The unexplained early deaths observed in our study of arsenic trioxide and other anecdotal evidence of serious, potentially arsenic-related cardiac toxicity presented here suggest that this agent, at the doses currently in use for the treatment of APL, may have more significant toxicity than previously recognized. Until the issues of acute and chronic arsenic toxicity have been better defined, we believe that arsenic trioxide should be used with caution in APL patients, particularly in those with pre-existing cardiac abnormalities, and that its use is best suited for patients with relapsed or refractory disease, preferably in a clinical trial setting.
We thank Dr Madeleine Kraus and Dr Jay Hess for review of the pathology material; Stacey Postma, Jennifer Moellering, and Tonya Wojka for expert data management; Carol Rush for administrative assistance; and Nancy Reidelberger for secretarial assistance.
Supported by Food and Drug Administration Orphan Drug Development grant FD-R-001657-01.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter Westervelt, Division of Bone Marrow Transplantation and Stem Cell Biology, Washington University School of Medicine, Campus Box 8007, 660 S Euclid Ave, St Louis, MO 63110; e-mail: pwesterv@im.wustl.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal