Abstract
This study was designed to assess the influence of highly active antiretroviral therapy (HAART) on non-Hodgkin lymphoma (NHL) among patients infected with human immunodeficiency virus (HIV). Within EuroSIDA, a multicenter observational cohort of more than 8500 patients from across Europe, the incidences of NHL and subtypes (Burkitt, immunoblastic, primary brain lymphoma [PBL], and other/unknown histology) were determined according to calendar time of follow-up, and for those who initiated HAART (≥3 drugs) also time on HAART. Potential predictive factors of NHL were evaluated in Cox proportional hazard models. Over 26 764 person-years of prospective follow-up (PYF) from May 1994 to December 2000, the incidence of NHL decreased from 1.99 (95% confidence interval, 1.51-2.47) before September 1995 to 0.30 (0.19-0.42) cases/100 (PYF) after March 1999 (P < .001). The incidence of all subtypes of NHL decreased significantly and most pronouncedly for PBL. Among patients who started HAART, the incidence of NHL decreased from 0.88 (0.60-1.16) within the first 12 months after starting HAART to 0.45 (0.31-0.60) cases/100 PYF after more than 24 months (P = .004). In an adjusted Cox model for patients on HAART, the latest CD4 cell count and plasma viral load were both significantly associated with diagnosis of NHL; the relative hazard was 1.39 (range, 1.14-1.69) per 50% lower CD4 cell count, and 1.51 (range, 1.21-1.88) per 1 log higher plasma viral load. In conclusion, the incidence of NHL among HIV-infected patients has decreased significantly after the introduction of HAART, and the decline was most pronounced for PBL. After starting HAART, patients with insufficient immunologic and virologic responses were at highest risk of NHL.
Introduction
Following the introduction of highly active antiretroviral therapy (HAART), the incidence of new acquired immunodeficiency syndrome (AIDS)–defining events and the mortality among patients infected with human immunodeficiency virus (HIV) have declined to less than one tenth of the level before the era of HAART.1-4 The incidence of AIDS-defining diseases associated with severe immune deficiency such as cytomegalovirus chorioretinitis, disseminated Mycobacterium avium infection, and Kaposi sarcoma have dropped dramatically, whereas other conditions such as non-Hodgkin lymphoma (NHL) and Mycobacterium tuberculosis infections have decreased to a much lesser extent.4-7 Most studies have reported a decreasing incidence of NHL, in particular for the primary brain lymphoma, coincident with the increasing uptake of HAART, although some studies reported no change or even an increase in the incidence of NHL.6 8-15
The impact of HAART on the clinical manifestation of NHL in broader terms, in particular on the clinical presentation and the clinical prognosis, is not sufficiently elucidated, as a natural consequence of the limited experience and follow-up since HAART was introduced into clinical practice in the mid-1990s.16 In some previous studies, but not all, the introduction of antiretroviral combination therapy did not seem to affect the survival time for patients with primary brain lymphoma (PBL) or Burkitt lymphoma.15,17-19Because NHL has constituted an increasing proportion of the new AIDS-defining events, there is a growing need for identifying factors associated with NHL and thereby patients at high risk of developing NHL in the era of HAART.4 15 It remains to be determined whether the risk of NHL changes with longer time on HAART and whether there is a difference in risk of NHL between patients responding adequately to HAART in terms of an increasing CD4 count and suppressed plasma viral load (pVL) relative to those who do not.
To investigate these questions further we have analyzed the influence of HAART and the level of immune deficiency on the occurrence of NHL and the different subtypes and assessed factors associated with NHL to identify patients at high risk of NHL in the era of HAART.
Patients, materials, and methods
Patients
EuroSIDA is a prospective, observational cohort study consisting of 8556 HIV-infected patients from 63 clinical centers in 20 European countries (). The study has been approved by local ethics committees at all participating sites in accordance with the Helsinki protocol. A prefixed number of consecutive HIV-infected patients older than 16 years seen in outpatient clinics were enrolled from May 1994 (EuroSIDA cohort I, 3118 patients), from December 1995 (EuroSIDA cohort II, 1367 patients), from February 1997 (EuroSIDA cohort III, 2844 patients), and from March 1999 (EuroSIDA cohort IV, 1227 patients). A CD4 cell count less than 500 cells/μL within the last 4 months of the date of enrollment was obligatory for cohorts I, II, and III.
Information was collected from patients' charts and by patient interviews onto a standardized data collection form at enrollment and at 6-month follow-up visits, as described in detail in previous publications from the study.20 21 At each follow-up, information on all CD4 cell count and viral load measurements as well as all clinical diagnoses and changes to treatment since last follow-up is requested. All time variables were collected as month and year.
The computerized data were validated manually (≥10%) as well as electronically by scientific staff at the coordinating office, and the centers were monitored to ensure uniform and correct patient enrollment and data transmission from patient charts to the data collection form.
For the present analysis, information from 13 follow-up forms for cohort I, 10 for cohort II, 7 for cohort III, and 3 for cohort IV was available. The latest follow-up to be included was performed in the period from December 2000 to February 2001.
Analysis
The analysis included all patients without NHL at enrollment in EuroSIDA. Characteristics of patients with NHL were assessed and compared according to whether HAART had been initiated, and characteristics of different subtypes of NHL diagnosed during prospective follow-up were also compared using χ2 and nonparametric tests such as the Kruskall-Wallis test. These characteristics included demographic characteristics, CD4 cell count, pVL, and previous exposure to antiretroviral therapy. HAART was broadly defined as therapy with 3 or more drugs.
The lymphoma was classified as Burkitt, immunoblastic, PBL, or other/unknown histology provided that a definitive histologic diagnosis based on a biopsy or autopsy was available. A presumptive diagnosis was acceptable for PBL and was defined as evidence by scanning of lesions having mass effect and no response to treatment against toxoplasmosis combined with recent onset of global/focal neurologic symptoms/signs and no evidence of lymphoma outside the brain.
Incidences of NHL and subtypes were assessed according to use of HAART and level of CD4 cell count using time from enrollment in EuroSIDA to date of diagnosis, last follow-up visit, or death. Patients who were diagnosed with one subtype of NHL were censored thereafter in all analyses.
The follow-up time without HAART was calculated as the time from recruitment in EuroSIDA until the time of initiation of HAART, death, last follow-up, or development of NHL, whereas the follow-up time on HAART was assessed as time from initiation of HAART (or recruitment in EuroSIDA, if HAART was initiated prior to recruitment) to development of NHL, death, or last follow-up. Incidences were also assessed in consecutive time intervals after starting HAART. Patients who started HAART before enrollment were censored up to the time of enrollment and did not contribute with follow-up before this time point. For example, a patient who started HAART in January 1997 and was enrolled into EuroSIDA in January 1999 was included in the analysis from 24 months onward, whereas data for the first 24 months was not included.
The CD4 cell count was divided into 5 groups (<50, 50-100, 100-200, 200-350, and >350 cells/μL), and incidences of NHL were calculated according to latest CD4 cell count. This method takes into account the changes in CD4 cell count caused by changing treatment regimens. Patient follow-up in different strata was calculated as the patient's CD4 cell count changed between the respective strata.
Incidences of NHL were compared and confidence intervals (95% CI) were calculated using a normal approximation, or when appropriate (<20 cases) a Poisson distribution.
Among patients who started HAART, the incidence of NHL was also assessed according to the CD4 cell (+/− increase of ≥50 cells/μL) and pVL response (+/− pVL ≤ 500 copies/mL) after 6 months of HAART. Follow-up in this supplementary analysis was from 6 months after starting HAART to date of diagnosis of NHL, last follow-up visit, or death.
Factors associated with NHL were assessed in Cox models. Variables tested included sex, age, HIV transmission category, ethnicity, body weight, hemoglobin, nadir CD4 cell count, CD4 cell count, pVL, present and previous use of each individual antiretroviral drug, and time since the first positive HIV antibody test. CD4 cell count variables, pVL, hemoglobin, weight, diagnosis of AIDS, and time since first positive HIV antibody test were all modeled as time-dependent variables. Variables with a P below .10 in unadjusted analyses were included in the final multivariate model. Follow-up was from recruitment in the study to time of last follow-up, death, or diagnosis of NHL. Because there were no differences in the risk of NHL between different regions of EuroSIDA in the preliminary multivariate analyses, all models were then stratified by center.
We also assessed predictive factors among patients who had initiated HAART; in this model, follow-up was from recruitment or from initiation of HAART, if patients started HAART after recruitment. This model also included calendar time of initiation of HAART (before versus after June 1997).
All analyses were run in accordance with the principle of intent-to-treat, so no adjustment for stopping treatment regimens was done. The analysis was performed in SAS, version 6.12 (SAS Institute, Cary, NC). P less than .05 was considered significant, and all tests of significance were 2-sided.
Results
Patient characteristics
The study population, 8471 patients without a diagnosis of NHL before recruitment into the EuroSIDA study, was heterogeneous with respect to HIV transmission group, ethnicity, sex, age, as well as treatment and disease status (Table 1). The median CD4 cell count for the entire study population increased from 160 (interquartile range [IQR], 36-196) cells/μL in September 1995 to 290 (IQR, 111-468) cells/μL in March 2000.
Characteristics of patients without NHL at enrollment into EuroSIDA
| Characteristics . | All patients without NHL at recruitment, n = 8471 . |
|---|---|
| Sex, % female | 20.7 |
| HIV transmission group | |
| Homosexual, % | 46.6 |
| Intravenous drug use, % | 24.1 |
| Heterosexual, % | 22.2 |
| Race, % Caucasian | 89.9 |
| Other AIDS event before recruitment, % | 30.7 |
| Antiretroviral treatment, % experienced | 77.4 |
| Ever exposed to PI/NNRTI, % | 28.2 |
| Age, y, median (IQR) | 36 (31-43) |
| CD4 count, cells/mm3, median (IQR) | 219 (94-351) |
| Plasma viral load, log10 copies/mL, median (IQR) | 3.3 (2.5-4.4) |
| Characteristics . | All patients without NHL at recruitment, n = 8471 . |
|---|---|
| Sex, % female | 20.7 |
| HIV transmission group | |
| Homosexual, % | 46.6 |
| Intravenous drug use, % | 24.1 |
| Heterosexual, % | 22.2 |
| Race, % Caucasian | 89.9 |
| Other AIDS event before recruitment, % | 30.7 |
| Antiretroviral treatment, % experienced | 77.4 |
| Ever exposed to PI/NNRTI, % | 28.2 |
| Age, y, median (IQR) | 36 (31-43) |
| CD4 count, cells/mm3, median (IQR) | 219 (94-351) |
| Plasma viral load, log10 copies/mL, median (IQR) | 3.3 (2.5-4.4) |
PI indicates protease inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor.
During prospective follow-up, 222 cases of NHL were diagnosed, of which immunoblastic, PBL, and other/unknown histology accounted for approximately 30% each and Burkitt lymphoma for the last 10% (Table2). There were some differences between the different subtypes; the median calendar time of diagnosis ranged from 1995 for PBL to 1997 for other/unknown histology, and the CD4 cell count at diagnosis was markedly lower for PBL relative to the other subtypes.
Characteristics of 222 patients with NHL diagnosed during prospective follow-up according to the subtype diagnosed
| . | Burkitt (n = 20) . | Immunoblastic (n = 63) . | Other unknown/histology (n = 76) . | PBL (n = 63) . | P* . |
|---|---|---|---|---|---|
| Time of diagnosis, mo/y, median (IQR) | 12/96 | 09/96 | 07/97 | 10/95 | < .001 |
| (04/95-08/98) | (07/95-01/98) | (02/96-03/99) | (01/95-03/97) | ||
| Method of diagnosis, % definitive/presumptive/autopsy | 100.0/0.0/0.0 | 90.5/0.0/9.5 | 92.1/0.0/7.9 | 41.3/44.4/14.3 | < .001 |
| Sex, % female | 5.0 | 15.9 | 17.1 | 20.6 | .44 |
| HIV transmission group | .79 | ||||
| Homosexual, % | 45.0 | 52.4 | 54.0 | 49.2 | |
| Intravenous drug use, % | 25.0 | 23.8 | 13.2 | 25.4 | |
| Heterosexual, % | 25.0 | 17.5 | 23.7 | 22.2 | |
| Race, % Caucasian | 100.0 | 92.1 | 92.1 | 90.5 | .58 |
| AIDS diagnosis before NHL, % | 35.0 | 49.2 | 60.5 | 88.9 | < .001 |
| Age, y, median (IQR) | 42 (37-50) | 42 (34-49) | 41 (34-51) | 36 (32-44) | .02 |
| CD4 count, cells/mm3, median (IQR) | 168 (100-460) | 70 (26-201) | 81 (20-193) | 15 (3-40) | < .001 |
| Plasma viral load, log10 copies/mL, median (IQR) | 3.3 (2.7-4.7) | 4.4 (2.7-5.4) | 4.3 (3.0-5.1) | 4.6 (3.7-5.5) | .62 |
| . | Burkitt (n = 20) . | Immunoblastic (n = 63) . | Other unknown/histology (n = 76) . | PBL (n = 63) . | P* . |
|---|---|---|---|---|---|
| Time of diagnosis, mo/y, median (IQR) | 12/96 | 09/96 | 07/97 | 10/95 | < .001 |
| (04/95-08/98) | (07/95-01/98) | (02/96-03/99) | (01/95-03/97) | ||
| Method of diagnosis, % definitive/presumptive/autopsy | 100.0/0.0/0.0 | 90.5/0.0/9.5 | 92.1/0.0/7.9 | 41.3/44.4/14.3 | < .001 |
| Sex, % female | 5.0 | 15.9 | 17.1 | 20.6 | .44 |
| HIV transmission group | .79 | ||||
| Homosexual, % | 45.0 | 52.4 | 54.0 | 49.2 | |
| Intravenous drug use, % | 25.0 | 23.8 | 13.2 | 25.4 | |
| Heterosexual, % | 25.0 | 17.5 | 23.7 | 22.2 | |
| Race, % Caucasian | 100.0 | 92.1 | 92.1 | 90.5 | .58 |
| AIDS diagnosis before NHL, % | 35.0 | 49.2 | 60.5 | 88.9 | < .001 |
| Age, y, median (IQR) | 42 (37-50) | 42 (34-49) | 41 (34-51) | 36 (32-44) | .02 |
| CD4 count, cells/mm3, median (IQR) | 168 (100-460) | 70 (26-201) | 81 (20-193) | 15 (3-40) | < .001 |
| Plasma viral load, log10 copies/mL, median (IQR) | 3.3 (2.7-4.7) | 4.4 (2.7-5.4) | 4.3 (3.0-5.1) | 4.6 (3.7-5.5) | .62 |
P is derived from an overall comparison among all 4 subtypes.
Among the 222 cases, 125 (56%) were patients who had not initiated HAART (pre-HAART), whereas 97 (44%) were patients who had initiated HAART (post-HAART). Differences in characteristics of patients with NHL in the pre-HAART and post-HAART groups are shown in Table3. In the post-HAART group, PBL constituted a smaller proportion of events diagnosed and in contrast there was a larger proportion of other/unknown histology relative to the pre-HAART group. In addition, patients were older and had a higher CD4 cell count and a higher percentage were infected by heterosexual contact and a lower percentage by intravenous drug use in this group.
Characteristics of patients diagnosed with NHL during prospective follow-up according to whether they had initiated HAART
| . | All patients (n = 222) . | NHL pre-HAART (n = 125) . | NHL post-HAART (n = 97) . | P3-150 . |
|---|---|---|---|---|
| Subtype of NHL, % Burkitt/immunoblastic/ PBL/other/unknown histology | 9/28/34/28 | 10/27/38/25 | 7/30/16/46 | < .001 |
| Method of diagnosis, definitive/presumptive/autopsy | 7813/9 | 71/16/13 | 87/8/5 | .022 |
| Sex, % female | 16.7 | 20.0 | 12.4 | .13 |
| HIV transmission group | .007 | |||
| Homosexual, % | 51.4 | 53.6 | 48.5 | |
| Intravenous drug use, % | 20.7 | 26.4 | 13.4 | |
| Heterosexual, % | 20.6 | 15.2 | 29.2 | |
| Race, % Caucasian | 92.3 | 96.0 | 87.6 | .020 |
| AIDS diagnosis before NHL, % | 63.1 | 67.2 | 57.7 | .15 |
| Antiretroviral treatment experienced, % | 95.3 | 92.0 | 100.0 | .003 |
| Ever exposed to PI/NNRTI, % | 44.6 | 5.6 | 94.9 | < .001 |
| Age, y, median (IQR) | 40 (34-48) | 38 (34-45) | 42 (34-51) | .010 |
| CD4 count, cells/mm3, median (IQR) | 50 (12-163) | 30 (8-82) | 108 (33-223) | < .001 |
| Plasma viral load, log10 copies/mL, median (IQR) | 4.3 (2.8-5.4) | 5.0 (4.6-5.9) | 4.0 (2.7-5.2) | .010 |
| . | All patients (n = 222) . | NHL pre-HAART (n = 125) . | NHL post-HAART (n = 97) . | P3-150 . |
|---|---|---|---|---|
| Subtype of NHL, % Burkitt/immunoblastic/ PBL/other/unknown histology | 9/28/34/28 | 10/27/38/25 | 7/30/16/46 | < .001 |
| Method of diagnosis, definitive/presumptive/autopsy | 7813/9 | 71/16/13 | 87/8/5 | .022 |
| Sex, % female | 16.7 | 20.0 | 12.4 | .13 |
| HIV transmission group | .007 | |||
| Homosexual, % | 51.4 | 53.6 | 48.5 | |
| Intravenous drug use, % | 20.7 | 26.4 | 13.4 | |
| Heterosexual, % | 20.6 | 15.2 | 29.2 | |
| Race, % Caucasian | 92.3 | 96.0 | 87.6 | .020 |
| AIDS diagnosis before NHL, % | 63.1 | 67.2 | 57.7 | .15 |
| Antiretroviral treatment experienced, % | 95.3 | 92.0 | 100.0 | .003 |
| Ever exposed to PI/NNRTI, % | 44.6 | 5.6 | 94.9 | < .001 |
| Age, y, median (IQR) | 40 (34-48) | 38 (34-45) | 42 (34-51) | .010 |
| CD4 count, cells/mm3, median (IQR) | 50 (12-163) | 30 (8-82) | 108 (33-223) | < .001 |
| Plasma viral load, log10 copies/mL, median (IQR) | 4.3 (2.8-5.4) | 5.0 (4.6-5.9) | 4.0 (2.7-5.2) | .010 |
P is derived from a comparison between the pre-HAART and post-HAART group.
Incidences
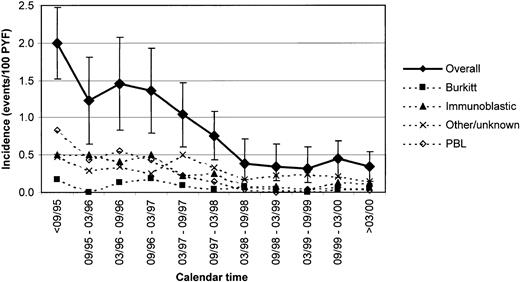
Over 26 764 person-years of prospective follow-up (PYF), we found an overall incidence of 0.83 (95% CI, 0.72-0.94) events/100 PYF, decreasing from 1.99 (95% CI, 1.51-2.47) before September 1995 (only minor variation in 1994-1995) to 0.30 (95% CI, 0.19-0.42) events/100 PYF after March 1999 (P < .001). The incidences of all 4 registered subtypes decreased, and most pronouncedly for PBL, from 0.83 before September 1995 to 0.04 cases/100 PYF after March 1999 (P < .001). Corresponding values for Burkitt lymphoma were 0.18/0.03 (P = .018), for immunoblastic lymphoma 0.50/0.10 (P < .001), and for unknown/other histology 0.48/0.19 cases/100 PYF (P = .008) (Figure 1).
Incidence of NHL and subtypes of NHL (events/100 PYF) among HIV-infected patients from 1995 to 2000.
The EuroSIDA study. Bars indicate 95% CIs.
Incidence of NHL and subtypes of NHL (events/100 PYF) among HIV-infected patients from 1995 to 2000.
The EuroSIDA study. Bars indicate 95% CIs.
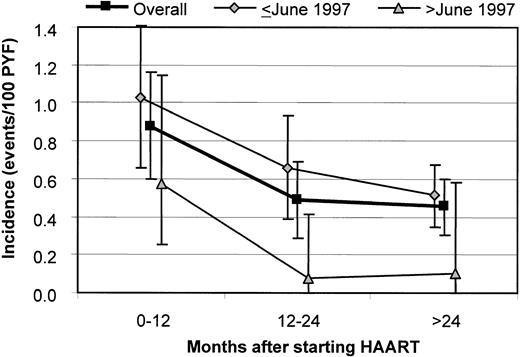
In the pre-HAART and post-HAART groups, the incidences were 1.29 (95% CI, 1.07-1.52) and 0.57 (95% CI, 0.45-0.68) events/100 PYF, respectively (P < .001). After starting HAART, the incidence decreased from 0.88 (95% CI, 0.60-1.16) within the first 12 months to 0.45 (95% CI, 0.31-0.60) events/100 PYF after more than 24 months (P = .004; Figure 2). When stratifying for the calendar time of starting HAART, the incidence of NHL was higher among patients starting HAART before June 1997 relative to those starting HAART later: 0.66 (95% CI, 0.52-0.80) versus 0.27 (95% CI, 0.13-0.49) events/100 PYF (P = .005), and this was true for all time intervals after initiation of HAART.
Incidence of NHL according to the calendar time of starting HAART and the time after starting HAART.
Overall: P = .027 for 0 to 12 months versus 12 to 24 months and P = .004 for 0 to 12 months versus more than 24 months. Bars indicate 95% CIs.
Incidence of NHL according to the calendar time of starting HAART and the time after starting HAART.
Overall: P = .027 for 0 to 12 months versus 12 to 24 months and P = .004 for 0 to 12 months versus more than 24 months. Bars indicate 95% CIs.
Furthermore, the incidence of NHL was significantly lower among patients who experienced a CD4 and pVL response (increase of ≥50 cells/μL and pVL ≤ 500 copies/mL) after 6 months of HAART relative to those who did neither have CD4 nor pVL response to HAART: 0.24 (95% CI, 0.08-0.0.52) (response) versus 0.99 (95% CI, 0.54-1.65) events/100 PYF (no response); (P = .003).
Overall, the incidence of NHL was approximately 20-fold higher among patients with their latest CD4 count less than 50 cells/μL compared with patients with a CD4 count more than 350 cells/μL: 5.54 (95% CI, 4.51-6.56) versus 0.23 (95% CI, 0.13-0.33) events/100 PYF (P < .001). The incidence at a latest CD4 count less than 50 cells/μL was significantly lower among patients in the post-HAART group relative to the pre-HAART group. The incidence also differed significantly, but less profoundly so, at a latest CD4 count between 50 and 100 cells/μL, whereas at higher CD4 cell counts there was little difference between the groups (Figure3A). For patients with their latest CD4 count above 200 cells/μL, the nadir CD4 cell count before recruitment in the study did not influence the incidence of NHL, regardless of whether the patients had initiated HAART or not (an overall incidence of 0.20 [95% CI, 0.07-0.44] among those with a nadir CD4 count < 50 cells/μL versus 0.42 [95% CI, 0.25-0.66] events/100 PYF among patients who had never had a CD4 < 200 cells/μL;P = .11).
Incidence of NHL and subtypes of NHL according to CD4 cell count before and after starting HAART.
(A) NHL. (B) Subtypes of NHL. P values indicated ifP < .05 in comparison between pre-HAART and post-HAART within CD4 stratum. Bars indicate 95% CIs.
Incidence of NHL and subtypes of NHL according to CD4 cell count before and after starting HAART.
(A) NHL. (B) Subtypes of NHL. P values indicated ifP < .05 in comparison between pre-HAART and post-HAART within CD4 stratum. Bars indicate 95% CIs.
When analyzing each subtype separately, the influence of HAART at low CD4 cell counts tended to be the same for all subtypes, though only significantly so for PBL; latest CD4 less than 50 cells/μL: 4.24 (95% CI, 2.96-5.52) versus 1.34 (95% CI, 0.58-2.10) events/100 PYF in the pre-HAART and post-HAART groups, respectively (P < .001). At higher CD4 levels the incidence was low for all subtypes of NHL, and there were no significant differences between the pre-HAART and post-HAART groups (Figure 3B).
Predictive factors
Predictive factors of NHL were assessed in Cox proportional hazards models (Table 4). In the overall model, sex (men at higher risk), higher age, a diagnosis of AIDS, lower hemoglobin, lower weight, higher pVL, lower nadir CD4 cell count, and lower latest CD4 cell count were all associated (P < .10) with a higher risk of NHL in univariate analysis. Age, sex, and the latest CD4 cell count remained associated with NHL in multivariate models that also included adjustment for present and duration of previous antiretroviral treatment experience. When repeating the model for patients starting HAART, the relative hazards (RHs) for all these factors remained similar with the possible exception of a previous diagnosis of AIDS. The CIs were wider in the post-HAART model because of the lower number of patients and shorter follow-up time (Table4).
Cox proportional hazards model for diagnosis of NHL within the entire study population and in the group of patients who have initiated HAART (post-HAART)
| . | Entire study population . | Post-HAART . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate . | Multivariate . | Multivariate . | Multivariate including pVL . | |||||||||
| RH . | 95% CI . | P . | RH . | 95% CI . | P . | RH . | 95% CI . | P . | RH . | 95% CI . | P . | |
| Sex (female vs male) | 0.73 | 0.51 -1.04 | .08 | 0.63 | 0.39 -1.03 | .07 | 0.35 | 0.15 -0.80 | .0127 | 0.33 | 0.13 -0.85 | .021 |
| Ethnicity (white vs nonwhite) | 0.64 | 0.39 -1.05 | .08 | 0.59 | 0.27 -1.27 | .18 | 0.68 | 0.25 -2.12 | .48 | 1.33 | 0.44 -3.43 | .69 |
| AIDS (td, yes vs no) | 3.61 | 2.59 -5.04 | < .001 | 1.48 | 0.99 -2.21 | .05 | 1.01 | 0.51 -2.02 | .98 | 0.87 | 0.39 -1.95 | .73 |
| Age (per 10 years older) | 1.25 | 1.10 -1.42 | < .001 | 1.32 | 1.13 -1.55 | < .001 | 1.43 | 1.14 -1.79 | .002 | 1.52 | 1.19 -1.95 | < .001 |
| Nadir CD4 cell count (td, per 50% lower) | 1.25 | 1.20 -1.30 | < .001 | 1.01 | 0.93 -1.10 | .79 | 0.95 | 0.83 -1.09 | .46 | 0.96 | 0.82 -1.11 | .56 |
| Latest CD4 cell count (td, per 50% lower) | 1.53 | 1.46 -1.60 | < .001 | 1.44 | 1.32 -1.59 | < .001 | 1.57 | 1.34 -1.83 | < .001 | 1.39 | 1.13 -1.69 | .001 |
| Latest haemoglobin (td, per 1 g/dL lower) | 1.23 | 1.14 -1.33 | < .001 | 1.06 | 0.97 -1.16 | .20 | 1.09 | 0.95 -1.25 | .20 | 1.15 | 0.99 -1.34 | .07 |
| Weight (td, per 5 kg lower) | 1.09 | 1.02 -1.15 | .006 | 1.05 | 0.97 -1.13 | .20 | 1.09 | 0.97 -1.22 | .16 | 1.05 | 0.93 -1.19 | .41 |
| Latest plasma viral load (td, per 1 log10 copies/mL higher)4-150 | 1.71 | 1.47 -1.99 | < .001 | — | — | — | — | — | — | 1.51 | 1.21 -1.88 | < .001 |
| Initiation of HAART (> June 1997 vs ≤ June 1997)4-150 | 0.41 | 0.21 -0.79 | .008 | — | — | — | 0.53 | 0.23 -1.24 | .14 | 0.71 | 0.30 -1.68 | .44 |
| . | Entire study population . | Post-HAART . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate . | Multivariate . | Multivariate . | Multivariate including pVL . | |||||||||
| RH . | 95% CI . | P . | RH . | 95% CI . | P . | RH . | 95% CI . | P . | RH . | 95% CI . | P . | |
| Sex (female vs male) | 0.73 | 0.51 -1.04 | .08 | 0.63 | 0.39 -1.03 | .07 | 0.35 | 0.15 -0.80 | .0127 | 0.33 | 0.13 -0.85 | .021 |
| Ethnicity (white vs nonwhite) | 0.64 | 0.39 -1.05 | .08 | 0.59 | 0.27 -1.27 | .18 | 0.68 | 0.25 -2.12 | .48 | 1.33 | 0.44 -3.43 | .69 |
| AIDS (td, yes vs no) | 3.61 | 2.59 -5.04 | < .001 | 1.48 | 0.99 -2.21 | .05 | 1.01 | 0.51 -2.02 | .98 | 0.87 | 0.39 -1.95 | .73 |
| Age (per 10 years older) | 1.25 | 1.10 -1.42 | < .001 | 1.32 | 1.13 -1.55 | < .001 | 1.43 | 1.14 -1.79 | .002 | 1.52 | 1.19 -1.95 | < .001 |
| Nadir CD4 cell count (td, per 50% lower) | 1.25 | 1.20 -1.30 | < .001 | 1.01 | 0.93 -1.10 | .79 | 0.95 | 0.83 -1.09 | .46 | 0.96 | 0.82 -1.11 | .56 |
| Latest CD4 cell count (td, per 50% lower) | 1.53 | 1.46 -1.60 | < .001 | 1.44 | 1.32 -1.59 | < .001 | 1.57 | 1.34 -1.83 | < .001 | 1.39 | 1.13 -1.69 | .001 |
| Latest haemoglobin (td, per 1 g/dL lower) | 1.23 | 1.14 -1.33 | < .001 | 1.06 | 0.97 -1.16 | .20 | 1.09 | 0.95 -1.25 | .20 | 1.15 | 0.99 -1.34 | .07 |
| Weight (td, per 5 kg lower) | 1.09 | 1.02 -1.15 | .006 | 1.05 | 0.97 -1.13 | .20 | 1.09 | 0.97 -1.22 | .16 | 1.05 | 0.93 -1.19 | .41 |
| Latest plasma viral load (td, per 1 log10 copies/mL higher)4-150 | 1.71 | 1.47 -1.99 | < .001 | — | — | — | — | — | — | 1.51 | 1.21 -1.88 | < .001 |
| Initiation of HAART (> June 1997 vs ≤ June 1997)4-150 | 0.41 | 0.21 -0.79 | .008 | — | — | — | 0.53 | 0.23 -1.24 | .14 | 0.71 | 0.30 -1.68 | .44 |
The multivariate models also included adjustment for present and previous use of individual antiretroviral drugs (if P≤.10 in an unadjusted analysis for the entire population).
RH indicates relative hazard; CI, confidence interval; td, time-dependent.
Univariate model restricted to patients starting HAART.
Factors such as HIV transmission category and time since first documented positive HIV test were also tested in univariate models for the entire population, but had P above .10 and were consequently not included in the multivariate model.
Interestingly, the nadir CD4 cell count was not significantly associated with NHL in multivariate models, which also included the latest CD4 cell count, and this remained true in exploratory analyses of each of the subtypes of NHL separately. The pVL was not included in the multivariate model for the entire population because measurements were only available for a subgroup of patients, preferentially followed in the most recent period. In the subgroup of patients with pVL available, this factor was a significant predictor in an unadjusted model and remained so in an adjusted model including this variable (RH = 1.61; 95% CI, 1.32-1.98; P < .001), whereas the other predictors remained the same in such a model. This was also the case when restricting the adjusted model to the post-HAART group (Table4).
In the post-HAART group, time of initiation of HAART was, as would be expected from the incidence data, significantly associated with development of NHL in a univariate model. However, when adjusting for changes in CD4 cell count and pVL, there was no longer any significant association between calendar time of starting HAART and risk of NHL, indicating that the temporal influence could at least in part be explained by differences in response to HAART depending on when HAART was initiated (Table 4).
Survival after NHL
The median survival time after a diagnosis was significantly lower for patients with PBL relative to the other subtypes: PBL 1 month (95% CI, 1-2), Burkitt 6 months (95% CI, 2-16 months) immunoblastic 6 months (95% CI, 4-10 months), and other/unknown histology 5 months (95% CI, 2-7 months); P < .001, log-rank test. Further, the median survival time for both PBL and other subtypes of NHL (grouped together) did not change significantly over time. The median survival time after a diagnosis of PBL was 1 month (95% CI, 1-2 months) before 1997, 1 month (95% CI, 1-2 months) in 1997, and 3 months (95% CI, 1-4 months) after 1997; P = .80, log-rank test (46, 12, and 5 patients, respectively). For patients with Burkitt, immunoblastic, or other/unknown histology, the corresponding values were 4 months (95% CI, 3-6 months), 13 months (95% CI, 5-32 months), and 5 months (95% CI, 3-9 months), respectively;P = .08 (74, 35, and 50 patients, respectively). In an exploratory analysis among the 50 patients diagnosed with systemic NHL after 1997, there was no significant change in the survival time according to the calendar year of diagnosis. The overall pattern did not change significantly when excluding patients who were diagnosed with NHL at autopsy.
Discussion
Within a large European multicenter study of HIV-infected patients, the incidence of NHL has decreased 6-fold after antiretroviral combination therapy was introduced in clinical practice (1995, dual therapy and 1996, HAART).16
In previous studies, there have been divergent results on the changes in the incidence of NHL in relation to the introduction of HAART, influenced by PBL being included in the studies or not, and by the limited numbers of patients and follow-up available after the introduction of HAART.9,10,12-15 However, a large multicohort study recently reported a 3-fold decline in the 1990s, primarily driven by significant decreases of PBL and immunoblastic lymphoma, whereas the incidence of Burkitt lymphoma did not seem to decrease.8 The present analysis adds further evidence to that by providing follow-up until the winter of 2000-2001, and by documenting that, in addition to PBL, the incidence of Burkitt, immunoblastic, and lymphoma of other/unknown histology also decreased significantly within the follow-up period now available.
Further differentiation within the category of other/unknown histology would have been of interest, but it is not possible at present. However, it is important that the incidence for this category also decreased, so the declining incidences of the 3 specific categories were not explained by NHLs in recent years being categorized rather in the group of other/unknown histology than as one of the specific subtypes. Only definitive diagnoses (a presumptive diagnosis of PBL allowed) based on biopsy or autopsy were accepted, and a lower rate of autopsy is unlikely to explain the overall decline of particularly PBL, because only one eighth of all PBLs were diagnosed by autopsy. Among patients who have initiated HAART, a slight and nonsignificant decline in the incidence of NHL within the 12 months after starting HAART has been reported from the Swiss HIV Cohort Study, contrasting the pronounced and rapid decline of other diseases occurring at low CD4 cell counts such as cytomegalovirus chorioretinitis, M aviumcomplex infection, and Kaposi sarcoma.6,22 In contrast to these, NHL is a heterogeneous entity with a multifactorial pathogenesis with no single causal agent documented so far, though Ebstein-Barr virus and human herpes virus type 8 have been associated with development of PBL and body cavity–based lymphoma.8 23-25 However, having accumulated longer follow-up we have found that the incidence of NHL does decrease with longer time on HAART.
In an epidemiologic sense, NHL therefore behaves as an opportunistic infection. The incidence of NHL declined approximately 1 year later than what was generally seen for AIDS-defining events,4,7,22 and the protective effect for NHL seems to arise after a longer period on HAART compared with opportunistic infections. This would at least in part explain why NHL constitutes an increasing proportion of the AIDS-defining events diagnosed in recent years.4
The declining incidence following initiation of HAART is reassuring, because life expectancies of HIV-infected patients have increased following the introduction of HAART, and duration of HIV infection in some studies in the pre-HAART era was a predictor of developing NHL. However, time since first positive HIV antibody test in our study was not associated with risk of NHL. Interestingly, the incidence of NHL remained considerably higher in patients receiving HAART for more than 1 year compared with the general population.26-28
The continuous decrease in the risk of NHL several years after initiation of HAART might reflect a latency in the pathogenesis of NHL and some events resulting from irreversible processes initiated before HAART was instituted. It is also possible that the HAART-induced immune restoration is partial and provides less protection against NHL than opportunistic infections.29 Also other factors (eg, prolonged immune activation by B-cell activation during ongoing HIV replication) might potentially be involved in the pathogenesis of NHL.27,30 31 Of note, a high pVL remained a predictive factor for NHL in all analyses and patients who did not respond adequately to initiation of HAART had a higher incidence of NHL than those who did not experience optimal CD4 and pVL responses.
In addition to the profound decline in the incidence of NHL following the HAART-induced CD4 lymphocyte increases, patients receiving HAART at persistently low CD4 cell counts were at lower risk of risk of NHL, primarily of PBL compared with patients with low CD4 cell count, who had not initiated HAART. Conversely, at higher CD4 cell count patients who had initiated HAART were not at a measurably lower risk of NHL, because the risk of NHL at CD4 cell count above 200 cells/μL was low among patients starting HAART or not.
The introduction of HAART does not seem to have affected the natural history of NHL, because the predictive factors of NHL (and values of RHs) in our study were very similar in the entire population and in the post-HAART group, and with few exceptions, consistent with previous studies. The associations of male sex and higher age with a higher risk of NHL were in agreement with previous studies of both HIV-infected patients and the general population.15 28
One exception from the similar pattern in the entire population and the post-HAART group was a previous diagnosis of AIDS, which overall was associated with higher risk of developing NHL, whereas this was not the case among patients who had initiated HAART. These findings are in agreement with previous reports of NHL as a predominantly secondary AIDS event in the pre-HAART era and a relatively common initial AIDS-defining event after HAART was introduced.4 32-34
Interestingly, in our study, nadir CD4 cell count was not significantly associated with the risk of NHL when adjusting for the latest or time-updated CD4 cell count, whereas other studies have reported a higher risk of NHL among patients with prior immune deficiency.15 27 Our finding indicates that the past immune deficiency in general had little influence on risk of NHL once the CD4 cell count increased.
Finally, the findings in the present study document that the prognosis of NHL has not improved substantially from the early part of the HIV epidemic in Europe and remains considerably worse for PBL relative to other subtypes of NHL.15,17,18,34 These results should be interpreted with caution because only few cases of NHL, especially of PBL, have been diagnosed in recent years. However, the results are of concern, because improvement of prognostic factors such as CD4 cell count and pVL among patients with NHL would have been expected to translate into an better clinical prognosis in recent years.15 The prognosis remained also worse than for non-HIV–infected patients possibly due to increased toxicity of chemotherapy in HIV-infected patients and overlapping toxicity with antiretroviral therapy.33 Therefore, dose reduction of chemotherapy has been attempted.35 A detailed analysis of the influence of type of chemotherapy is not possible within the present study because such data have not been collected, nor have data on complete and partial responses.
Because of the low incidences of most HIV-related events in recent years, a continuous monitoring in large-sized observational studies is required to detect potential changes in incidences of and survival from AIDS-defining events and the appearance of new HIV-related diseases. These may include several malignancies that are being followed closely (eg, anal cancer, cervical dysplasia/cancer). Because of its design and size, the EuroSIDA study has the capability of assessing such changes in the coming years. Importantly, all new AIDS-defining events are reviewed and sites monitored to ensure a correct and uniform data collection.
In conclusion, the incidence of NHL has declined gradually in patients initiating HAART and even at persistent severe immune deficiency, HAART protects against NHL, especially against PBL. The natural history including the clinical prognosis of NHL has not changed after the introduction of HAART. In the era of HAART, patients at highest risk of developing NHL are those who did not respond adequately to HAART, that is, remained at low CD4 cell count and insufficiently suppressed viral replication.
The EuroSIDA Study Group (national coordinators in parenthesis).
Austria (N. Vetter) Pulmologisches Zentrum der Stadt Wien, Vienna. Belgium (N. Clumeck) P. Hermans, B. Sommereijns, Saint-Pierre Hospital, Brussels; R. Colebunders, Institute of Tropical Medicine, Antwerp. Czech Republic (L. Machala) H. Rozsypal, Faculty Hospital Bulovka, Prague. Denmark (J. Nielsen) J. Lundgren, T. Benfield, O. Kirk, Hvidovre Hospital, Copenhagen; J. Gerstoft, T. Katzenstein, B. Røge, P. Skinhøj, Rigshospitalet, Copenhagen; C. Pedersen, Odense University Hospital, Odense. France (C. Katlama) C. Rivière, Hôpital de la Pitié-Salpétière, Paris; J.-P. Viard, Hôpital Necker-Enfants Malades, Paris; T. Saint-Marc, Hôpital Edouard Herriot, P. Vanhems, University Claude Bernard, Lyon; C. Pradier, Hôpital de l'Archet, Nice. Germany (M. Dietrich) C. Manegold, Bernhard-Nocht-Institut for Tropical Medicine, Hamburg; J. van Lunzen, Eppendorf Medizinische Kernklinik, Hamburg; V. Miller, S. Staszewski, J. W. Goethe University Hospital, Frankfurt; F.-D. Goebel, Medizinische Poliklinik, Munich; Bernd Salzberger, Universität Köln, Cologne; J. Rockstroh, Universitäts Klinik Bonn. Greece (J. Kosmidis) P. Gargalianos, H. Sambatakou, J. Perdios, Athens General Hospital, Athens; G. Panos, M. Astriti, 1st IKA Hospital, Athens. Hungary (D. Banhegyi) Szent Lásló Hospital, Budapest. Ireland (F. Mulcahy) St. James's Hospital, Dublin. Israel (I. Yust) D. Turner, Ichilov Hospital, Tel Aviv; S. Pollack, Z. Ben-Ishai, Rambam Medical Center, Haifa; Z. Bentwich, Kaplan Hospital, Rehovot; S. Maayan, Hadassah University Hospital, Jerusalem. Italy (S. Vella, A. Chiesi) Istituto Superiore di Sanita, Rome; C. Arici, Ospedale Riuniti, Bergamo; R. Pristerá, Ospedale Generale Regionale, Bolzano; F. Mazzotta, F. Vichi, Ospedale S. Maria Annunziata, Florence; R. Esposito, A. Bedini, Universitàdi Modena, Modena; A. Chirianni, E. Montesarchio, Presidio Ospedaliero A. D. Cotugno, Naples; V. Vullo, P. Santopadre, Universitàdi Roma La Sapienza, Rome; P. Narciso, A. Antinori, P. Franci, M. Zaccarelli, Ospedale Spallanzani, Rome; A. Lazzarin, R. Finazzi, Ospedale San Raffaele, Milan; A. D'Arminio Monforte, Osp. L. Sacco, Milan. Luxembourg (R. Hemmer), T. Staub, Centre Hospitalier, Luxembourg. Netherlands (P. Reiss) Academisch Medisch Centrum bij de Universiteit van Amsterdam, Amsterdam. Norway (J. Bruun) A. Maeland, Ullevål Hospital, Oslo. Poland (B. Knysz) J. Gasiorowski, Medical University, Wroslaw; A. Horban, Centrum Diagnostyki i Terapii AIDS, Warsaw; R. Rogowska-Szadkowska, Medical University, Bialystok; A. Boron-Kaczmarska, M. Pynka, Medical Univesity, Szczecin; M. Beniowski, Osrodek Diagnostyki i Terapii AIDS, Chorzow; H. Trocha, Medical University, Gdansk. Portugal (F. Antunes) Hospital Santa Maria, Lisbon; K. Mansinho, Hospital de Egas Moniz, Lisbon; R. Proenca, Hospital Curry Cabral, Lisbon. Spain (J. González-Lahoz) R. Polo, V. Soriano, Hospital Carlos III, Madrid; B. Clotet, A. Jou, J. Conejero, C. Tural, Hospital Germans Trias i Pujol, Badalona; J. M. Gatell, J. M. Miró, Hospital Clinic i Provincial, Barcelona. Sweden (A. Blaxhult) Karolinska Hospital; B. Heidemann, Södersjukhuset; P. Pehrson, Huddinge Sjukhus, Stockholm. Switzerland (B. Ledergerber) R. Weber, University Hospital, Zürich; P. Francioli, A. Telenti, Centre Hospitalier Universitaire Vaudois, Lausanne; B. Hirschel, V. Soravia-Dunand, Hospital Cantonal Universitaire de Geneve, Geneve. United Kingdom (S. Barton) St. Stephen's Clinic, Chelsea and Westminster Hospital, London; A. M. Johnson, D. Mercey, Royal Free and University College London Medical School, London (University College Campus); A. Phillips, C. Loveday, M. A. Johnson, A. Mocroft, Royal Free and University College Medical School, London (Royal Free Campus); A. Pinching, J. Parkin, Medical College of Saint Bartholomew's Hospital, London; J. Weber, G. Scullard, Imperial College School of Medicine at St. Mary's, London; M. Fisher, Royal Sussex County Hospital, Brighton; R. Brettle, City Hospital, Edinburgh.
Steering committee: J. Nielsen (chair), N. Clumeck, M. Dietrich, J. M. Gatell, A. Horban, A. M. Johnson, C. Katlama, B. Ledergerber, C. Loveday, A. Phillips, P. Reiss, S. Vella.
Coordinating center staff: J. Lundgren (project leader), I. Gjørup, O. Kirk, N. Friis-Moeller, A. Mocroft, D. Mollerup, M. Nielsen, A. Sørensen, H. Buch, L. Madsen, L. Hansen, L. Teglbjærg.
Supported by the European Commission BIOMED 1 (CT94-1637), BIOMED 2 (CT97-2713) programs, and the 5th framework program (QLK2-2000-00773) were the primary sponsors of the EuroSIDA study. Unrestricted grants were also provided by GlaxoSmithKline, Roche, and Boehringer Ingelheim. The participation of centers from Switzerland was supported by a grant from the Swiss Federal Office for Education and Science. The study group participants are listed in the .
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ole Kirk, Coordinating Center of EuroSIDA, Department of Infectious Diseases 144, Hvidovre Hospital, University of Copenhagen, 2650 Hvidovre, Denmark; e-mail: oki@cphiv.dk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal