Abstract
Chemokines are a large family of cytokines that direct normal leukocyte migration. They also have been implicated in leukocyte development and in the pathogenesis of many diseases. The CC chemokine CCL21, also known as Exodus-2, SLC, 6Ckine, and TCA4 induces both the adhesion and migration of human T cells. CCL21 is hypothesized to regulate the trafficking of T cells through secondary lymphoid tissues. To test this hypothesis, a transgenic mouse model was generated that placed the expression of mouse CCL21 (mCCL21) under the control of the T cell-specific lck promoter to abrogate the concentration gradient to which T cells normally respond. Overexpression of mCCL21 in T cells resulted in defects in CCL21- and CCL19-induced T-cell chemotaxis, node T-cell subpopulations, and lymph node architecture. The regulation of T-cell trafficking in secondary lymphoid tissues by CCL21 is therefore a tightly regulated system that can be altered by changes in the level of environmental CCL21 protein.
Introduction
Chemokines are a large family of cytokines that direct leukocyte migration and have been implicated in the regulation of leukocyte development, angiogenesis, tumor growth, and metastasis.1-4 They play a key role in both innate and acquired immunity.5-8 The number and spacing of the first cysteines in the amino acid sequence are used to characterize chemokines into 4 subfamilies: XCL1-2, CCL1-27, CXCL1-14, and CX3CL1.9,10 Chemokines act through chemokine receptors (XCR1, CCR1-11, CXCR1-5, CX3CR1), which are a subfamily of G protein-coupled 7-transmembrane receptors.9-11
Chemokines are reported to be mediators of tissue destruction in a wide variety of human diseases, including autoimmune disease, postinfarct reperfusion injury, atherosclerosis, adult respiratory distress syndrome, and infectious disease.1-8,12-17 For example, CCL11, CCL7, and CCL5 are all suspected mediators of asthma due to their localized presence at the disease site and function in attracting eosinophils.5 Chemokines also stimulate neutrophil infiltration of infarcted tissue, mediating reperfusion tissue damage.18 In addition, the human immunodeficiency virus (HIV) coreceptors that function with CD4 are chemokine receptors, suggesting a mechanism for the inhibition of HIV infection by specific chemokines.19-21
Chemokines have been implicated in angiogenesis, tumor growth, and metastasis.3,5,8 It has been reported that ELR-CXC chemokines are angiogenic and most non–ELR-CXC chemokines are angiostatic.3 In addition, the chemokine receptor CXCR4 mediates metastasis in breast cancer.22 Chemokines have generated interest as tumor vaccines for their chemoattractant and T-cell and natural killer (NK)–cell stimulatory properties. The CC chemokine CCL1 can induce tumor-specific and long-lasting immunity in mice that have been immunized with tumor cells expressing CCL1.23 Tumor cells transduced with the CC chemokine CCL2 also stimulate a specific immune response that protects immunized rats against further tumor cell challenge.24 XCL1 transduced into cells, simultaneously with IL-2 as a T cell-stimulatory molecule, was reported to be effective as a tumor vaccine.25CCL1926,27 has been shown to inhibit breast cancer cell growth in a murine model by an NK and CD4+ cell-mediated mechanism.28 We recently found that CCL19, 20, and 21 inhibited chronic myelogenous leukemia progenitors.29CCL21 has also been reported to stimulate lymphocyte antitumor responses in mouse tumor models and cell line-based models.30-32
Our laboratory and others cloned CCL21, thought to be a critical regulator of T-cell trafficking through lymphoid tissue.33-36 We and others found that CCL21 expression in the high endothelial venule (HEV) of the lymph node stimulates the adherence and migration of T cells into the secondary lymphoid tissue where antigen presentation can occur.37-41 CCL21 expression attracts and colocalizes both T cells and antigen-presenting dendritic cells to the nodal T-cell zone where antigen presentation can occur.42-46 The importance of CCL21 and CCL19 in T-cell trafficking has also been implied by studies involvingplt-mice and CCR7-null mice. The plt (paucity of lymph node T cells) mouse lacks CCL19 and CCL21 expression and displays an inability of T cells and activated dendritic cells to traffic to lymph nodes or T-cell zones of the spleen.47-53 CCL19 and CCL21 both bind CCR7.40,42-46 Data analysis of CCR7-deficient mice shows the disordered migration of lymphocytes to secondary lymphoid organs and a failure of skin dendritic cells to migrate into draining lymph nodes.54
In this study, a transgenic mouse overexpressing CCL21 in T cells was generated to abrogate the CCL21 concentration gradient T cells encounter and migrate toward. We found that overexpression of CCL21 by T cells in the CCL21 transgenic mice resulted in defects in mCCL21- and mCCL19-induced T-cell chemotaxis, lymphocyte subpopulations in lymphoid tissues, and nodal architecture.
Materials and methods
Generation of the lck-mCCL21 construct
The complementary DNA (cDNA) of murine CCL21 (accession no. MMU88322) was amplified by polymerase chain reaction (PCR) using the primers mEx2-5′BamHI (5′ TATGGATCCATGGCTCAGATGATGACTCTGAGCCTC 3′) and mEx2-3′BamHI (5′ TATGGATCCATGGAGAGCAGGTTCAGGTCT 3′). The PCR product was subcloned into pBluescript II KS+ (Strategene, La Jolla, CA) using BamHI and clones were sequenced. After ensuring the proper sequence, the DNA fragment representing the CCL21 open reading frame was then ligated into the BamHI site of the lck transgenic cassette located in the plasmid p1017 (Figure1) (gift of Dr Roger Perlmutter, Amgen, Thousand Oaks, CA55 56).
The lck transgene cassette contains a
BamHI restriction site behind the lck proximal promoter. The proximal lck promoter directs T cell-specific expression.55 56BamHI was used to subclone the mCCL21 cDNA into the p1017 plasmid behind the lck proximal promoter.NotI restriction digest was then used to isolate the transgene cassette from the plasmid.
The lck transgene cassette contains a
BamHI restriction site behind the lck proximal promoter. The proximal lck promoter directs T cell-specific expression.55 56BamHI was used to subclone the mCCL21 cDNA into the p1017 plasmid behind the lck proximal promoter.NotI restriction digest was then used to isolate the transgene cassette from the plasmid.
Microinjection and screening of lck-mCCL21 construct
The lck-mCCL21 transgene cassette was then separated from the plasmid (p1017) (Figure 1) by NotI digest and then agarose gel purified. The purified lck-mCCL21 construct was then injected into the pronucleus of diploid one-celled C3HeBFeJ fertilized mouse eggs. These were cultured to the 2-cell stage to ensure viability and then implanted into the oviducts of pseudopregnant mice and pups were born approximately 20 days later.57 Pups from these mice were then screened by PCR for the presence of the lck-mCCL21 transgene using the primers mEx2lck5′PCR (5′ CAGGGGAGTTGTAATGAAGAGGGACAGG 3′) and mEx2lck3′PCR (5′ GATGGACAGCTAACTTGGTTCTTGC 3′). Southern blot analysis for the lck-mCCL21 transgene was used to confirm the presence of the transgene and establish the relative number of integration events per mouse genome. Isolated mouse genomic DNA was digested withBamHI and the full-length mCCL21 cDNA fragment was used as a probe. Surviving mice showing the presence and expression of the transgene by Southern and Western blotting analysis were used as founders to establish a breeding colony.
Protein analysis
The CCL21 cellular protein levels were quantitated in transgenic mice versus wild-type (WT) mice by Western blot. Polyvinylidene difluoride (PVDF) membranes were blocked in 5% dry milk for 1 hour and probed with a 1:1000 dilution of antimouse CCL21 (m6Ckine) IgG-goat (R & D Systems, Minneapolis, MN) for 1 hour in 5% dry milk + Tween20. After washing, membranes were then probed with a 1:5000 dilution of antigoat IgG-horseradish peroxidase (HRP; Santa Cruz, Santa Cruse, CA) for 1 hour in 5% dry milk plus Tween20 and detected by enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ). Equal amounts of protein were added to each lane and a β-actin antibody was used to confirm equal loading.
Chemotaxis
Chemotaxis assays were performed using 96-well chemotaxis chambers (Neuroprobe, Gaithersburg, MD) in accordance with manufacturer's instructions with minor variations as described.58-61 Briefly, 0, 200, 400, and 800 ng/mL of the appropriate murine chemokine was added to 300μL RPMI without phenol media supplemented with 10% fetal bovine serum (FBS) in the lower chamber. Two hundred thousand fluorescent-tagged (4 μg/mL Calceinam, Molecular Probes, Eugene, OR) isolated lymphocytes or splenocytes in 50 μL media were added to the upper side of the membrane (5.7-mm diameter, 5-μm pore size, polycarbonate membrane).
Total cell migration was obtained by measuring fluorescence (excitation, 485 nm; emission, 530 nm) and calculating cell number in the lower well by comparison to a cell number standard curve after 3 hours of incubation at 37°C, 5% CO2. Percent migration was calculated by dividing the number of the cells in the lower well by the total cell input multiplied by 100 and subtracting random migration to the lower chamber without chemokine presence.
Cell subtype migration was obtained by staining the input cell population and migrating cell population with fluorochrome-conjugated monoclonal antibodies to CD3, CD19, CD4, CD8, and 62L as described below. The numbers of each cell type in the input and migrating populations were then used to obtain the percent migration of each cell type. This was done by dividing the absolute number of cells of a given cell type in the lower well by the total input cell number of that same cell type, multiplied by 100, and then subtracting random transwell migration to the lower chamber without chemokine presence. At least 4 WT and transgenic individual mice were analyzed separately in triplicate, and then the data averaged for statistical analysis.
Adhesion
Static adhesion assays were performed as we previously described.37 43 Briefly, intercellular adhesion molecule 1 (ICAM-1) was coated on glass slides and lymphocytes were allowed to settle for 10 minutes on the surface of the slide. CCL21 was added and nonadherent cells were washed away. Adherent cells were then fixed and counted. Adhesion assays were performed on splenocytes from lck-mCCL21 transgenic mice and compared to normal control mouse splenocytes.
Flow cytometry
Cells were stained with fluorochrome-conjugated monoclonal antibodies to CD3, CD19, CD4, CD8, and 62L (BD Pharmingen, San Diego, CA) in accordance with the manufacturer's specifications and then counted by flow cytometric analysis. CD3/62L was used to identify lymph node–homing murine T cells. Harvested cells are washed in phosphate-buffered saline (PBS)/penicillin/streptomycin/1% bovine serum albumin (BSA) and resuspended in 100 μL PBS/BSA. Then 0.5 μg of the appropriate antibody was added and mixed, and the cells were incubated at 4°C in the dark for 30 minutes. The cells were then washed twice in PBS/BSA and fixed in PBS/1% paraformaldehyde for flow cytometric analysis at 488 nm. Ten thousand events were accumulated for each analysis. At least 4 WT and transgenic individual mice were analyzed separately in triplicate and the data averaged for statistical analysis.
Immunohistochemistry
Comparisons of T-cell and B-cell localization between normal and transgenic mice within lymphoid tissue architecture were made using immunohistology as we described.62 Freshly isolated adult mouse spleen, thymus, and lymph node tissues were frozen and cut into tissue sections. Sections were stained with biotinylated CD3 and CD19 cell surface marker antibodies (BD Pharmingen). Slides were stained with antibody and signals were developed by peroxidase/3,3′-diamobenzidine (DAB) staining in accordance with manufacturer's specifications (Vector Labs, Burlingame, CA). Slides were counterstained with hematoxylin nuclear counterstain (Vector Labs, Burlingame, CA).
Results
DNA and protein analysis of transgenic expression of CCL21
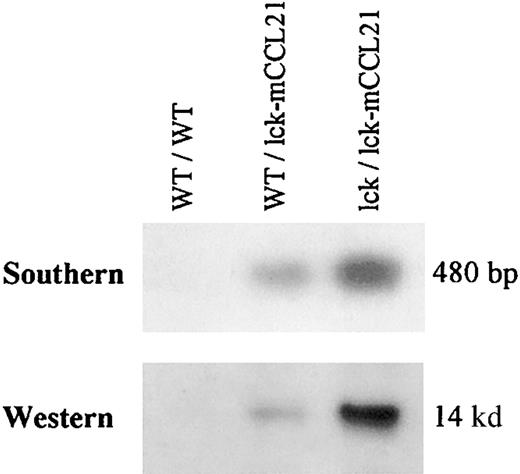
Homozygous transgenic (lck/lck-mCCL21) and heterozygous transgenic (WT/lck-mCCL21) mice were compared to wild-type (WT/WT) mice. A Southern blot analysis of isolated mouse genomic DNA using a radiolabeled mCCL21 cDNA probe revealed the presence of the lck-mCCL21 construct in the heterozygous WT/lck-mCCL21 mice. The lck/lck-mCCL21 homozygous transgenic mice had 2 copies of the transgene (Figure2).
Southern blot of isolated mouse genomic DNA and Western blot of isolated mouse thymocyte cell lysate.
Homozygous transgenic (lck/lck-mCCL21) and heterozygous transgenic (WT/lck-mCCL21) mice were compared to wild-type (WT/WT) mice. Southern analysis of BamHI-digested mouse genomic DNA was performed using radiolabeled mCCL21 cDNA as a probe. Western analysis was performed using antimouse CCL21 IgG-goat as a primary antibody, antigoat IgG-HRP as a secondary antibody, and detected by ECL. On prolonged exposure appropriate endogenous CCL21 expression can be seen on Western analysis, but is not seen on the exposure shown here.
Southern blot of isolated mouse genomic DNA and Western blot of isolated mouse thymocyte cell lysate.
Homozygous transgenic (lck/lck-mCCL21) and heterozygous transgenic (WT/lck-mCCL21) mice were compared to wild-type (WT/WT) mice. Southern analysis of BamHI-digested mouse genomic DNA was performed using radiolabeled mCCL21 cDNA as a probe. Western analysis was performed using antimouse CCL21 IgG-goat as a primary antibody, antigoat IgG-HRP as a secondary antibody, and detected by ECL. On prolonged exposure appropriate endogenous CCL21 expression can be seen on Western analysis, but is not seen on the exposure shown here.
The well-characterized T cell–specific lck promoter has been shown by lck-green fluorescent protein (GFP) expression to be expressed in the earliest thymic T-cell population and in splenic T cells.56 Western analysis of isolated mouse thymocyte cell lysates showed overexpression of the CCL21 protein by the lck-mCCL21 construct (Figure 2). Endogenous CCL21 protein was undetectable in the normal mouse thymocytes by Western blot analysis at this exposure, but can be seen on prolonged exposure. A copy number–dependent increase in protein expression can be seen when comparing heterozygous (WT/lck-mCCL21) and homozygous (lck/lck-mCCL21) transgenic expression of CCL21. Two mouse lineages were obtained for study that resulted from 2 distinct integration events. Both lines showed transmission of the transgene and transgenic overexpression of CCL21 protein at equal levels. Homozygous mice from both lineages were used for phenotypic analysis to ensure that observations are the direct result of CCL21 overexpression and were not dependent on the integration site.
Chemotaxis
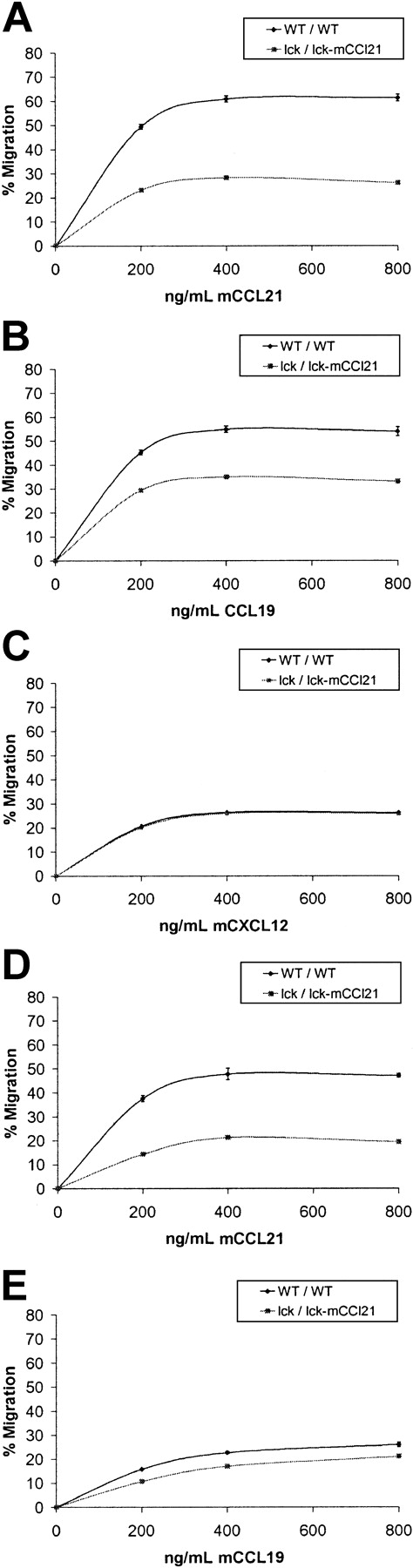
To analyze total T-cell migratory function, chemotaxis assays were performed comparing WT and homozygous transgenic CD3+ mouse splenocytes and thymocytes. Flow cytometric analysis was performed to characterize starting and migrating T-cell populations. This analysis found significant defects in transgenic CD3+ splenocyte and thymocyte chemotaxis in response to mCCL21 and mCCL19 but not CXCL12 (Figure 3). Transgenic spleen T cells had a maximum decrease of 57% of their CCL21-induced chemotactic ability as compared to normal spleen T cells (P = .0004). In a similar manner, transgenic spleen T cells had a maximum decrease of 37% of their CCL19-induced chemotactic ability as compared to normal spleen T cells (P = .0014). However, no appreciable difference was seen between the ability of transgenic versus normal spleen CD3+ cells to migrate in response to CXCL12 (Figure3, P = .445). Transgenic thymic CD3+ cells also had defects in CCL19- and CCL21-induced chemotaxis as compared to WT controls (Figure 3). Transgenic thymic T cells had maximum decreases of 60% (P = .0055) and 33% (P = .012) compared to WT/WT for CCL21- and CCL19-induced chemotaxis, respectively. Thymic T cells expressed the receptor for CXCL12 poorly and, as a result, there was little thymic T-cell chemotaxis with which to compare transgenic to WT mice.
CCL21 and CCL19 chemotaxis of total T cells is reduced in homozygous lck/lck-mCCL21 transgenic versus normal mice.
Chemotaxis assays were performed comparing normal (WT/WT) and homozygous transgenic (lck/lck-mCCL21) mouse splenocytes (A-C) and thymocytes (D,E). Dose responses to 0, 200, 400, and 800 ng/mL chemokine were obtained. Flow cytometric analysis was performed to characterize starting and migrating cell populations. Severe deficiencies in transgenic CD3+ splenocyte and thymocyte chemotaxis in response to CCL21 (A,D) and CCL19 (B,E) are observed. Splenocyte chemotactic response to CXCL12 was unchanged (C).
CCL21 and CCL19 chemotaxis of total T cells is reduced in homozygous lck/lck-mCCL21 transgenic versus normal mice.
Chemotaxis assays were performed comparing normal (WT/WT) and homozygous transgenic (lck/lck-mCCL21) mouse splenocytes (A-C) and thymocytes (D,E). Dose responses to 0, 200, 400, and 800 ng/mL chemokine were obtained. Flow cytometric analysis was performed to characterize starting and migrating cell populations. Severe deficiencies in transgenic CD3+ splenocyte and thymocyte chemotaxis in response to CCL21 (A,D) and CCL19 (B,E) are observed. Splenocyte chemotactic response to CXCL12 was unchanged (C).
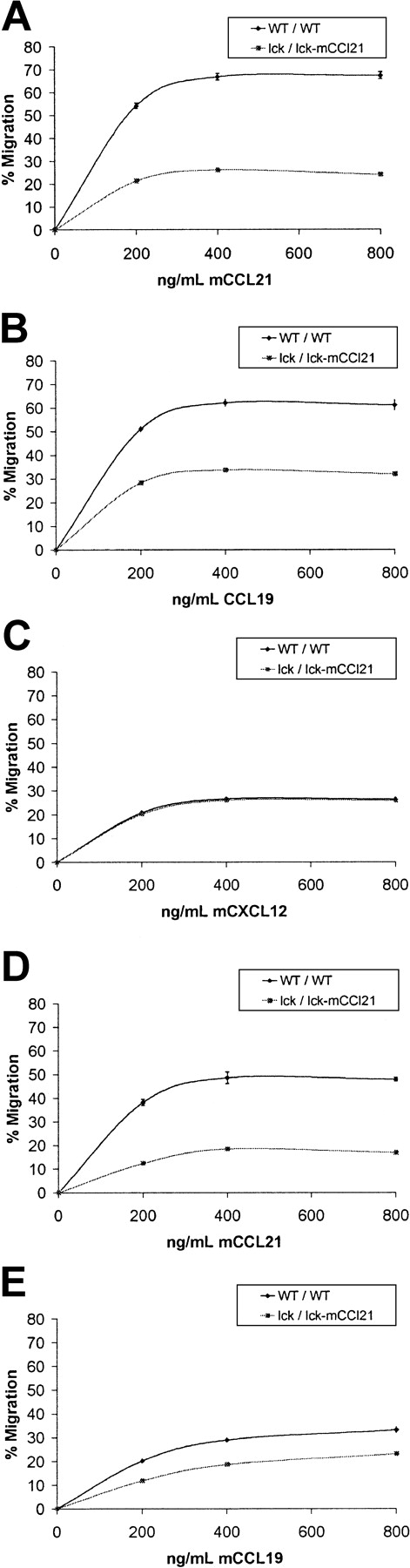
CCL21 has been reported to mediate T-cell homing to lymph nodes via stimulation of HEV adhesion and then chemotaxis into specific regions within the node.42-45 To analyze lymph node– homing T-cell migratory function, chemotaxis assays were performed comparing normal and homozygous transgenic CD3+/CD62L+splenocytes and thymocytes (Figure 4). CD62L expression on T cells is an absolute requirement for HEV lymph node homing. Significant deficiencies in transgenic CD3+/CD62L+ splenocyte and thymocyte chemotaxis in response to CCL21 and CCL19 were observed. There was a 61% decrease in the chemotactic response to CCL21 of CD3+/CD62L+ splenocytes in transgenic mice as compared to normal controls (P = .001, Figure 4). Chemotaxis to CCL19 was also reduced by 49% in transgenic CD3+/CD62L+ splenocytes as compared to normal controls (P = .001, Figure 4). Chemotactic response to CXCL12 was unchanged (Figure 4).
CCL21 and CCL19 chemotaxis of CD3+/CD62L+ T cells is reduced in homozygous lck/lck-mCCL21 transgenic versus normal mice.
Chemotaxis assays were performed comparing normal (WT/WT) and homozygous transgenic (lck/lck-mCCL21) mouse splenocytes (A-C) and thymocytes (D,E). Dose responses to 0, 200, 400, and 800 ng/mL chemokine were obtained. Flow cytometric analysis was performed to characterize starting and migrating cell populations. Severe deficiencies in transgenic CD3+/CD62L+splenocyte and thymocyte chemotaxis in response to CCL21 (A,D) and CCL19 (B,E) are observed. Splenocyte chemotactic response to CXCL12 was unchanged (C).
CCL21 and CCL19 chemotaxis of CD3+/CD62L+ T cells is reduced in homozygous lck/lck-mCCL21 transgenic versus normal mice.
Chemotaxis assays were performed comparing normal (WT/WT) and homozygous transgenic (lck/lck-mCCL21) mouse splenocytes (A-C) and thymocytes (D,E). Dose responses to 0, 200, 400, and 800 ng/mL chemokine were obtained. Flow cytometric analysis was performed to characterize starting and migrating cell populations. Severe deficiencies in transgenic CD3+/CD62L+splenocyte and thymocyte chemotaxis in response to CCL21 (A,D) and CCL19 (B,E) are observed. Splenocyte chemotactic response to CXCL12 was unchanged (C).
Mouse thymocyte CD3+/CD62L+ chemotactic response in homozygous transgenic mice was also reduced. CD3+/CD62L+ thymocytes from transgenic mice had a 62% loss in chemotactic response to 400 ng/mL CCL21 compared to normal controls (P = .003, Figure 4). Migration toward 400 ng/mL CCL19 was reduced 34% in transgenic compared to WT mice (P = .001, Figure 4).
Adhesion
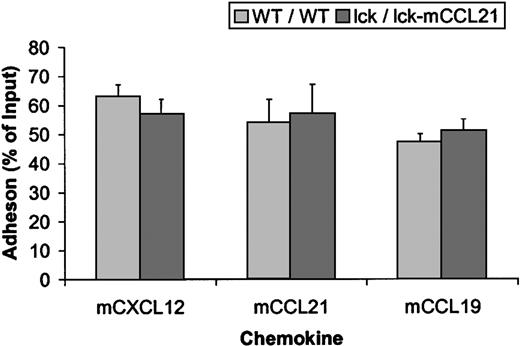
Adhesion studies using mCXCL12, mCCL21, and mCCL19 show no significant difference between the adhesion of normal (WT/WT) or transgenic (lck/lck-mCCL21) splenocytes or thymocytes to capillary tubes coated with ICAM-1 (Figure5).
Adhesion is unchanged in normal (WT/WT) versus transgenic (lck/lck-mCCL21) mice.
Adhesion studies using mCXCL12, CCL21, and CCL19 showed no significant difference between the adhesion of WT/WT or lck/lck-mCCL21 splenic T cells to capillary tubes coated with ICAM-1 under sheer conditions.
Adhesion is unchanged in normal (WT/WT) versus transgenic (lck/lck-mCCL21) mice.
Adhesion studies using mCXCL12, CCL21, and CCL19 showed no significant difference between the adhesion of WT/WT or lck/lck-mCCL21 splenic T cells to capillary tubes coated with ICAM-1 under sheer conditions.
Lymphocyte populations
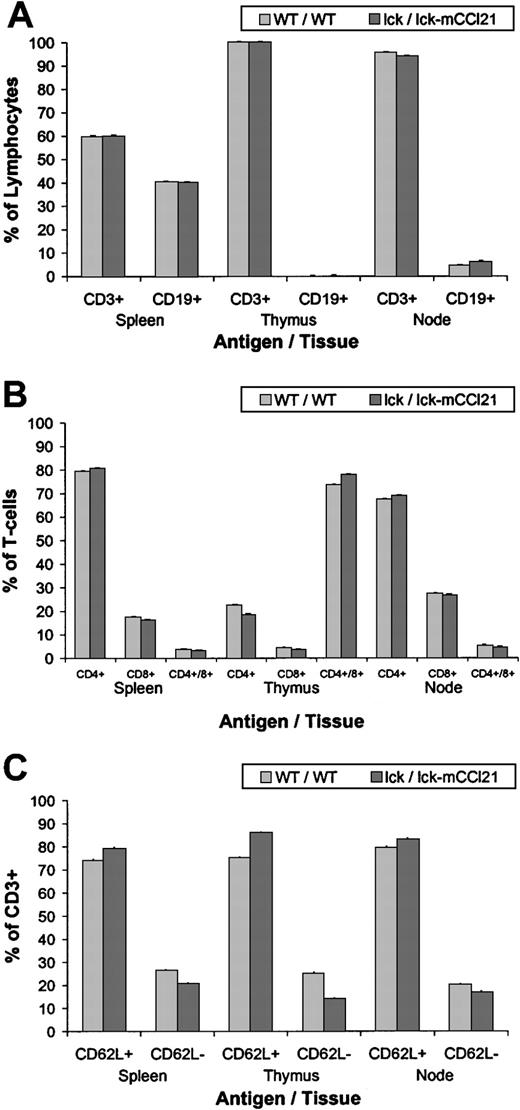
Cells were stained with fluorochrome-conjugated monoclonal antibodies (mAbs) to CD3, CD19, CD62L, CD4, and CD8 and then counted using flow cytometry. Analysis of CD3+ versus CD19+ (Figure 6A) showed no difference in the relative percent of T cells versus B cells in the spleen, thymus, or lymph nodes. There were no size differences between WT and transgenic nodes.
Flow cytometric analysis reveals an increase in 62L+ naı̈ve T-cells in homozygous lck/lck-mExodus-2 transgenic nodes.
Cells were stained with fluorochrome-conjugated mAbs to CD3, CD19, CD4, CD8, and CD62L and then counted by flow cytometry. Analysis of CD3+ versus CD19+ (A) shows no difference in the relative percent of T cells versus B cells in the spleen, thymus, or lymph nodes. Analysis of CD4+, CD8+, and CD4+/CD8+ (B) shows a slight increase (P = .012) in CD4+/CD8+ T cells in the thymus of the transgenic mice (lck/lck-mCCL21) when compared to normal mice (WT/WT). Analysis of CD3+/CD62L+versus CD3+/CD62L− (C) shows a statistically significant increase in CD62L+ T cells in the spleen, thymus, and lymph nodes of lck/lck-mCCL21 transgenic mice, when compared to normal WT mice. Corresponding decreases in CD62L− T-cells in the spleen, thymus, and node are also noted in transgenic mice versus normal mice.
Flow cytometric analysis reveals an increase in 62L+ naı̈ve T-cells in homozygous lck/lck-mExodus-2 transgenic nodes.
Cells were stained with fluorochrome-conjugated mAbs to CD3, CD19, CD4, CD8, and CD62L and then counted by flow cytometry. Analysis of CD3+ versus CD19+ (A) shows no difference in the relative percent of T cells versus B cells in the spleen, thymus, or lymph nodes. Analysis of CD4+, CD8+, and CD4+/CD8+ (B) shows a slight increase (P = .012) in CD4+/CD8+ T cells in the thymus of the transgenic mice (lck/lck-mCCL21) when compared to normal mice (WT/WT). Analysis of CD3+/CD62L+versus CD3+/CD62L− (C) shows a statistically significant increase in CD62L+ T cells in the spleen, thymus, and lymph nodes of lck/lck-mCCL21 transgenic mice, when compared to normal WT mice. Corresponding decreases in CD62L− T-cells in the spleen, thymus, and node are also noted in transgenic mice versus normal mice.
Analysis of CD4+, CD8+, and CD4+/CD8+ double-positive cells (Figure 6B) showed a slight increase (P = .012) in CD4+/CD8+ double-positive T cells in the thymus of the transgenic mice (77.8% ± 0.4%) when compared to normal mice (WT/WT) (73.5% ± 0.3%). Analysis of CD3+/CD62L+ versus CD3+/CD62L− (Figure 6C) found a small but statistically significant increase in CD62L+ T cells in the spleen (P = .004), thymus (P = .0004), and lymph nodes (P = .016) of lck/lck-mCCL21 transgenic mice (79.3% ± 0.3%, 85.9% ± 0.4%, 83.0% ± 0.3% respectively), when compared to normal (WT/WT) mice (73.8% ± 0.4%, 75.2% ± 0.4%, 79.6% ± 0.4%, respectively). Corresponding decreases in CD62L−/CD3+ in the spleen, thymus, and lymph node are also noted in transgenic mice (20.7% ± 0.3%, 14.0% ± 0.3%, 16.9% ± 0.3%, respectively) versus normal mice (26.2% ± 0.4%, 24.8% ± 0.3%, 20.1% ± 0.3%, respectively).
Lymphoid architecture
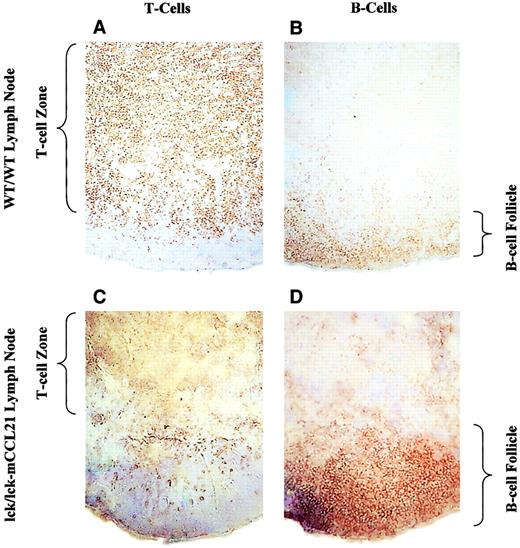
Immunohistochemistry of WT/WT and lck/lck-mCCL21 transgenic mice revealed that mCCL21 transgenic mice had abnormal lymph node architecture (Figure 7). Normal mouse and transgenic mouse lymph node frozen sections were stained for T cells (CD3) and B cells (CD19). Normal nodal architecture contained a large T-cell zone surrounded by a typical B-cell follicle. Overexpression of mCCL21 specifically in T cells results in a much smaller T-cell zone and a significantly larger B-cell follicle.
Immunohistochemistry of WT/WT and lck/lck-mCCL21 transgenic mice shows abnormal lymph node architecture in transgenic mice.
Normal mouse (A,B) and transgenic mouse (C,D) lymph node frozen sections were stained for T cells (CD3; A,C) and B cells (CD19; B,D). Normal nodal architecture (A,B) contains a large T-cell zone (shown by CD3 staining in panel A) reaching out toward a relatively smaller B-cell follicle (shown by CD19 staining in panel B). Overexpression of mExodus-2 specifically in T cells results in a more compact T-cell zone (shown by CD3 staining in panel C) and a large B-cell follicle (shown by CD19 staining in panel D). Original magnification × 10.
Immunohistochemistry of WT/WT and lck/lck-mCCL21 transgenic mice shows abnormal lymph node architecture in transgenic mice.
Normal mouse (A,B) and transgenic mouse (C,D) lymph node frozen sections were stained for T cells (CD3; A,C) and B cells (CD19; B,D). Normal nodal architecture (A,B) contains a large T-cell zone (shown by CD3 staining in panel A) reaching out toward a relatively smaller B-cell follicle (shown by CD19 staining in panel B). Overexpression of mExodus-2 specifically in T cells results in a more compact T-cell zone (shown by CD3 staining in panel C) and a large B-cell follicle (shown by CD19 staining in panel D). Original magnification × 10.
Discussion
For an appropriate acquired cellular immune response, it is important for T cells to survey secondary lymphoid organs thereby allowing contact with antigen-presenting dendritic cells that colocalize there.42-46 Such a nodal survey by T cells may be mediated by CCL21. In vitro evidence from us and others suggest that CCL21 is an extremely potent chemoattractant for normal human T cells, especially naı̈ve T cells.33-36,61In addition, data show that CCL21 is expressed in node endothelial cells and induces rapid adhesion of human T cells to endothelial ICAM-1.37-41 Lastly, the plt mouse mutation and the CCR7-deficient mouse model show disordered trafficking of T cells into nodal tissue.47-53
In this study transgenic mice were generated that placed the expression of mCCL21 under the control of the T cell–specific lck proximal promoter. Overexpression of CCL21 in T cells will abrogate any CCL21 chemokine concentration gradient that T cells might experience. Overexpression of mCCL21 by T cells in the lck/lck-mCCL21 transgenic mice resulted in defects in CCR7 ligand chemotaxis, the concentration of lymphocyte subpopulations in lymphoid structures, and nodal architecture.
Deficiencies in transgenic CD3+/CD62L+splenocyte and thymocyte chemotaxis in response to both mCCL21 and mCCL19 but not mCXCL12 were also observed. Because CCL21 and CCL19 share the CCR7 receptor, and CXCL12 interacts with CXCR4, it implies that this finding is receptor-specific. One possible mechanism for this chemotactic inhibition is that T-cell overexpression of CCL21 down-regulates the CCR7 receptor, thereby reducing its ability to respond to a CC21 gradient. This down-regulation could have occurred by either intracellular binding of the CCR7 receptor before it reached the surface or by endocytosis of the CCR7 receptor at the surface via clatharin- coated pits after it was bound by the secreted overexpressed CCL21. A second mechanism for this inhibition of cell migration is the destruction of a directional chemokine gradient to which the T cell would normally respond. Transgenic T cells would experience increased CCL21 protein levels completely surrounding the cell and no directional concentration gradient would exist.
Adhesion mediated by CXCL12, CCL21, and CCL19 was unchanged in transgenic mice when compared to normal mice. Because T cells require CCL21 for the activation of integrins on their cell surface for adhesion, it is possible that transgenic overexpression of CCL21 provided that signal constitutively. This would produce transgenic T cells that are already primed for adherence, similar to the T cells exposed to exogenous CCL21. Adhesion may be unchanged by the overexpression of CCL21 in T cells because adhesion does not require a gradient, like chemotaxis, but simply the local presence of the chemokine for activation of CCR7. However, this finding does imply that the CCR7 receptor is reaching the cell surface in the lck/lck-mCCL21 T cells, and that the CCL21 ligand is not binding the CCR7 receptor intracellularly.
Flow cytometry data revealed a small but statistically significant increase in the percent of CD62L+ T cells in the spleen and lymph nodes of lck/lck-mCCL21 transgenic mice when compared to WT/WT mice. One possible mechanism for this defect is that CD62L+node-homing T cells are more sensitive to the CCL21 gradients. Thus, if any gradient in lymphoid tissue still existed, this subtype of T cell would sense it. Another possibility is that the mass of T cells collecting near the HEV produce large quantities of CCL21, resulting in a new relative gradient that circulating T cells preferentially sense, and localize there. This potential mechanism could also explain the abnormal node architecture observed in the transgenic mice.
Examining nodal B- and T-cell architecture with immunohistology found that the transgenic mouse had a reduction in the size of the T-cell zone and an enlargement of the B-cell follicle. This is in contrast to the CCR7 knockout mice, where there was a lack of T-cell zones and B-cell follicles in the nodal architecture.55 Thus, modifying the CCL21 gradient a T cell would experience is not equivalent to deleting the CCR7 receptor. Either there still exists some small nodal CCL21 local gradient in the transgenic mice that allows T cells to enter the node, or other chemokines play a compensatory role in directing nodal T-cell migration.
However, the adhesion and chemotaxis data together supply a more satisfying explanation for the node architecture data. T cells do not distribute normally throughout the node—they exit the HEV normally but then fail to migrate properly throughout the node. Yet flow cytometry data indicated that the number of T cells relative to B cells remains unchanged in the lck/lck-CCL21 transgenic mice compared to normal mice. A potential explanation for this is that T-cell/endothelial cell adhesion (normal in these transgenic mice) is the key requirement for HEV node entry,40-45 but distribution throughout the node requires a chemotactic response to a CCL21 gradient, which is decreased in these transgenic mice. This may also explain the difference between this transgenic model and the plt/CCR7 knockout mouse models.
The data presented here provide additional evidence that CCL21 concentration gradients play an important role intranodal T-cell migration. However, these data also imply that other properties of CCR7 ligands besides a chemotactic concentration gradient (eg, adhesion) are important in naı̈ve T-cell trafficking. In addition, this study implies that pharmacologically saturating the CCR7 receptor may decrease T-cell chemotaxis sufficiently to disrupt T cell-mediated autoimmune diseases. These transgenic mice provide a model to validate that hypothesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert A. Hromas, Departments of Biochemistry/Molecular Biology and Hematology/Oncology, Indiana University Cancer Center, Indiana University School of Medicine, 1044 W Walnut, Bldg R4-202, Indianapolis, IN 46202; e-mail:rhromas@iupui.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal