Abstract
The human blood group i and I antigens are characterized as linear and branched repeats of N-acetyllactosamine, respectively. Conversion of the i to the I structure requires the activity of I-branching β-1,6-N-acetylglucosaminyltransferase (IGnT). Thus the blood group I gene is assigned to encode a β-1,6-N-acetylglucosaminyltransferase; however, its identity has not been confirmed. The null phenotype of I, the adult i phenotype, provides a means to identify the I gene. Interestingly, the adult i phenotype has been noted to be associated with congenital cataracts in Asians. Molecular genetic studies of 3 adult i pedigrees are reported here. The results obtained on mutation detection within the 2 I-branching enzyme encoding genes, segregation analyses, and enzyme function assays identify molecular changes associated with the adult i phenotype. The adult i phenotype in 2 of the pedigrees studied resulted from 1043G→A and 1148G→A mutations, which predict Gly348Glu and Arg383His alterations, respectively, in theIGnT gene. These amino acid changes abolished the original GlcNAc-transferase activity. Deletion of the IGnT gene was observed in the person with adult i phenotype in the third pedigree. These findings suggest that the IGnT gene, first reported in 1993, is the candidate for the blood group I gene. Confirmation of the blood group I gene will further assist in the investigations of the molecular genetics that control I antigen expression in secretions and the molecular basis for the association of the adult i phenotype with congenital cataracts in Asians.
Introduction
In 1956, Wiener et al1 first gave the name I to an antigen detected by a cold agglutinating autoantibody called anti-I. They found that red blood cells (RBCs) of only a small percentage of persons (5 of 22 000) were nonreactive to anti-I, and this phenotype was called I-negative. Later studies found that cord blood cells contained a very weak I antigen.2,3 In 1960, Marsh and Jenkins4 described the first cold agglutinating antibody, named anti-i, which behaved in an opposite manner to anti-I—reacting strongly with cord blood cells and RBCs with the I-negative phenotype but weakly with normal adult RBCs—and thus established the i antigen.5,6 Expression of the I and i antigens was soon found to have a reciprocal relationship and to be developmentally regulated. Adult human RBCs fully express I antigen and contain only a few i antigens, whereas the i antigen is predominantly present on fetal and neonatal RBCs. After birth, the quantity of I antigen gradually increases as the i antigen decreases, until the normal Ii status of adult RBCs is reached after approximately 18 months of life.5,6 Like ABH antigens, Ii antigens are also referred to as histo-blood group antigens7 because they are detected not only on RBCs but also on the surfaces of most human cells and on soluble glycoproteins in various body fluids, including saliva,8 plasma,8 milk,9amniotic fluid, urine, and ovarian cyst fluid.10 The developmentally regulated expression of Ii antigens is exhibited on RBCs and in many other tissues. High expression of the i antigen has been shown to be characteristic of immature and less differentiated cells.7 Altered expression patterns of I and i antigens have often been observed during oncogenesis processes,11 and thus the Ii antigens are considered as onco-developmental antigens.12
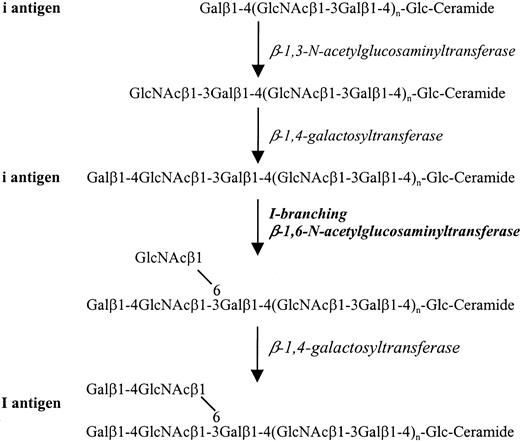
The Ii antigenic determinants have been elucidated through various studies.13-16 They are carbohydrate structures carried on glycolipids and glycoproteins and are present on the interior structures of the complex carbohydrate chains bearing ABH and Lewis antigens. Based on type 2 Galβ1-4GlcNAc chains, the basic i and I structures are characterized as linear and branched repeats ofN-acetyllactosamine, Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAc-R, and Galβ1-4GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)Galβ1-4GlcNAc-R, respectively (Figure 1). TheN-acetyllactosamine repeats are synthesized by the sequential action of β-1,3-N-acetylglucosaminyltransferase and β-1,4-galactosyltransferase. Conversion of i antigen into an I-active structure requires the activity of a third enzyme, the I-branching β-1,6-N-acetylglucosaminyltransferase (β6GlcNAc-T).14,16 17 Thus the blood group Ilocus is assigned to encode a β6GlcNAc-T. The reciprocal relationship of i- and I-antigen expression is believed to result from the appearance of the active I-branching enzyme after birth. However, I and i antigens cannot be considered products of alleles because the i antigen is determined by the action of β-1,3-N-acetylglucosaminyltransferase and β-1,4-galactosyltransferase. Consequently, the Ii antigens do not satisfy the criteria for designation as a blood group system and are categorized to comprise collection 207 according to the International Society of Blood Transfusion terminology.
Structures and biosynthetic pathways of the i and I antigens.
The i antigen is characterized by a linear chain of repeatingN-acetyllactosamine units and is converted to the branched I antigenic structure through the activity of I-branching β-1,6-N-acetylglucosaminyltransferase.
Structures and biosynthetic pathways of the i and I antigens.
The i antigen is characterized by a linear chain of repeatingN-acetyllactosamine units and is converted to the branched I antigenic structure through the activity of I-branching β-1,6-N-acetylglucosaminyltransferase.
In 1993, Bierhuizen et al18 reported the expression cloning of a cDNA encoding an I-branching β6GlcNAc-T. The gene, designatedIGnT, is located on chromosome 6p24.19,20Another gene, located on chromosome 15q21-22 and designatedC2GnT-M19 or C2/4GnT,20was identified and shown to encode another I-branching–forming enzyme.19 The blood group I locus has not been confirmed to date because more than one I-branching β6GlcNAc-T, coded by different loci, has been identified, and it is thought there may be other β6GlcNAc-Ts still awaiting elucidation.
The adult i phenotype should provide a means of demonstrating the gene responsible for blood group I antigen expression. Similar investigations have been of great value in confirming the identities of several blood group genes—among them Lewis,21Secretor,22 andPk,23—as those responsible for controlling their respective blood group antigen expression. Like fetal RBCs and cord cells, RBCs of persons with adult i phenotype are rich in i antigen but contain little I antigen. The phenotype is inherited as an autosomal recessive trait and is believed to result from lack of I-branching transferase activity.15,16 The frequency of the adult i phenotype is low, with only few occurrences in thousands or tens of thousands.10 Despite its rareness, the adult i phenotype has attracted considerable attention because of its association with congenital cataracts. Yamaguchi et al24-26first reported the observation of an association between the adult i phenotype and congenital cataracts among Japanese. Linkage of adult i phenotype and congenital cataracts has been observed in 3 Taiwanese pedigrees.27 However, the association does not seem to be as pronounced in the white population.28-30
Molecular genetic analysis of the adult i phenotype may provide a means of identifying the blood group I gene, but it also may facilitate progress in uncovering the basis for the association of the adult i phenotype with a frequent occurrence of congenital cataracts in Asians. Three Taiwanese adult i pedigrees were previously identified27; the 5 members with adult i phenotype all have congenital cataracts, whereas none of the other 17 members with I phenotype do. In this report we describe the findings of molecular genetic studies of these 3 pedigrees, in which the 2 reported I-branching β6GlcNAc-T encoding genes, IGnT andC2GnT-M, were targeted.
Materials and methods
Samples
Three adult i pedigrees (pedigrees S, W, and C), including 5 members with adult i phenotype, the rest of the members with common I phenotype, and 51 randomly selected controls served as the study subjects. Informed consent was obtained from all participants. Identification of the 3 families has been reported.27Before the current studies, the Ii phenotypes of the members in the 3 families were confirmed by a tube method using anti-I antibody (a gift from Dr Y. Okubo, Red Cross Blood Center, Osaka, Japan). Total RNA and genomic DNA from these persons were prepared from their peripheral blood cells using the QIAamp RNA Blood Mini Kit (Qiagen GmbH, Hilden, Germany) and the QIAamp DNA Blood Mini Kit (Qiagen), respectively.
Reverse transcription–polymerase chain reaction and cloning of the IGnT and C2GnT-M genes
Complementary DNA (cDNA) to the IGnT transcript was amplified from the RNA sample by reverse transcription–polymerase chain reaction (RT-PCR) using the OneStep RT-PCR Kit (Qiagen) and the synthetic oligonucleotide primer pair of IFb (AACAGGGCAGGAGTGAGTGGAGTATGTTGC, nucleotides −117 through −88 ofIGnT cDNA, codon for initiation methionine as nucleotides 1-3) and IRc (AGCTGCAGTTTCCCTTCAGTCATGAGTAGC, complementary of nucleotides 1215-1244). Then 1.5 μg total RNA and 15 pmol each primer were combined in a final volume of 25 μL RT-PCR master mix and were subjected to an RT-PCR program consisting of 30 minutes at 50°C and then 15 minutes at 95°C followed by 40 cycles of 1 minute at 94°C and 2.5 minutes at 72°C. The coding sequence of theC2GnT-M gene is located in a single exon and, therefore, could be amplified from genomic DNA by PCR. One hundred nanograms genomic DNA and 10 pmol each primer, MF1 (GGATTGTGTCCTCCTCCACCTTCCCTGTGC, nucleotides −61 through −32 ofC2GnT-M, codon for initiation methionine as nucleotides 1-3) and MR3 (CCACCCACACTGTCCCAGCAAGTTCTGAGC, complementary to nucleotides 1369-1398), were combined in PCR buffer containing 0.2 mM dNTP and 0.5 U hot-start Taq polymerase (Qiagen). The PCR program included 15 minutes at 95°C followed by 30 cycles of 1 minute at 94°C and 2.5 minutes at 72°C. PCR products of 1361 bp and 1459 bp, encompassing the coding regions of IGnT andC2GnT-M, respectively, were cloned into the pCRII-TOPO vectors using a TOPO TA Cloning Kit (Invitrogen, Groningen, The Netherlands), and DNA sequences were determined using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). Multiple clones from 2 batches of PCR products were sequenced to distinguish any PCR error from actual sequence polymorphism.
RT-PCR for the β-actin cDNA was performed using the same OneStep RT-PCR Kit (Qiagen) and the primers with sequences of CCTCGCCTTTGCCGATCC and GGATCTTCATGAGGTAGTCAGTC (antisense). The RT-PCR program was similar to that described above, except that annealing occurred at 55°C for 1 minute and extension at 72°C for 1 minute.
PCR amplifications of the 3 exon regions of the IGnT gene were achieved by using primer pairs of IFb and IRd (CCTAATAAAAAGTGGCTGGTTATTCTAAAGCC, antisense sequence, 50 nucleotides downstream of exon 1), IFh (TCCCTTCTCTCATGACTCTCATCTCTACGC, 22 nucleotides upstream of exon 2) and IRk (ACACCAACAGGCAGCGGCCT-AGAAGCATGG, antisense sequence, 14 nucleotides downstream of exon 2), and IFg (GTCGGAGAGTACCTCTAGTATTCTGTAAGTTC, 57 nucleotides upstream of exon 3) and IRc. PCR conditions were similar to those described above, except that annealing occurred at 60°C for 1 minute and extension at 72°C for 1 minute.
PCR-sequence specific primer–restriction fragment length polymorphism analysis
A PCR-sequence specific primer (SSP) conjoining the restriction fragment length polymorphism (RFLP) method was developed to detect the 1043G→A and 1148G→A mutations identified in the IGnTalleles. Wild-type and mutant oligonucleotide primers, w (GGTATTTGTATCTATGGA) and m (GGTATTTGTATCTATGAA), were designed and annealed to the IGnT gene with wild-type G nucleotide at position 1043 and to the mutant Ii1allele with A nucleotide at position 1043 under appropriate temperatures, respectively. One hundred nanograms genomic DNA and 10 pmol each forward (w or m) and reverse (IRc) primer were combined in PCR buffer containing 0.2 mM dNTP and 0.5 U hot-start Taqpolymerase. The PCR program included 15 minutes at 95°C followed by 30 cycles of 30 seconds at 94°C, 30 seconds at 54°C (for w+IRc primers) or 50°C (for m+IRc primers), and 30 seconds at 72°C. The amplified 218-bp fragment encompasses the 1148 nucleotide position. As the 1148G→A mutation of the mutant Ii2allele destroys a BstUI recognition sequence (CGCG), the PCR products were subjected to digestion by BstUI restriction endonuclease and then were analyzed by 2.0% agarose gel electrophoresis. The 218-bp PCR product amplified from the allele with wild-type 1148 nucleotide was cleaved into 122- and 96-bp fragments byBstUI digestion, whereas that from the mutant allele with 1148G→A mutation was resistant to digestion.
Functional analyses of the wild-type and mutantIGnT genes
cDNA fragments encompassing the region of nucleotides 88 to 1200, which encode the amino acid residues 30 to 400, of theI, Ii1, andIi2 genes were prepared using the OneStep RT-PCR Kit (Qiagen) and primers of IFw (aattggcccagccggccGATCCAAGCTTCCAAAGGCTAAATATC) and IRx (ttaagggcccAAAATACCAGCTGGGTTGTATCGCAG, antisense sequence), which contained SfiI and ApaI recognition sequences (underlined) at their 5′ ends, respectively. Total RNAs obtained from W-1 and W-2 members of pedigree W served as templates. Amplified cDNA fragments were cloned into SfiI andApaI sites of the mammalian expression vector pSecTaq2A (Invitrogen), which is designed for the secretion of the expressed protein by the N-terminal secretion signal from the V-J2-C region of mouse immunoglobulin κ chain. Vectors bearing wild-type I,Ii1, andIi2 cDNAs were selected and sequence-confirmed, leading to the construction of pSecTaq2A-I, pSecTaq2A-Ii1, and pSecTaq2A-Ii2, respectively. The 3 constructed and the mock pSecTaq2A plasmids were prepared for transfection using the EndoFree Plasmid Kit (Qiagen).
COS-7 cells (purchased from the American Type Culture Collection, Manassas, VA) were grown in 90% Dulbecco modified eagle medium and 10% fetal bovine serum containing 50 U/mL penicillin and 50 μg/mL streptomycin. The day before transfection, cells were split into 60-mm culture dishes at a density of 5 × 104/mL; after culture for 24 hours, cells were transfected with 1 μg expression vector. Transfection of the cells was performed using Effectene Transfection Reagent (Qiagen). After culture for an additional 72 hours, the medium was harvested and then concentrated 50-fold by Centriplus YM-10 (Millipore Intertech, Bedford, MA) and directly used for GlcNAc-transferase assay.
The GlcNAc-transferase assay was performed in a 25-μL reaction mixture containing 50 mM cacodylic acid (sodium salt, pH 7.0), 20 mM MnCl2, 4 mM ATP, 10 mM D-galactonic acid γ-lactone, 1 mM EDTA, 0.5 mM UDP-GlcNAc, 0.25 μCi (9250 Bq) UDP-[3H]GlcNAc (60 Ci/mmol; American Radiolabeled Chemicals, St Louis, MO), 7.5 μL concentrated medium, and with or without 2 mM acceptor substrate, LS–tetrasaccharide c (NeuNAcα2-6Galβ1-4GlcNAcβ1-3Galβ1-4Glc; Oxford GlycoSystems, Abingdon, United Kingdom). After incubation at 37°C for 2.5 hours, the reaction mixtures were passed through a mixed bed of AG1-X8 (AcO−) and AG50W-X8 (H+) resins (Bio-Rad Laboratories, Hercules, CA), and the run-through was scintillation-counted.
Results
Missense mutations were identified in the IGnTgene, but not in the C2GnT-M gene, of adult i propositi
The coding regions of the IGnT and C2GnT-Mof the adult i propositus, member 6 of pedigree S (denoted as S-6), were amplified and cloned, and the sequences were determined. EightIGnT clones from the S-6 propositus were analyzed, and all were found to have a nucleotide substitution of G to A at position 1043, which predicts an amino acid alteration of Gly to Glu at residue 348 (Figure 2). Direct sequencing of the RT-PCR product of IGnT cDNA also demonstrated the 1043G→A substitution. Taken together, these results suggest that the S-6 propositus was most likely homozygous for the 1043G→A mutation in theIGnT gene. Analysis of sequences of 4 C2GnT-Mclones from S-6 revealed nucleotide substitutions in these clones, but none had identical mutations. Direct sequencing of the PCR product of the C2GnT-M gene yielded the expected sequence of the wild-type gene and suggested that the mutations in the clones were due to PCR errors. These results support the proposition that theC2GnT-M gene of the adult i propositus (S-6) had a wild-type coding sequence.
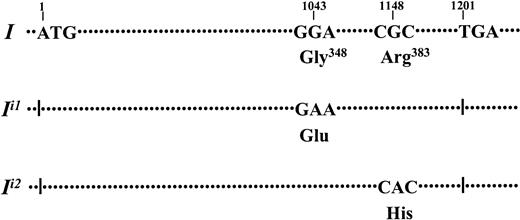
Schematic representation of the mutations identified in the
IGnT genes of persons with adult i phenotype.I indicates the coding region of the IGnT gene reported by Bierhuizen et al18 in 1993. ATG and TGA correspond to the enzyme's translation initiation and termination codons, respectively. Mutant alleles identified in the persons with adult i phenotype, designated as Ii1 andIi2, had 1043G→A and 1148G→A nucleotide substitutions, respectively, which predict amino acid alterations of Gly348Glu and Arg383His, respectively.
Schematic representation of the mutations identified in the
IGnT genes of persons with adult i phenotype.I indicates the coding region of the IGnT gene reported by Bierhuizen et al18 in 1993. ATG and TGA correspond to the enzyme's translation initiation and termination codons, respectively. Mutant alleles identified in the persons with adult i phenotype, designated as Ii1 andIi2, had 1043G→A and 1148G→A nucleotide substitutions, respectively, which predict amino acid alterations of Gly348Glu and Arg383His, respectively.
The IGnT gene of another adult i propositus, member 3 of pedigree W (denoted as W-3), was also analyzed. Three of the 5IGnT clones from this propositus also demonstrated the 1043G→A substitution; however, the other 2 had the wild-type G nucleotide at position 1043 and another nucleotide substitution of G to A at position 1148. This substitution led to an amino acid change of Arg to His at residue 383 (Figure 2). Her parents were further analyzed, and both were shown to be heterozygotes at theIGnT locus. The IGnT allele with 1043G→A mutation was demonstrated in the mother, and the IGnT with the 1148G→A mutation was demonstrated in the father. Both parents had another IGnT allele with a wild-type coding sequence identical to that previously reported.18
The identified mutant IGnT alleles with the 1043G→A and 1148G→A mutations and their corresponding amino acid changes are illustrated in Figure 2 and are designated asIi1 andIi2, respectively. The wild-type allele is indicated as I.
Linkage of double-dose mutant IGnT alleles with i members, but not with I members, in the 2 pedigrees
A PCR-SSP-RFLP analysis was developed to detect the mutations of the Ii1 andIi2 alleles. By using the plasmid clones bearing the wild-type I, Ii1, and Ii2 cDNA segments as control templates, the system showed specificity in distinguishing the 3 alleles (Figure 3A-B, lower panels). The wild-type I allele yielded a PCR product when the wild-type primer set (w+IRc) was used, but not when the mutant primer set (m+IRc) was used. The amplified 218-bp fragment was cleaved to 122- and 96-bp products by BstUI digestion. PCR product was produced from the Ii1 allele only when the mutant primer set was used. The product was also cleaved into 122- and 96-bp fragments by BstUI. The Ii2allele yielded 218-bp product from wild-type primer, and the fragment was resistant to the BstUI digestion because of the 1148G→A mutation.
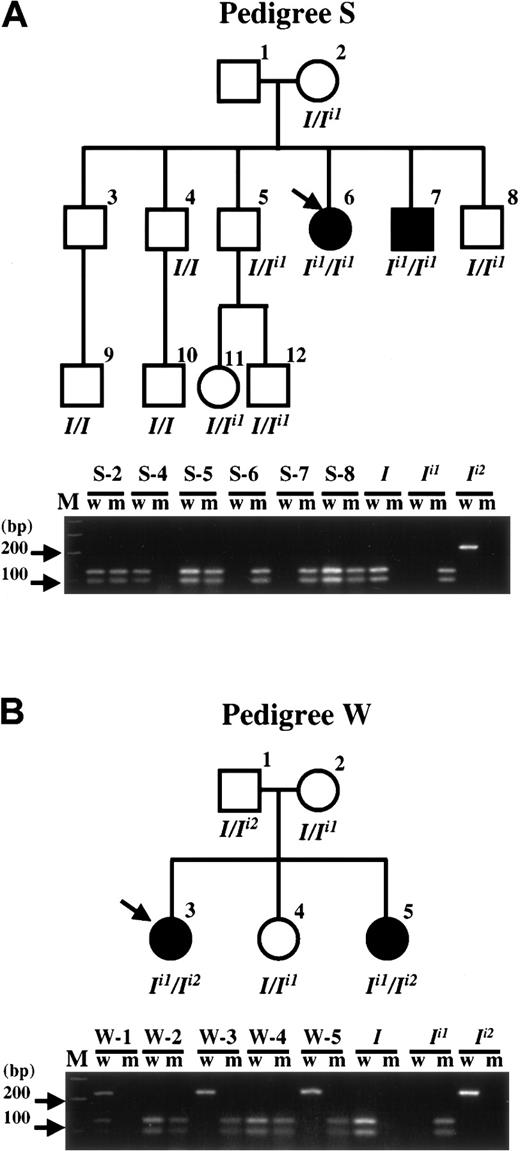
Segregation of wild-type
I, Ii1, andIi2 alleles in 2 adult i pedigrees. Pedigree drawings (upper) and PCR-SSP-RFLP analysis of the family members (lower) of pedigree S (A) and pedigree W (B). Pedigree drawing: To avoid confusing the blood group I phenotype and the I gene, the standard symbols for generations I, II, and III, are not used. Open and solid symbols for male (square) and female (circle) denote an person with common I and adult i phenotypes, respectively. I locus genotypes under each symbol are inferred from the results of PCR-SSP-RFLP analysis for each person. The adult i propositus in each pedigree is indicated by an arrow. Samples from S-1 and S-3 were unavailable in the current study to determine their I genotype; however, these persons were previously demonstrated to have I phenotype.27 PCR-SSP-RFLP analysis: w and m represent the PCR amplifications using the wild-type primer set and mutant primer set, respectively, for distinguishing theI and Ii1 alleles (described in “Materials and methods” and “Results”). The 218-bp PCR products were then digested with BstUI restriction endonuclease for distinguishing the I andIi2 alleles. Plasmid clones bearingI, Ii1, andIi2 cDNA segments served as control templates. The results for the third generation of pedigree S, S-9 to S-12, are not shown. Lane M shows the molecular mass standards of the 100-bp ladder.
Segregation of wild-type
I, Ii1, andIi2 alleles in 2 adult i pedigrees. Pedigree drawings (upper) and PCR-SSP-RFLP analysis of the family members (lower) of pedigree S (A) and pedigree W (B). Pedigree drawing: To avoid confusing the blood group I phenotype and the I gene, the standard symbols for generations I, II, and III, are not used. Open and solid symbols for male (square) and female (circle) denote an person with common I and adult i phenotypes, respectively. I locus genotypes under each symbol are inferred from the results of PCR-SSP-RFLP analysis for each person. The adult i propositus in each pedigree is indicated by an arrow. Samples from S-1 and S-3 were unavailable in the current study to determine their I genotype; however, these persons were previously demonstrated to have I phenotype.27 PCR-SSP-RFLP analysis: w and m represent the PCR amplifications using the wild-type primer set and mutant primer set, respectively, for distinguishing theI and Ii1 alleles (described in “Materials and methods” and “Results”). The 218-bp PCR products were then digested with BstUI restriction endonuclease for distinguishing the I andIi2 alleles. Plasmid clones bearingI, Ii1, andIi2 cDNA segments served as control templates. The results for the third generation of pedigree S, S-9 to S-12, are not shown. Lane M shows the molecular mass standards of the 100-bp ladder.
Using this method, the I locus genotypes of the members of pedigrees S and W were demonstrated. As shown in Figure 3A, another adult i member of the pedigree S, S-7, was demonstrated to be homozygous for the Ii1 allele, as had been shown for the S-6 propositus. All the other members were I phenotype and had at least one wild-type I allele, having either I/I orI/Ii1 genotypes. In the pedigree W, theIi1/Ii2genotype was also demonstrated in another i member, W-5 (Figure 3B). Her sister, W-4, was a heterozygote with theI/Ii1 genotype and had the common I phenotype. The father and mother hadI/Ii2 andI/Ii1 genotypes, respectively, as demonstrated by cloning and PCR-SSP-RFLP analyses. Obviously the heterozygousIi1/Ii2genotype of the W-3 and W-5 i adults resulted from the segregation of the mutant IGnT alleles from her mother and father with their respective 1043G→A and 1148G→A mutations. Results obtained from the 2 pedigrees show the correlation of segregation of double-dose mutant IGnT alleles, Ii1and Ii2, with the formation of the adult i phenotype.
The Ii1andIi2mutant alleles are rare in the general population
The PCR-SSP-RFLP method was used to inspect the incidence of theIi1 and Ii2alleles in the general population. Genomic DNA obtained from 51 randomly selected persons were screened, and none of them had the mutations of 1043G→A or 1148G→A in their IGnT genes (data not shown). The result indicates that theIi1 and Ii2alleles are infrequent, and agrees with the observation of rareness of the adult i phenotype.
Deletion of the IGnT gene was observed in a person with adult i phenotype
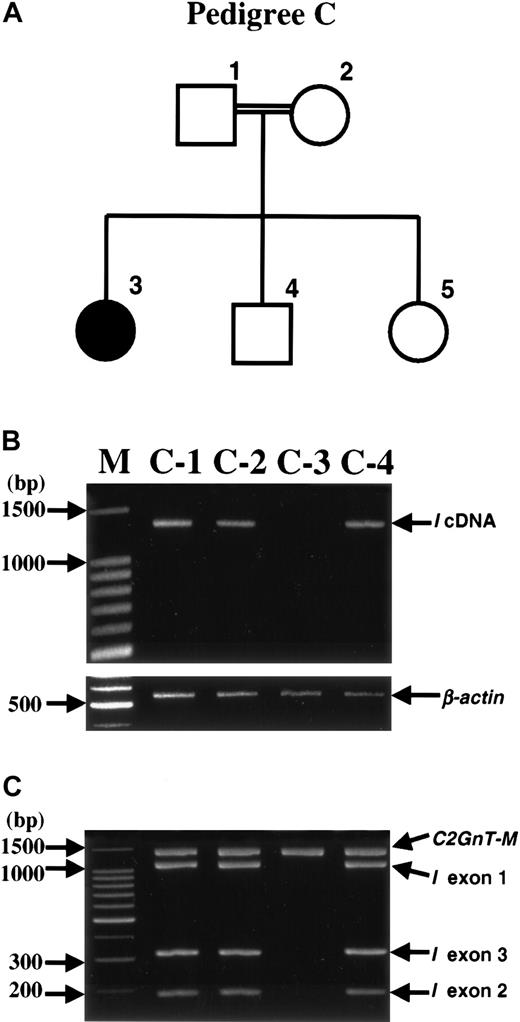
A different molecular basis for the adult i phenotype was observed in the pedigree C (Figure 4). RT-PCR of peripheral blood cell RNA of the member with adult i phenotype (C-3) failed to amplify the IGnT cDNA (Figure 4B). However, RNA samples from other members with I phenotype, C-1, C-2, and C-4, yielded products of IGnT cDNA. Each of the 3 exon regions of theIGnT gene of the families was examined by PCR. PCR products with the expected sizes for IGnT exon 1, exon 2, and exon 3 regions were produced from genomic DNA samples of the 3 I members, whereas genomic DNA sample of C-3 failed to yield any product for theIGnT exon regions (Figure 4C). The β-actin and the C2GnT-M genes served as controls for the RT-PCR and PCR reactions, respectively, and demonstrated the integrity of RNA and genomic DNA samples of C-3.
Deletion of
I gene in the i member of pedigree C. (A) Pedigree drawing. A sample from C-5 was unavailable in the current study; however, she had been shown to have common I phenotype previously.27 The parents were first cousins. (B) RT-PCR for I cDNA. The expected size of the RT-PCR product ofI cDNA is 1361 bp. Lane M shows the molecular mass standards of the 100-bp ladder. (C) PCR amplifications for the 3 exon regions of the I gene. PCR amplifications for the DNA segments, including I exon 1 (1118 bp), I exon 2 (189 bp), and I exon 3 (321 bp), and C2GnT-M gene (1459 bp), which served as control, were performed separately, and then the 4 products from each person's sample were analyzed in the same lane.
Deletion of
I gene in the i member of pedigree C. (A) Pedigree drawing. A sample from C-5 was unavailable in the current study; however, she had been shown to have common I phenotype previously.27 The parents were first cousins. (B) RT-PCR for I cDNA. The expected size of the RT-PCR product ofI cDNA is 1361 bp. Lane M shows the molecular mass standards of the 100-bp ladder. (C) PCR amplifications for the 3 exon regions of the I gene. PCR amplifications for the DNA segments, including I exon 1 (1118 bp), I exon 2 (189 bp), and I exon 3 (321 bp), and C2GnT-M gene (1459 bp), which served as control, were performed separately, and then the 4 products from each person's sample were analyzed in the same lane.
The results showed that the chromosome region of the IGnTgene was totally absent in the C-3 i adult but appeared intact (at least for one allele) and was expressed normally in the other I members. This evidence further supports that the IGnT gene is responsible for the expression of blood group I antigen.
Enzyme activity of the I β6GlcNAc-T is abolished by the Gly348Glu and Arg383His alterations
The effects of the Gly348Glu and Arg383His changes, resulting from the 1043G→A and 1148G→A mutations, respectively, on the enzyme activity of I β6GlcNAc-T were inspected and compared using a functional assay. Table 1 lists the amounts of GlcNAc transferred to the acceptor substrate, LS–tetrasaccharide c, from the donor substrate UDP-GlcNAc by the medium concentrates harvested from the cells transfected with the respective expression vectors. LS–tetrasaccharide c has been shown to be a good acceptor substrate for β6GlcNAc-T transferase assay.31 Medium harvested from the cells transfected with the expression vector bearing the wild-type I cDNA segment displayed GlcNAc-transferring activity in the assay. In contrast, medium from the cells transfected with vectors constructed with theIi1 and Ii2cDNAs, respectively, had virtually no detectable activity because the amounts of GlcNAc transferred were at the same level as the mock control pSecTaq2A. These findings indicate that the Gly348Glu or Arg383His alterations in I β6GlcNAc-T totally abolished the original GlcNAc-transferase activity.
Comparison of the GlcNAc-transferase activities of the enzymes expressed from the I,Ii1, andIi2 cDNAs
| . | pSecTaq2A . | pSecTaq2A-I . | pSecTaq2A-Ii1 . | pSecTaq2A-Ii2 . |
|---|---|---|---|---|
| GlcNAc transferred (pmol) | 36.0 ± 26.3 | 841.4 ± 61.0 | 38.2 ± 34.8 | 37.8 ± 21.9 |
| . | pSecTaq2A . | pSecTaq2A-I . | pSecTaq2A-Ii1 . | pSecTaq2A-Ii2 . |
|---|---|---|---|---|
| GlcNAc transferred (pmol) | 36.0 ± 26.3 | 841.4 ± 61.0 | 38.2 ± 34.8 | 37.8 ± 21.9 |
Results are the average and SD of 4 tests. Endogenous transfer of GlcNAc in the absence of acceptor substrate was corrected for each test. Amounts of the transferred GlcNAc in the mock control, pSecTaq2A, indicate the background levels of the assay, which are believed to result from the addition of the acceptor substrate.
Discussion
Molecular genetic analyses of the 3 adult i pedigrees demonstrated 3 different molecular origins for the adult i phenotype and suggest that the IGnT gene is the locus responsible for the expression of the human blood group I antigen. Although the I antigen was first identified more than 40 years ago, it is one of the few human blood groups for which the responsible gene locus remains unconfirmed. Even though the IGnT gene has been identified and its protein product was demonstrated several years ago to have I-branching forming capability, it has not been approved as the human blood groupI gene because it is believed that more than one I-branching enzymes may exist. It has been suggested that different I-branching enzymes may be responsible for the synthesis of I antigens in different tissues.10 The identification of another I-branching enzyme encoding gene, C2GnT-M, has further complicated efforts to identity the blood group I gene, though it is not surprising that another I-branching βGlcNAc-T exists. Therefore, further evidence from the genetic analysis of the null phenotype of the I blood group, the adult i phenotype, has been awaited to confirm the identity of the human blood group I gene.32 The current study has provided this evidence.
The IGnT gene, identified by Bierhuizen et al18in 1993, encodes a β6GlcNAc-T enzyme composed of 400 amino acid residues. The gene is located on chromosome 6p24,19,20 and its 1200 nucleotides of coding sequence are divided into 3 exon regions.33 Two possible enzymatic pathways have been proposed for I-branching activity: centrally acting34,35and distally acting.17 Through enzyme characterization using different acceptor substrates, the β6GlcNAc-T encoded by theIGnT gene was shown to have a majority of centrally acting activity, transferring GlcNAc to the internal Gal in Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-R sequence, and a minority of distally acting activity, transferring GlcNAc to predistal Gal in the acceptor GlcNAcβ1-3Galβ1-4GlcNAcβ1-R.19 36
Confirmation of the I gene locus will allow further investigation of the gene regulation mechanism(s) for differential expression of I antigen during developmental and oncogenesis processes, and it will further assist in the investigations of the molecular genetics that control I antigen expression in secretions and the molecular basis for the association of the adult i phenotype with congenital cataracts in Asians. Synthesis of I antigens in different tissues has been suggested to result from different I-branching enzymes given that normal quantities of I antigen in saliva, milk, and plasma of persons with adult i phenotype has been observed.37,38A similar situation has been demonstrated in the formation of blood group H antigens in RBC membrane and in saliva, which are synthesized by the action of different α-1,2-fucosyltransferases encoded by theH and Secretor genes, respectively.39 Whether the other I-branching enzyme encoding gene, C2GnT-M, or another unidentified gene is responsible for the I antigen expression in secretions remains to be determined.
Identification of the molecular origins of the adult i phenotype in the 3 pedigrees still leaves the molecular genetic basis of its association with congenital cataracts in Asians obscure for the present. The association can be explained by either a close linkage between independent I- and cataract-related genes or a pleiotropic effect of the gene responsible for the adult i phenotype on the development of cataracts.24,25 Because of the reduced association between adult i phenotype and congenital cataracts in the white population, the former hypothesis of a close linkage of 2 independent genes was suggested to be the tenable one.28It has been proposed that the adult i phenotype in Asians, and in some whites, may result from the deletion of a small chromosomal region that also encompasses a nearby gene, and this results in the development of cataracts.10 In our study, chromosomal deletion was detected in one person with adult i in 1 of our 3 pedigrees; however, the molecular basis of the adult i phenotype in the other 2 pedigrees consisted of single nucleotide substitutions in the I gene that occurred at 2 different positions. It is unlikely that 2 different mutational changes would be linked to the same nearby gene that had, by chance, also mutated to a form that resulted in the development of cataracts. Elucidation of the molecular genetic basis of persons with adult i without congenital cataracts may help explain the association in Asians.
Supported in part by National Health Research Institute grant NHRI-EX90-8601SL (M.L.) and National Science Council grant NSC 89-2314-B-195-014 (L.-C.Y.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marie Lin, Transfusion Medicine Laboratory, Mackay Memorial Hospital, 45 Ming-San Rd, Tamshui, Taipei County 251, Taiwan; e-mail: marilin@ms2.mmh.org.tw.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal