Abstract
Notch signaling is involved in cell fate decisions in many systems including hematopoiesis. It has been shown that expression of an activated form of Notch1 (aNotch1) in 32D mouse myeloid progenitor cells inhibits the granulocytic differentiation induced by granulocyte colony-stimulating factor (G-CSF). Results of the current study show that aNotch1, when expressed in F5-5 mouse erythroleukemia cells, also inhibits erythroid differentiation. Comparison of the expression levels of several transcription factors after stimulation for myeloid and erythroid differentiation, in the presence or absence of aNotch1, revealed that aNotch1 did not change its regulation pattern with any of the transcription factors examined, except for GATA-2, despite its inhibitory effect on differentiation. GATA-2 was down-regulated when the parental 32D and F5-5 were induced to differentiate into granulocytic and erythroid lineages, respectively. In these induction procedures, however, the level of GATA-2 expression was sustained when aNotch1 was expressed. To ascertain whether maintenance of GATA-2 is required for the Notch-induced inhibition of differentiation, the dominant-negative form of GATA-3 (DN-GATA), which acted also against GATA-2, or transcription factor PU.1, which was recently shown to be the repressor of GATA-2, was introduced into aNotch1-expressing 32D (32D/aNotch1) cells that do not express GATA family proteins other than GATA2. Both DN-GATA and PU.1 reversed the phenotype of 32D/aNotch1 inducing its differentiation when G-CSF was added. Furthermore, enforced expression of HES-1, which is involved in Notch signaling, delayed differentiation of 32D, and again this phenotype was neutralized by DN-GATA. These results indicate that GATA-2 activity is necessary for the Notch signaling in hematopoietic cells.
Introduction
Hematopoietic stem cells maintain themselves by self-renewal and by generating cells committed to differentiation into the various hematopoietic lineages.1 The molecular mechanism by which hematopoietic stem cells are kept undifferentiated while retaining their proliferative ability remains poorly understood. However, the criticality of the cell-cell interaction in the hematopoietic environment has been well recognized,2 and in this context, hematopoietic expression of Notch receptors and stromal expression of their ligands are of particular interest.3-6
The Notch genes constitute a family of genes that are highly conserved from the nematode through the vertebrates.7-9They play important roles in determining cell fate in multiple systems. In general, it is well established that Notch signal activation exerts the inhibitory effect on cell differentiation. In vertebrates, 4 members of this gene family, Notch1 throughNotch4, have been isolated.10-14 Each Notch protein is a transmembrane protein consisting of an extracellular domain that contains tandem epidermal growth factor (EGF)–like repeats and 3 lin-12/Notch repeats, a single transmembrane domain, and an intracellular domain containing 6 cdc10/SWI16/ankyrin repeats, putative nuclear localization signals, and a C-terminal OPA/PEST region. Studies of Notch using various experimental systems demonstrated that deletion of all or most of their extracellular portion results in the constitutive activation of the Notch receptor.15-17
Among the Notch family proteins, Notch1 and Notch2 have been shown to be expressed in the hematopoietic progenitor cells.18,19In human acute lymphocytic leukemia, the human Notch1 homologue TAN-1 is truncated and converted into activated Notch1 (aNotch1).10 Previously, it was reported that aNotch1 and activated Notch2 (aNotch2) inhibited myeloid differentiation of 32D cells19,20 and that infection of mouse bone marrow cells with a retrovirus containing aNotch1 resulted in the establishment of immortalized, cytokine-dependent cell lines that generated progeny with either lymphoid or myeloid characteristics both in vitro and in vivo.21 Furthermore, Notch ligand Jagged1, Jagged2, and Delta1 can inhibit differentiation of hematopoietic progenitor cells.3-6 Finally, Jagged1 was recently shown to be a novel growth factor for hematopoietic stem cells.22 When these findings are taken together, the Notch signal is likely to be important for hematopoiesis in preventing progenitor cells from differentiation.23
However, the molecular mechanism by which Notch inhibits differentiation of hematopoietic cells remains totally unknown. Here we show that the level of GATA-2 expression is maintained in the aNotch1-expressing F5-5 and 32D (F5-5/aNotch1 and 32D/aNotch1) cells, although it is down-regulated in the control cells. Moreover, the fact that dominant-negative GATA-3 (DN-GATA)24,25 and PU.1,26 which can suppress GATA-2 function, reversed the aNotch1-induced differentiation inhibition of differentiation in 32D/aNotch1 cells. Hairy and enhancer of split homolog-1 (HES-1), which is involved in Notch signaling,27 28 can delay the differentiation of 32D cells, whereas DN-GATA could also cancel this phenotype. These results indicated that GATA-2 is required for the Notch signaling in hematopoiesis.
Material and methods
Plasmid construction
Mouse Notch1 complementary DNA (cDNA) was isolated from a mouse brain cDNA library. Myc-tagged aNotch1 was constructed as described previously.29,30 Myc-aNotch1 was subcloned into the EcoRI site of the expression vector pME18Sneo and the retrovirus vector pMX/IRES-EGFPpuro, which was made by the insertion into pMXpuro of an internal ribosomal entry site-enhanced green fluorescent protein (IRES-EGFP) fragment derived from pIRES-EGFP (Clontech, Palo Alto, CA).31 Mouse HES-1 cDNA, a gift from Dr R. Kageyama (Kyoto University), was tagged with a Flag sequence at the 5′ end and subcloned into pME18Sneo. An HA-tagged dominant-negative form of GATA-3 (KRR) was prepared as previously described24 25 and was subcloned into the EcoRI site of pMX/IRES-EGFPpuro. PU.1 cDNA was obtained by reverse transcription–polymerase chain reaction (RT-PCR) using total RNA obtained from 32D cells stimulated with granulocyte colony-stimulating factor (G-CSF). The following primer pair was used: sense, AAGGATCCATGTTACAGGCGTGCAAAATGGAAGGG; antisense, GGCGACGGGTTAATGCTATG. After the sequence of the amplified product was verified, a Flag sequence was tagged at the 5′ end and subcloned into theEcoRI site of pMX/IRES-EGFPpuro.
Cell culture
Recombinant human Activin A was a gift from Dr Y. Eto (Ajinomoto, Kawasaki, Japan). Recombinant mouse interleukin-3 (IL-3) and human G-CSF were provided by Kirin Brewery, Takasaki, Japan. A mouse erythroleukemia cell line F5-5 was maintained in RPMI 1640 medium with 10% fetal calf serum (FCS). For erythroid differentiation of F5-5 cells, dimethyl sulfoxide (DMSO) was added at a concentration of 1.5% or Activin A at 100 ng/mL. After a 7-day culture period, cytospin and benzidine staining were performed as described elsewhere.32 The ratio of benzidine-positive cells was calculated by counting 300 cells under a microscope. A mouse myeloid progenitor 32D cell line was maintained in RPMI 1640 medium with 10% FCS and 0.25 ng/mL recombinant mouse IL-3. For the granulocytic differentiation, IL-3 was removed by washing twice with phosphate-buffered saline (PBS), and the cells were resuspended in RPMI 1640 containing 10% FCS and 5 ng/mL G-CSF. After 4 days of culture, Wright-Giemsa staining was performed. The proportion of cells with a lobulated nucleus was determined under a microscope. The ecotropic packaging cell line GP+E86 was maintained in Dulbecco modified Eagle medium (DMEM) containing 10% FCS.
Stable transductions and selection procedures
Stable aNotch1 or HES-1–expressing 32D cells were generated by electroporation (960 μF, 250 V) and G418 selection (800 μg/mL) as described previously.33 Packaging cells producing retrovirus containing aNotch1, KRR, and PU.1 were generated by the transfection of these cDNAs in pMX/IRES-EGFPpuro using Superfect (Qiagen, Valencia, CA) according to the manufacturer's instructions. Conditioned media containing high-titer retrovirus derived from stable GP+E86 transfectants were used to transduce 32D and F5-5 in the presence of 4 μg/mL polybrene. After incubation for 48 hours, puromycin was added to the medium at a final concentration of 1 ng/mL. Puromycin-resistant clones were screened for the expression of EGFP by FACS caliber (Becton Dickinson, San Jose, CA) after limiting dilution. The GFP-positive clones were analyzed for the expression of aNotch1, KRR, or PU.1 by Western blotting using antibodies against the respective tags.
RNA extraction and semiquantitative RT-PCR
Total RNA was isolated from cells as indicated using Isogen (Nihon gene, Tokyo, Japan) as stated in the manufacturer's instructions. Random hexamer-primed cDNA was prepared from 5 μg total RNA, using mouse mammary leukemia virus reverse transcriptase (Gibco BRL, Grand Island, NY) in a total volume of 25 μL. The synthesized cDNA was subsequently diluted up to 100 μL with water. Semiquantitative RT-PCR was performed as described previously.34 Briefly, to standardize the template cDNA, a series of dilutions was subjected to 15 cycles of PCR using a primer pair specific for hypoxanthine phosphoribosyltransferase (HPRT). After determination of the relative cDNA concentrations, specific primer pairs listed in Table 1 were used to perform PCR for the analysis of the expression levels of messenger RNA (mRNA) encoding the respective transcription factors. The number of PCR cycles was determined for each primer pair to cause the PCR yield to depend on the amount of template cDNA. The rationale of the semiquantification by this method was previously verified by comparing the results derived therefrom with those derived from the real-time PCR.34
Oligonucleotide primers used for gene expression analysis by RT-PCR
| Gene . | Size (bp) . | Annealing temperature (°C) . | 5′ primer . | 3′ primer . |
|---|---|---|---|---|
| SCL | 744 | 55 | ATTGCACACACGGGATTCTG | CATACAGTACGACACTGACG |
| GATA-1 | 581 | 55 | ATGCCTGTAATCCCAGCACT | TCATGGTGGTAGCTGGTAGC |
| GATA-2 | 720 | 60 | CGGAATTCGACACACCACCCGATACCCACCTAT | CGGAATTCGCCTACGCCATGGCAGTCACCATGCT |
| EKLF | 359 | 55 | TCGCCGGAGACGCAGGCT | CCCAGTCCTTGTGCAGGA |
| NF-E2(p45) | 391 | 62.5 | GAGCCCTGGCCATGAAGATTCC | CACCATCAGCAGCCTGTTGCAG |
| MafK(p18) | 491 | 55 | GACAGGGCCCGGGTTATGACGACTAATCCC | GGATCCGGAGGCGGCTGAGAAGGGTACAGAGGT |
| c-myb | 681 | 55 | TTCAAGGCCAGCATTCTTGC | CCTCTAGGAGCTCATTTGTG |
| AML1b | 292 | 55 | CCAGCAAGCTGAGGAGCGGCG | CCGACAAACCTGAGGTCGTTG |
| C/EBPα | 191 | 58 | AAGGCCAAGAAGTCGGTGGA | CAGTTCACGGCTCAGCTGTT |
| PU.1 | 615 | 55 | TGGAAGGGTTTTCCCTCACC | TGCTGTCCTTCATGTCGCCG |
| β-globin | 578 | 55 | CTGACAGATGCTCTCTTGGG | CACAACCCCAGAAACAGACA |
| MPO | 379 | 55 | GGTGCTGAAGAACCTGGAGTTG | CTAGGTCTCCTTCCAGGAAGTC |
| HPRT | 249 | 50 | CACAGGACTAGAACACCTGC | GCTGGTGAAAAGGACCTCT |
| Gene . | Size (bp) . | Annealing temperature (°C) . | 5′ primer . | 3′ primer . |
|---|---|---|---|---|
| SCL | 744 | 55 | ATTGCACACACGGGATTCTG | CATACAGTACGACACTGACG |
| GATA-1 | 581 | 55 | ATGCCTGTAATCCCAGCACT | TCATGGTGGTAGCTGGTAGC |
| GATA-2 | 720 | 60 | CGGAATTCGACACACCACCCGATACCCACCTAT | CGGAATTCGCCTACGCCATGGCAGTCACCATGCT |
| EKLF | 359 | 55 | TCGCCGGAGACGCAGGCT | CCCAGTCCTTGTGCAGGA |
| NF-E2(p45) | 391 | 62.5 | GAGCCCTGGCCATGAAGATTCC | CACCATCAGCAGCCTGTTGCAG |
| MafK(p18) | 491 | 55 | GACAGGGCCCGGGTTATGACGACTAATCCC | GGATCCGGAGGCGGCTGAGAAGGGTACAGAGGT |
| c-myb | 681 | 55 | TTCAAGGCCAGCATTCTTGC | CCTCTAGGAGCTCATTTGTG |
| AML1b | 292 | 55 | CCAGCAAGCTGAGGAGCGGCG | CCGACAAACCTGAGGTCGTTG |
| C/EBPα | 191 | 58 | AAGGCCAAGAAGTCGGTGGA | CAGTTCACGGCTCAGCTGTT |
| PU.1 | 615 | 55 | TGGAAGGGTTTTCCCTCACC | TGCTGTCCTTCATGTCGCCG |
| β-globin | 578 | 55 | CTGACAGATGCTCTCTTGGG | CACAACCCCAGAAACAGACA |
| MPO | 379 | 55 | GGTGCTGAAGAACCTGGAGTTG | CTAGGTCTCCTTCCAGGAAGTC |
| HPRT | 249 | 50 | CACAGGACTAGAACACCTGC | GCTGGTGAAAAGGACCTCT |
Results
The effect of aNotch1 on erythroid differentiation
It has been reported that Notch1 signaling inhibits G-CSF–induced granulocytic differentiation in 32D myeloid progenitor cells.19,20 To ascertain whether the erythroid differentiation is also inhibited, we expressed aNotch1 in a mouse erythroleukemia line, F5-5, and investigated its effect on the erythroid differentiation. F5-5 differentiates into hemoglobin-producing cells in the presence of DMSO or Activin A.32 Approximately 80% of wild-type and mock virus–infected F5-5 cells became positive for benzidine staining 7 days after the stimulation with DMSO. In contrast, only 10% to 30% of F5-5/aNotch1 cells turned benzidine positive (Figure1A,Bi). When Activin A was added instead of DMSO, about 30% of mock virus–infected control cells became benzidine positive, whereas less than 5% of F5-5/aNotch1 did so (Figure 1Bii). Thus, we concluded that aNotch1 can inhibit erythroid differentiation as well as differentiation into other lineages.
Inhibition of erythroid differentiation by aNotch1 in F5-5 cells.
(A) F5-5 cells were infected with mock virus or aNotch1-containing pMX/IRES-EGFPpuro virus. The morphology of clone 33 and a control clone 6 days after the induction for differentiation is demonstrated with 1.5% DMSO or Activin A. Cells were stained with benzidine. Original magnification × 200. (B) Erythroid differentiation was evaluated by the ratio of benzidine-positive cells. Cells were differentiated by DMSO (i) or Activin A (ii). After 6 days, the ratio of benzidine-positive cells was calculated. The results show the mean values of 3 independent experiments and the error bars show SD.
Inhibition of erythroid differentiation by aNotch1 in F5-5 cells.
(A) F5-5 cells were infected with mock virus or aNotch1-containing pMX/IRES-EGFPpuro virus. The morphology of clone 33 and a control clone 6 days after the induction for differentiation is demonstrated with 1.5% DMSO or Activin A. Cells were stained with benzidine. Original magnification × 200. (B) Erythroid differentiation was evaluated by the ratio of benzidine-positive cells. Cells were differentiated by DMSO (i) or Activin A (ii). After 6 days, the ratio of benzidine-positive cells was calculated. The results show the mean values of 3 independent experiments and the error bars show SD.
Impact of aNotch1 on the regulation of expression of various transcription factors after differentiation induction
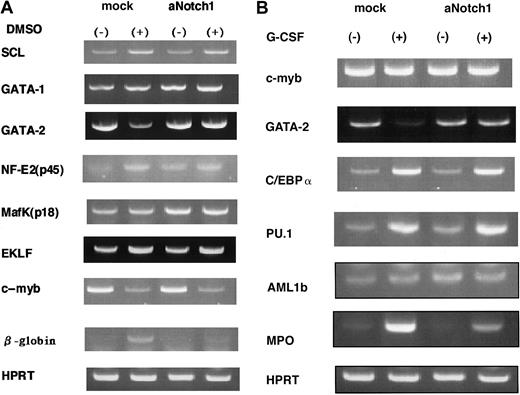
To elucidate the mechanism of differentiation inhibition by aNotch1, we compared the expression levels of several transcription factors that were known to be involved in erythroid and myeloid differentiation,35 36 before and after the differentiation induction in the presence or absence of aNotch1 by a semiquantitative RT-PCR method. In F5-5 cells, we compared the expression levels of SCL, GATA-1, GATA-2, NF-E2 (p45), MafK (p15), EKLF, and c-myb. Of these, SCL, GATA-1, NF-E2, and EKLF were up-regulated, MafK was unchanged, and c-myb was down-regulated after the DMSO stimulation in the control retrovirus-transduced F5-5 cells, in which the erythroid differentiation was demonstrated by the up-regulation of theβ-globin gene. In F5-5/aNotch1 cells, whose erythroid differentiation was inhibited, these transcription factors were regulated in a similar manner when stimulated with DMSO. In contrast, expression of GATA-2 was sustained in F5-5/aNotch1, although it was sharply down-regulated in the control F5-5 cells by the differentiation stimulation with DMSO (Figure 2A).
Regulation of hematopoietic transcription factors.
A semiquantitative RT-PCR method was performed to compare the expression level of the transcription factors shown, which are involved in erythroid (A) and myeloid (B) differentiation in the presence or absence of aNotch1. The results were confirmed using 2 independent aNotch1-expressing and control clones.
Regulation of hematopoietic transcription factors.
A semiquantitative RT-PCR method was performed to compare the expression level of the transcription factors shown, which are involved in erythroid (A) and myeloid (B) differentiation in the presence or absence of aNotch1. The results were confirmed using 2 independent aNotch1-expressing and control clones.
To investigate the regulation of transcription factors in myeloid differentiation, we similarly analyzed the expression levels of transcription factors (c-myb, GATA-2, C/EBPα, PU.1, and AML1b) using mouse myeloid progenitor cells 32D, before and after the stimulation with G-CSF. We obtained 10 clones of 32D cells expressing aNotch1 (32D/aNotch1) and myeloid differentiation was inhibited in all of them. The expression of C/EBPα, PU.1, and AML1b was up-regulated, and c-myb was unchanged in both control vector-transfected 32D (32D/pME) and 32D/aNotch1 after the stimulation with G-CSF, but the expression level of GATA-2 was again maintained only in the 32D/aNotch1 (Figure 2B). These results suggest that the maintenance of GATA-2 expression is a phenomenon commonly observed in the aNotch1-induced inhibition of differentiation of hematopoietic cells.
DN-GATA neutralizes the aNotch1-induced phenotype
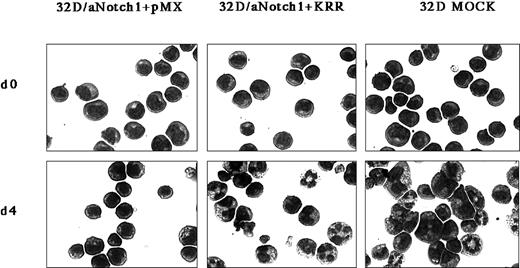
To investigate whether maintenance of GATA-2 expression is a requirement for, not a consequence of, the aNotch1-induced inhibition of differentiation, we used the dominant-negative form of GATA (DN-GATA), KRR.24,25 KRR has 3 amino acid substitutions in GATA-3 and can inhibit the transcriptional activity of all the GATA family proteins in a dominant-negative fashion.24 Myeloid cells such as 32D, in which either GATA-1 or GATA-3 is not expressed,37 should be better than erythroid cells such as F5-5, in which GATA-1 plays an indispensable role in growth and differentiation. Therefore, we transduced 32D/aNotch1 with a retrovirus containing KRR (32D/aNotch1+KRR). In the presence of IL-3, there was no difference in proliferation rate or in viability among parental 32D, 32D/KRR, 32D/aNotch1, and 32D/aNotch1+KRR (data not shown). In both 32D/aNotch1+KRR and the control retrovirus-transduced 32D/aNotch1 (32D/aNotch1+pMX), the immature morphology was common to original 32D in the presence of IL-3 (Figure 3). As we expected, granulocytic differentiation was observed after removal of IL-3 and addition of G-CSF in 32D/aNotch1+KRR, but not in 32D/aNotch1+pMX, to an extent similar to that in the original 32D cells. This result indicates that KRR neutralized the aNotch1-induced inhibition of differentiation. However, enforced expression of GATA-2 in the original 32D did not inhibit its granulocytic differentiation (data not shown), indicating that the maintenance of GATA-2 expression is necessary but insufficient to display the phenotype of aNotch1.
Reversal by DN-GATA of the effect of aNotch1 on the granulocytic differentiation in 32D cells.
Activated Notch1-expressing 32D cells were infected with a mock virus or a DN-GATA–containing pMX/IRES-EGFPpuro virus. The cells are shown stained with Wright-Giemsa stain 4 days after the addition of G-CSF. Original magnification × 400. Three independent DN-GATA–expressing clones and control clones derived from 2 independent aNotch1-expressing clones were investigated.
Reversal by DN-GATA of the effect of aNotch1 on the granulocytic differentiation in 32D cells.
Activated Notch1-expressing 32D cells were infected with a mock virus or a DN-GATA–containing pMX/IRES-EGFPpuro virus. The cells are shown stained with Wright-Giemsa stain 4 days after the addition of G-CSF. Original magnification × 400. Three independent DN-GATA–expressing clones and control clones derived from 2 independent aNotch1-expressing clones were investigated.
Overexpression of HES-1 delays the G-CSF–induced granulocytic differentiation of 32D and cancellation of this phenotype by further expression of DN-GATA
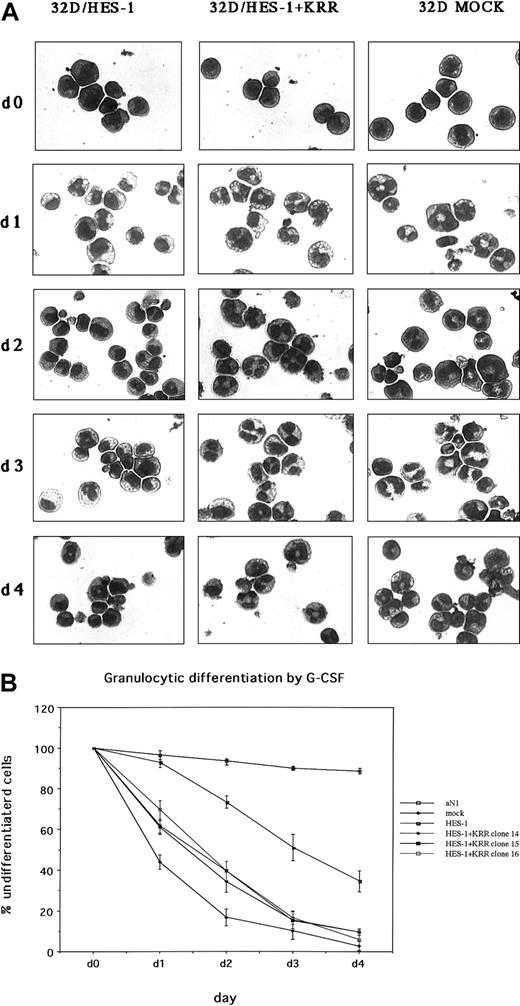
It has been shown that HES-1 is involved in Notch signaling in various systems such as neurogenesis,27myogenesis,38 and thymocyte development28 as well as the development of the embryo. To confirm whether HES-1 also functions as a protein downstream of Notch in the block of myeloid differentiation, we overexpressed HES-1 protein in 32D cells (32D/HES-1). Although removal of IL-3 and addition of G-CSF eventually allowed 32D/HES-1 to differentiate into granulocytes, the rate of differentiation was obviously slower in 32D/HES-1 than in the control 32D/pME (Figure 4A). This phenotype was neutralized when KRR was introduced into 32D/HES-1 (32D/HES-1+KRR). In detail, 32D/HES-1+KRR differentiated into granulocytes when stimulated with G-CSF at a similar rate to the original 32D cells. The rate of differentiation in 32D/HES-1+pMX was the same as that in 32D/HES-1 (Figure 4A-B). These observations indicate that GATA-2 acts downstream of HES-1.
Delay of granulocytic differentiation by HES-1 in 32D cells and abolishment of this phenotype by DN-GATA.
The original 32D cells were transfected with the SRα promoter-driven HES-1 cDNA and selected for G418. Some G418-resistant clones were further infected with a mock virus or a DN-GATA–containing pMX/IRES-EGFPpuro virus. The morphology of the cells 4 days after the addition of G-CSF is shown in panel A (Wright-Giemsa staining). Original magnification × 400. Three independent DN-GATA–expressing clones derived from each of 2 independent HES-1–expressing clones were investigated. (B) The ratio of undifferentiated cells after successive days of culture is shown. The results are expressed as the mean values of 3 independent experiments, with error bars showing SD.
Delay of granulocytic differentiation by HES-1 in 32D cells and abolishment of this phenotype by DN-GATA.
The original 32D cells were transfected with the SRα promoter-driven HES-1 cDNA and selected for G418. Some G418-resistant clones were further infected with a mock virus or a DN-GATA–containing pMX/IRES-EGFPpuro virus. The morphology of the cells 4 days after the addition of G-CSF is shown in panel A (Wright-Giemsa staining). Original magnification × 400. Three independent DN-GATA–expressing clones derived from each of 2 independent HES-1–expressing clones were investigated. (B) The ratio of undifferentiated cells after successive days of culture is shown. The results are expressed as the mean values of 3 independent experiments, with error bars showing SD.
PU.1 can cancel the aNotch1-induced phenotype
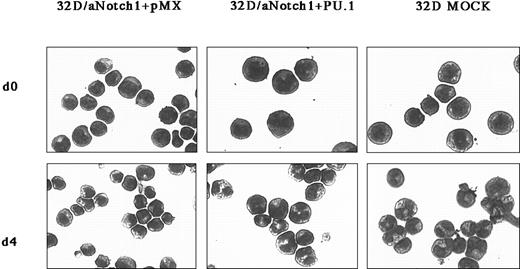
It is reported that PU.1 physically interacts with GATA-1 and GATA-2.26,39-41 It is also known that PU.1 represses the transcriptional activity of each GATA protein, and vice versa.26,39-41 In addition, PU.1 inhibits erythroid differentiation through interference with GATA-1 function.39 This information prompted us to expect that PU.1 could also inhibit the function of GATA-2 when overexpressed in 32D/aNotch1, and eventually reverse its phenotype, if GATA-2 plays a major role in the Notch signaling in these cells. We therefore transduced 32D/aNotch1 with a retrovirus containing PU.1 (32D/aNotch1+PU.1) and observed its phenotype. Again as expected, 32D/aNotch1+PU.1, but not 32D/aNotch1+pMX, underwent granulocytic differentiation when stimulated with G-CSF (Figure5). Overexpression of PU.1 by itself did not change the morphology in the presence of IL-3 (data not shown), as was previously shown.42
Reversal by PU.1 of the effect of aNotch1 on the granulocytic differentiation in 32D cells.
The 32D/aNotch1 cells were infected with a mock virus or a PU.1-containing pMX/IRES-EGFPpuro virus. The cells are shown 4 days after the addition of G-CSF and after Wright-Giemsa staining. Original magnification × 400. Three independent PU.1-expressing clones and control clones derived from each of 2 independent aNotch1-expressing clones were investigated.
Reversal by PU.1 of the effect of aNotch1 on the granulocytic differentiation in 32D cells.
The 32D/aNotch1 cells were infected with a mock virus or a PU.1-containing pMX/IRES-EGFPpuro virus. The cells are shown 4 days after the addition of G-CSF and after Wright-Giemsa staining. Original magnification × 400. Three independent PU.1-expressing clones and control clones derived from each of 2 independent aNotch1-expressing clones were investigated.
Discussion
Several lines of evidence showed the role of Notch signaling in hematopoietic23 as well as neurogenic and myogenic differentiation.8,9 In contrast to the fact that Mash1 and MyoD, key regulatory transcription factors in neurogenesis and myogenesis, respectively, are found to be the target genes of Notch in neurogenic43 and myogenic30 38 cells, the target gene of Notch in hematopoietic cells has not been clarified at all.
Differentiation of hematopoietic cells is regulated by a number of transcription factors.35 In the current study, the expression level of several transcription factors involved in myeloid and erythroid differentiation in the aNotch1-expressing cells was compared with that in the control cells. Previously reported up-regulation and down-regulation patterns36 were reproduced in the control F5-5 and 32D cells. A striking feature was that the same regulation patterns were present for most of the transcription factors examined in the aNotch1-expressing cells when stimulation of the differentiation of these cells was attempted, and yet differentiation was blocked. These observations indicate that the regulation of SCL, GATA-1, NF-E2 (p45), MafK (p15), and EKLF in F5-5, and c-myb, C/EBPα, PU.1, and AML1b in 32D is correlated with the stimulatory signal to the cells, but not with the differentiation phenotype that is an outcome of the cellular response. A similar observation was reported in K562 cells with GATA-1 and SCL, when induction of erythroid differentiation was attempted in the presence or absence of aNotch1.44
In contrast, a difference in regulation pattern between the control and the aNotch1-expressing cells was detected only in GATA-2 in both F5-5 and 32D cells, with reproducible results in the independent clones. GATA-2 is highly expressed in hematopoietic stem cells and immature progenitor cells,45 and is down-regulated during their differentiation.36 In both the control and aNotch1-expressing cells, our results were consistent with this known fact. In other words, the regulation of GATA-2 expression correlates with the differentiation phenotype rather than with the stimulatory signal for differentiation.
It is not known whether the down-regulation of GATA-2 is a result or a cause of hematopoietic cell differentiation. Therefore, we could not conclude whether the sustained expression of GATA-2 in the aNotch1-expressing cells was a result or a cause of the aNotch1-induced inhibition of differentiation. To address this issue, we intended to down-regulate the function of GATA-2 in 32D/aNotch1 by expressing KRR, a known mutant of GATA-3 that works in a dominant-negative fashion24,25 against GATA-1, GATA-2, and GATA-3, which are expressed in hematopoietic cells and by expressing PU.1, a well-described myeloid-specific transcription factor that inhibits the function of GATA family transcription factors.26 This strategy should work because both transcription factors inhibit only GATA-2 in 32D/aNotch1, in which GATA-2 is the only GATA family protein expressed.37 The retroviral expression of both proteins clearly showed reversion to the phenotype without aNotch1, because we found that both 32D/aNotch1+KRR and 32D/aNotch1+PU.1 reacquired their responsiveness to G-CSF and differentiated into granulocytic cells. We also transfected DN-GATA or PU.1 into wild-type 32D, but found that these stable transfectants showed no difference compared to parental cells in the presence of IL-3. There was also no obvious difference in the rate or extent of differentiation in the presence of G-CSF (data not shown). We think that because the down-regulation of GATA-2 and the up-regulation of PU.1 in the parental cells by G-CSF was sufficient, the effect of overexpression was not visible. These results indicate that the functional down-regulation of GATA-2 is sufficient to cancel the phenotype induced by aNotch1 in 32D, or that maintenance of GATA-2 expression is the cause, not the result, of the differentiation inhibition in 32D/aNotch1. However, the maintenance of GATA-2 expression is necessary but insufficient for the phenotype of aNotch1 in 32D, because enforced expression of GATA-2 did not inhibit the G-CSF–induced differentiation in 32D. Therefore, in addition to the sustained expression of GATA-2, there should be another pathway that is necessary for the execution of aNotch1 signaling in 32D.
A short discussion of the role of PU.1 in 32D/aNotch1+PU.1 is now appropriate. In 32D/aNotch1, G-CSF up-regulated the expression of PU.1 in a manner similar to that in the control 32D cells, although the differentiation is blocked in 32D/aNotch1, which might appear to be in conflict with the fact that the overexpression of PU.1 abolished the differentiation inhibition. However, the phenotype of 32D/aNotch1+PU.1 can be explained if a sufficiently high PU.1/GATA2 ratio for G-CSF–induced differentiation despite the presence of aNotch1 is achieved only by cDNA-based PU.1 expression, and not by an induction of PU.1 by G-CSF stimulation in the 32D/aNotch1.
Recently, it was reported that aNotch1 promoted the differentiation of 32D cells,46 which was inconsistent with the previous data19,20 as well as the result presented here. The authors46 argued that the discrepancy may be attributed to the difference in the RBP-Jκ–mediated transactivation capacities of the different Notch1 products, though each of them is a constitutive active form. However, the aNotch1 that we used was the same as their construct in terms of the integrity of the RAM domain, which was lacking in the aNotch1 construct used by the group that obtained data similar to ours.19,20 Indeed, the aNotch1 that we used can activate the RBP-Jκ–responsive reporter substantially (data not shown). Furthermore, KyoT2, which is an inhibitor of RBP-Jκ,47 abolished the aNotch1-induced differentiation inhibition (data not shown). Delayed differentiation of 32D cells resulted from enforced expression of HES-1, which is involved in Notch signaling in many cells,27,28 and the cancellation of this phenotype by DN-GATA (Figure 4A) collectively indicates that the Notch1-RBP-Jκ–HES-1 signaling is involved in the inhibition, rather than promotion, of 32D cell differentiation. There was also discussion46 on the difference resulting from the different expression system. A gene-inducible system was used by the group obtaining the apparently conflicting result,46whereas we used stable transfectants. However, coculture of full-length Notch1-transfected 32D (32D/fN1) with wild-type or full-length Jagged1-transfected Chinese hamster ovary cells (fJ1/CHO) disclosed that 32D/fN1 did not induce differentiation in the presence of IL-3 when cocultured with fJ1/CHO, but that G-CSF–induced differentiation of 32D/fN1 was inhibited when cocultured with fJ1/CHO (data not shown). We therefore propose that the apparently opposing results produced in the different laboratories using 32D cells by the expression of aNotch1 probably reflects the difference in the 32D subline maintained in each laboratory.
Although we demonstrated the maintenance of GATA-2 expression by aNotch1, the direct mechanism of this phenomenon has yet to be elucidated. Our findings that DN-GATA abolishes similar phenotypes induced by aNotch1 and HES-1 suggest that HES-1 or its downstream transcription factors block the down-regulation of GATA-2 by directly acting on its regulatory sequences. It is also possible that another molecule independent of an RBP- Jκ–HES-1 pathway may play a role in this.
The function of Notch1 described in this paper does not necessarily indicate the physiologic role of Notch1 in myelopoiesis or erythropoiesis, given the recent report on the phenotype of Notch1-conditional knockout mice.48 In these mice, in which Notch1 was disrupted after birth, only T-cell developmental deficiency was observed, whereas nonlymphoid hematopoiesis was apparently normal. However, immature hematopoietic progenitor cells express not only Notch1 but also Notch2.18 19 They can be stimulated by the same ligand and may have functional redundancy. It is possible that the signaling through Notch receptors other than Notch1 has a significant role in hematopoiesis in the physiologic settings, and the results shown here may represent such signaling. It is of great interest to study the role of Notch in the context of hematopoiesis and to analyze the molecular mechanism of Notch signaling, in which GATA-2 plays a role.
We thank Dr R. Kageyama for providing mouse HES-1 cDNA, Dr T. Kitamura for a retrovirus vector pMX, and Dr Y. Eto for Activin A. We are also grateful to Mr C. W. P. Reynolds for assistance with the English for this manuscript.
Supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Ministry of Health, Labour and Welfare of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hisamaru Hirai, Department of Hematology and Oncology, University of Tokyo Hospital, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan; e-mail: hhirai-tky@umin.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal