Abstract
Erythropoietin (Epo) controls red cell production in the basal state and during stress. Epo binding to its receptor, EpoR, on erythroid progenitors leads to rapid activation of the transcription factor Stat5. Previously, fetal anemia and increased apoptosis of fetal liver erythroid progenitors were found in Stat5a−/−5b−/− mice. However, the role of Stat5 in adult erythropoiesis was not clear. The present study shows that some adult Stat5a−/−5b−/− mice have a near-normal hematocrit but are deficient in generating high erythropoietic rates in response to stress. Further, many adult Stat5a−/−5b−/− mice have persistent anemia despite a marked compensatory expansion in their erythropoietic tissue. Analysis of erythroblast maturation in Stat5a−/−5b−/− hematopoietic tissue shows a dramatic increase in early erythroblast numbers, but these fail to progress in differentiation. Decreased expression of bcl-xLand increased apoptosis in Stat5a−/−5b−/−early erythroblasts correlate with the degree of anemia. Hence, Stat5 controls a rate-determining step regulating early erythroblast survival.
Introduction
Formation of red blood cells is continuous throughout life. The primary erythropoietic regulator is the cytokine hormone erythropoietin (Epo). Binding of Epo to its receptor, EpoR, expressed by erythroid progenitors, is essential for the formation of red blood cells1 and for the maintenance of basal erythropoiesis. In addition, Epo regulates the erythropoietic stress response in conditions that lower tissue oxygen tension.2The molecular and cellular mechanisms that allow Epo to regulate erythropoiesis through a wide dynamic range are largely unknown.
Stat5 is a latent cytoplasmic transcription factor activated by EpoR as well as many other hematopoietic and nonhematopoietic cytokine receptors.3-6 Following EpoR activation, Stat5 binds to phosphorylated tyrosines on EpoR, and itself becomes tyrosine-phosphorylated. This results in its dimerization and translocation to the nucleus where it initiates transcription of target genes (reviewed by Bromberg and Darnell7). Known Stat5 targets include tissue-specific genes as well as genes regulating cell growth.8-10 Recently, we and others11-14 have shown that in a number of erythroid and hematopoietic cell lines Stat5 induces the immediate early expression of the antiapoptotic genebcl-xL, by directly binding to Stat5 consensus sites in the bcl-x gene. Consistent with this, Stat5 has an antiapoptotic effect in erythroid cell lines,11 and dominant-negative Stat5 molecules increased apoptosis and inhibited growth of cultured fetal liver erythroid progenitors.11 15
Mice mutant for both Stat5 isoforms, Stat5a and Stat5b, were initially reported to have apparently normal hematocrit and hemoglobin concentrations.16 However, we found that Stat5 is critical during fetal erythropoiesis.11 The first wave of erythropoiesis in the embryo generates large nucleated red cells in the yolk sac. This is replaced by definitive erythropoiesis in fetal liver, generating smaller, adult-type enucleated red cells. At embryonic day 13.5, definitive erythropoiesis predominates and becomes essential for survival. At this stage of development, we found that Stat5a−/− 5b−/− embryos are severely anemic due to decreased survival of Stat5a−/−5b−/− fetal liver erythroid progenitors. Stat5a−/−5b−/− fetal liver cells gave rise to fewer erythroid colonies in vitro, showed a 2.5-fold increase in TUNEL-positive cells in vivo, and a marked increase in apoptosis when cultured in vitro in the presence of Epo. These findings were consistent with Stat5 mediating an Epo-dependent antiapoptotic effect in fetal erythroid progenitors. However, it remained unclear whether Stat5 continued to mediate this function beyond fetal life. Further, the precise stage of erythroid terminal differentiation when the antiapoptotic effect of Stat5 is exerted was not identified. Consequently, it also remained unknown whether the induction of bcl-xL by Stat5, identified in cultured cell lines, was also true in differentiating erythroblasts in vivo.
Two possible mechanisms might account for fetal anemia in Stat5a−/−5b−/− mice resolving into an apparently normal hematocrit in adult mice. First, Stat5 may be specifically required for fetal erythropoiesis but not for the production of adult red cells. Indeed, several molecular differences between fetal and adult erythropoiesis are known.17 18 The second possibility is that Stat5 has a similar role in both adult and fetal erythropoiesis. However, its absence manifests as anemia in the fetus but not in the adult because of the large difference in erythropoietic reserve capacity between adult and fetus. This latter model predicts that the ability of Stat5a−/−5b−/− mice to further increase their erythropoietic rate in response to stress would be impaired.
To distinguish these 2 models, we assessed erythropoiesis in adult Stat5a−/−5b−/− mice. Approximately half the Stat5a−/−5b−/− adult mice had near-normal steady-state hematocrit values, but could not efficiently increase erythropoietic rate in response to stress. In addition, many adult Stat5a−/−5b−/− mice had chronic anemia, suggesting a severe deficit in steady-state erythropoietic rate. To identify the deficit in Stat5a−/−5b−/−erythropoiesis, we assessed the degree of maturation of erythroid progenitors in Stat5a−/−5b−/− hematopoietic tissue, using a novel flow cytometry assay. This identified a clear block to erythroid differentiation in Stat5a−/−5b−/− mice. Despite a compensatory increase in the number of early erythroid progenitors, their progression through terminal differentiation is impaired, resulting in a dramatic decrease in the number of late erythroblasts. Decreased bcl-xL expression and increased apoptosis of early erythroblasts correlated with the severity of anemia and can account for the failure of early erythroblasts to progress in differentiation. Therefore, Stat5 controls a rate-determining step required for survival of early erythroblasts and for normal erythropoiesis.
Materials and methods
Mice
Stat5a−/−5b−/− mice were provided by Dr J. Ihle. All Stat5a−/−5b−/− animals and wild-type controls were progeny of the original colony of 129xC57BL/6 mice. Genotyping was performed by polymerase chain reaction (PCR) as described.11 VHL−/− mice (Vhl2lox/2lox;PEPCK-Cre) have a tissue-specific deletion of the von Hippel-Lindau (VHL) tumor suppressor in the proximal renal tubule and a subgroup of periportal hepatocytes (Volker H. Haase and Rudolf Jaenisch, manuscript in preparation).
Hematocrit, hemoglobin, and red cell indices
Neonates (<24 hours old) were decapitated and blood was obtained directly into heparinized microhematocrit tubes (40 μL, Drummond Microcaps, Fisher Scientific, Pittsburgh, PA). Older animals were bled from the tail vein. Hematocrit was measured on a hematocrit (Micro-MB, IEC, Needham Heights, MA) centrifuge. Hemoglobin concentration was determined colorimetrically (Total Hemoglobin, Sigma Diagnostics, St Louis, MO). The size and cytoplasmic content of circulating erythroid cells was assessed with the Bayer H*3 flow cytometer (Bayer Diagnostics, Tarrytown, NY) using the reticulocyte channel.19 With this method, the mean cell size of the circulating erythroid cells is measured in arbitrary femtoliter units and the mean cell cytoplasmic content is measured in arbitrary picogram units/cell. The values provided in the text are for the entire pool of circulating erythroid cells and do not distinguish between mature red cells, reticulocytes, or nucleated red cells.
Flow cytometry
Spleens were mechanically dissociated by pushing with a syringe plunger through a 70-μ strainer in the presence of phosphate-buffered saline and 0.5% bovine serum albumin (PBS/0.5% BSA). Bone marrow cells were resuspended in PBS/0.5% BSA. Freshly isolated bone marrow or spleen cells were immunostained at 4°C in PBS/0.5% BSA in the presence of either mouse IgG (200 μg/mL, Pharmingen, San Diego, CA) or 5% mouse serum, to block Fc receptors. Cells were incubated with phycoerythrin (PE)–conjugated anti-Ter119 (Pharmingen, 1 μg/mL) and biotin-conjugated anti-CD71 (Pharmingen, 1 μg/mL) antibodies for 20 minutes, followed by a 15-minute incubation with allophycocyanin (APC)–conjugated streptavidin (Molecular Probes, Eugene, OR). Cells were also stained with propidium iodide to exclude dead cells from analysis. Where apoptosis was also measured immunostaining for Ter119 and CD71 was followed by a 15-minute incubation with fluorescein isothiocyanate (FITC)–conjugated annexin V and propidium iodide (Pharmingen) as per the manufacturer's protocol. To measure bcl-xL, cells were first surface immunostained for Ter119 and CD71. Cells were then fixed with 3% paraformaldehyde in PBS/2% sucrose at 4°C for 1 hour, permeabilized with 0.1% Triton X-100 for 5 minutes, and stained in PBS/1%BSA at 37°C for 30 minutes with polyclonal rabbit anti–bcl-x antiserum (1:200, Pharmingen) and Alexa Fluor 488 goat anti–rabbit IgG (Molecular Probes, A-11034, 10 μg/mL). Flow cytometry was carried out on a Becton Dickinson FACSCalibur (Franklin Lakes, NJ).
Cytospins
Freshly isolated hematopoietic cells (from spleen, bone marrow, or fetal liver) were labeled for Ter119 and CD71, and sorted according to regions as indicated in Figure 4. Cytospin preparations of cells from each region were stained with May-Grunwald Giemsa (Sigma Diagnostics).
Phenylhydrazine stress test
Mice with a baseline hematocrit of at least 35% were used. Mice were injected subcutaneously on each of days 0,1, and 3 with 40 mg/kg of phenylhydrazine hydrochloride solution in PBS. Blood (40-150 μL) was obtained from the tail vein on days 0, 3, 6, and 9 for hematocrit and reticulocyte count measurements. Reticulocyte count was measured by counting the number of reticulocytes and red cells on blood smears made after staining of freshly drawn blood with new methylene blue “N” (Ricca Chemical, Arlington TX). At least 500 cells per mouse were counted for each time point. The corrected reticulocyte count was calculated as described in the legend to Figure 2.
Histopathology
Freshly isolated neonatal or adult spleens were fixed in buffered neutral 10% formalin (VWR, West Chester, PA). Paraffin-embedded tissue was sectioned, mounted, and stained with hematoxylin and eosin.
Mouse serum Epo was measured by a sandwich enzyme-linked immunosorbent assay (ELISA) as described.20
Results
Developmental anemia and anemia in adults in Stat5a−/−5b−/− mice
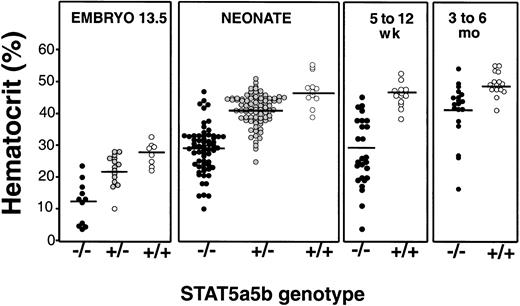
Stat5a−/−5b−/− mice are anemic by embryonic day 13.511 (Figure1). To assess whether anemia persists postnatally, we looked at Stat5a−/−5b−/−neonates within 24 hours of birth. We found that more than 95% of Stat5a−/−5b−/− neonates are born anemic. The Stat5a−/−5b−/− neonatal hematocrit is 28.9% ± 0.9% (mean ± SE, n = 65), compared with 41.3% ± 0.5% for Stat5a+/−5b+/− neonates (n = 88) and 47.6% ± 1.6% for wild-type neonates in the same litters (n = 12, Figure 1 and Table 1). Anemia persists until weaning, at approximately 3.5 weeks (Table 1). By 5 weeks of age, approximately 30% of Stat5a−/−5b−/− mice have recovered a near-normal hematocrit, whereas the rest remain anemic. The mean hematocrit of Stat5a−/−5b−/− mice at 5 to 12 weeks is 27.9% ± 2.0%, compared with 45.2% ± 0.9% in the wild-type mice (Table 1). The proportion of Stat5a−/−5b−/− mice with near-normal hematocrit increases to more than 50% with older mice (Figure 1). This may reflect decreased survival of anemic young adult mice (M.S., H.-S.N., H.F.L., unpublished observations, 1999-2001). In addition to a lower hematocrit, Stat5a−/−5b−/− mice show a small decrease in mean red blood cell volume (MCV) (Table 1).
Developmental anemia in Stat5a−/−5b−/−mice.
Hematocrit measurement on Stat5a−/−5b−/−, Stat5a+/−5b+/− or wild-type littermates at the ages indicated. The same hematocrit data are tabulated in Table 1. Some of the embryo hematocrit data were published previously.11
Hematologic parameters of Stat5a−/−5b−/− mice
| . | Age . | Genotype (no. of animals) . | Mean ± SEM . | P . |
|---|---|---|---|---|
| Hematocrit (%) | Neonates | 5a−/−5b−/−(65) | 28.9 ± 0.9*,† | *P< .0001 †P < .0001 |
| 5a+/−5b+/−(88) | 41.3 ± 0.5* | *P = .0014 | ||
| Wild-type (12) | 47.6 ± 1.6 | |||
| 3 wk | 5a−/−5b−/−(12) | 32.6 ± 1.0*,† | *P< .0001 †P = .03 | |
| 5a+/−5b+/−(23) | 36.5 ± 1.2* | *P = .0006 | ||
| Wild-type (5) | 45.7 ± 1.2 | |||
| 5-12 wk | 5a−/−5b−/−(26) | 27.9 ± 2.0* | *P < .0001 | |
| 5a+/−5b+/−(3) | 29.5 ± 6.2* | *P = .008 | ||
| Wild-type (15) | 45.2 ± 0.9 | |||
| 3-6 mo | 5a−/−5b−/−(21) | 41.1 ± 1.9* | *P = .0014 | |
| 5a+/−5b+/−(5) | 44.0 ± 2.6 | |||
| Wild-type (15) | 49.2 ± 1.0 | |||
| MCV (μ3) | Neonates | 5a−/−5b−/−(14) | 89.7 ± 1.6*,† | *P< .0001 †P = .0003 |
| 5a+/−5b+/−(25) | 97.3 ± 1.25 | |||
| Wild-type (9) | 102.8 ± 1.5 | |||
| 3 wk | 5a−/−5b−/−(10) | 56.1 ± 0.73*,† | *P= .004 †P = .002 | |
| 5a+/−5b+/−(18) | 59.1 ± 0.45 | |||
| Wild-type (5) | 60.0 ± 1.47 | |||
| 5 wk | 5a−/−5b−/−(10) | 51.8 ± 2.75 | ||
| 5a+/−5b+/−(18) | 54.6 ± 1.1 | |||
| Wild-type (5) | 54.6 ± 1.3 | |||
| Total bilirubin (mg/dL) | >6 wk | 5a−/−5b−/−(9) | 0.46 ± 0.19 | |
| Wild-type (6) | 0.12 ± 0.02 | |||
| LDH (IU/L) | 5a−/−5b−/−(9) | 715 ± 372 | ||
| Wild-type (6) | 282 ± 120 | |||
| Serum iron (μg/dL) | 5a−/−5b−/−(6) | 161.3 ± 15.1 | ||
| Wild-type (6) | 152.7 ± 17.9 | |||
| TIBC (μg/dL) | 5a−/−5b−/−(6) | 617 ± 40.0* | *P < .0001 | |
| Wild-type (6) | 427 ± 18.8 |
| . | Age . | Genotype (no. of animals) . | Mean ± SEM . | P . |
|---|---|---|---|---|
| Hematocrit (%) | Neonates | 5a−/−5b−/−(65) | 28.9 ± 0.9*,† | *P< .0001 †P < .0001 |
| 5a+/−5b+/−(88) | 41.3 ± 0.5* | *P = .0014 | ||
| Wild-type (12) | 47.6 ± 1.6 | |||
| 3 wk | 5a−/−5b−/−(12) | 32.6 ± 1.0*,† | *P< .0001 †P = .03 | |
| 5a+/−5b+/−(23) | 36.5 ± 1.2* | *P = .0006 | ||
| Wild-type (5) | 45.7 ± 1.2 | |||
| 5-12 wk | 5a−/−5b−/−(26) | 27.9 ± 2.0* | *P < .0001 | |
| 5a+/−5b+/−(3) | 29.5 ± 6.2* | *P = .008 | ||
| Wild-type (15) | 45.2 ± 0.9 | |||
| 3-6 mo | 5a−/−5b−/−(21) | 41.1 ± 1.9* | *P = .0014 | |
| 5a+/−5b+/−(5) | 44.0 ± 2.6 | |||
| Wild-type (15) | 49.2 ± 1.0 | |||
| MCV (μ3) | Neonates | 5a−/−5b−/−(14) | 89.7 ± 1.6*,† | *P< .0001 †P = .0003 |
| 5a+/−5b+/−(25) | 97.3 ± 1.25 | |||
| Wild-type (9) | 102.8 ± 1.5 | |||
| 3 wk | 5a−/−5b−/−(10) | 56.1 ± 0.73*,† | *P= .004 †P = .002 | |
| 5a+/−5b+/−(18) | 59.1 ± 0.45 | |||
| Wild-type (5) | 60.0 ± 1.47 | |||
| 5 wk | 5a−/−5b−/−(10) | 51.8 ± 2.75 | ||
| 5a+/−5b+/−(18) | 54.6 ± 1.1 | |||
| Wild-type (5) | 54.6 ± 1.3 | |||
| Total bilirubin (mg/dL) | >6 wk | 5a−/−5b−/−(9) | 0.46 ± 0.19 | |
| Wild-type (6) | 0.12 ± 0.02 | |||
| LDH (IU/L) | 5a−/−5b−/−(9) | 715 ± 372 | ||
| Wild-type (6) | 282 ± 120 | |||
| Serum iron (μg/dL) | 5a−/−5b−/−(6) | 161.3 ± 15.1 | ||
| Wild-type (6) | 152.7 ± 17.9 | |||
| TIBC (μg/dL) | 5a−/−5b−/−(6) | 617 ± 40.0* | *P < .0001 | |
| Wild-type (6) | 427 ± 18.8 |
All measurements were carried out on Stat5a−/−5b−/− mice of the ages indicated, and on Stat5a−/−5b−/−, Stat5a+/−5b+/− or wild-type controls from the same mouse colony. Hematocrit data corresponds to Figure 1.P values are indicated only when P < .05. The increase in total iron-binding capacity (TIBC) in Stat5a−/−5b−/− mice is consistent with ineffective erythropoiesis. Red cell indices in Stat5a−/−5b−/− mice other than MCV were not significantly different than control values. P was calculated using Fisher protected least significant difference (PLSD) post hoc analysis.
Significantly different from wild-type value.
Significantly different from Stat5a+/−5b+/− value.
Anemic Stat5a−/−5b−/− mice had some increase in bilirubin and lactic dehydrogenase (LDH) levels, but this did not reach statistical significance, suggesting that hemolysis is not a significant cause of their anemia (Table 1). Further, normal serum iron levels in Stat5a−/−5b−/− mice suggested that the mice were not iron deficient and that their anemia was not due to marked blood loss (Table 1).
Adult Stat5a−/−5b−/−mice with near-normal hematocrit have a sluggish response to erythropoietic stress
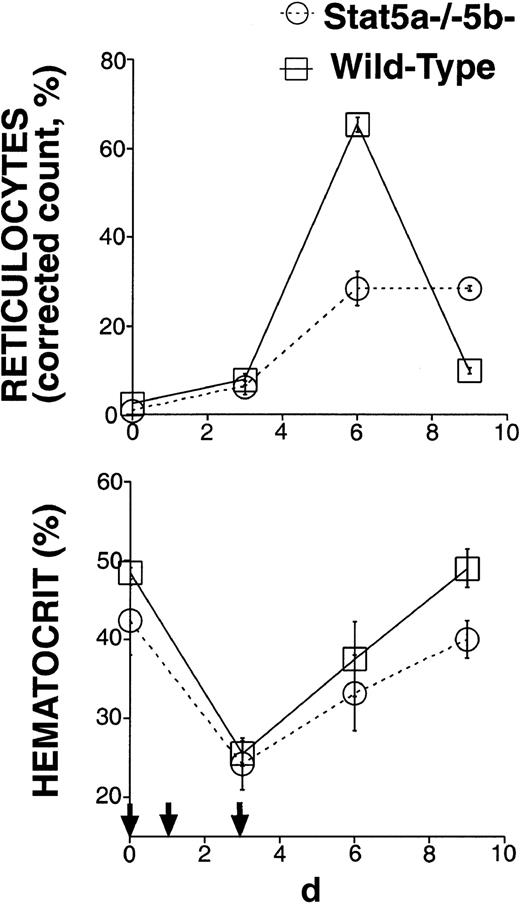
The rapid growth rate of the fetus and young mouse requires a high erythropoietic rate. We proposed that fetal anemia in Stat5a−/− 5b−/− mice may reflect the inability of erythroid progenitors lacking Stat5 to generate the high erythropoietic rate required during development.11 The near-normal hematocrit of some adult Stat5a−/−5b−/− mice (Figure 1) may be explained by the relatively low rate of erythropoiesis required to maintain the steady-state hematocrit in adult mice. Alternatively, qualitative differences between fetal/neonatal erythropoiesis and erythropoiesis in the adult may make Stat5 relatively redundant in adult erythropoiesis. To distinguish these 2 possibilities, we selected adult Stat5a−/− 5b−/− mice with a near-normal hematocrit and tested their ability to generate high erythropoietic rates in response to stress. The Stat5a−/−5b−/− mice used in this experiment had an initial hematocrit of at least 36% and a mean initial hematocrit of 41%. We subjected these mice and wild-type controls to a brief, chemically induced hemolytic anemia (Figure2) by injecting them with phenylhydrazine subcutaneously on days 0, 1, and 3. Their hematocrit and reticulocyte counts were monitored over the following 9 days. Reticulocytes are newly generated red blood cells and typically become mature red cells within 2 days. Therefore, the proportion of reticulocytes in the red cell population allows an assessment of erythropoietic rate.21 In the basal state, 2% or less of total red cells were reticulocytes in either wild-type mice or in Stat5a−/−5b−/− mice with near-normal hematocrit. After administration of phenylhydrazine, the reticulocyte count in wild-type mice increased sharply, reaching a maximum of 65.3% ± 1.7% (mean ± SEM) on day 6 (Figure 2). However, in Stat5a−/−5b−/− mice the maximal reticulocyte count was only 28.4% ± 3.9%. By day 9, the reticulocyte count in the wild-type mice had decreased to less than 10%, suggesting they had recovered from their blood loss and down-regulated their erythropoietic response. In contrast, Stat5a−/− 5b−/− mice on day 9 still maintained an elevated reticulocyte count of 28.4% ± 0.6%, clearly showing they had not yet recovered from loss in red cell mass. Therefore, Stat5a−/−5b−/− mice with a near-normal hematocrit at the basal state are deficient in generating high erythropoietic rates and show a sluggish, blunted response to erythropoietic stress.
Stat5a−/−5b−/−-mice with near-normal hematocrit have a sluggish response to erythropoietic stress.
Six adult Stat5a−/−5b−/− mice with initial hematocrit more than 35% and 6 wild-type controls were injected with phenylhydrazine on days 0, 1, and 3 (indicated by small arrows). The corrected reticulocyte count (also known as reticulocyte index21) allows an assessment of erythropoietic rate, and was calculated assuming a normal hematocrit of 45%, as follows: Corrected reticulocyte count (%) = reticulocyte count (%) × (hematocrit/45).
Stat5a−/−5b−/−-mice with near-normal hematocrit have a sluggish response to erythropoietic stress.
Six adult Stat5a−/−5b−/− mice with initial hematocrit more than 35% and 6 wild-type controls were injected with phenylhydrazine on days 0, 1, and 3 (indicated by small arrows). The corrected reticulocyte count (also known as reticulocyte index21) allows an assessment of erythropoietic rate, and was calculated assuming a normal hematocrit of 45%, as follows: Corrected reticulocyte count (%) = reticulocyte count (%) × (hematocrit/45).
Increased spleen size in Stat5a−/−5b−/− mice correlates with the severity of anemia
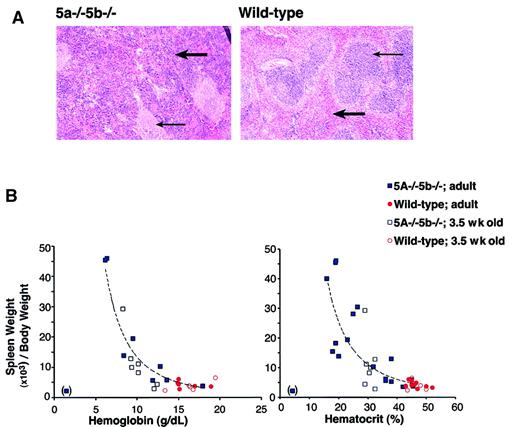
Anemia is expected to trigger a compensatory response mediated principally through increased serum Epo levels. The raised Epo promotes recovery from anemia by acting on erythroid progenitors in bone marrow and spleen, stimulating them to increase the rate at which red cells are generated. Over half of adult Stat5a−/−5b−/− mice have a chronic, persistent anemia (Figure 1), in the absence of continuous blood loss or hemolysis. This suggests a defect in the Epo-mediated compensatory feedback mechanism. We examined serum Epo levels in anemic Stat5a−/−5b−/− mice. Serum Epo in wild-type mice was recently found to be in the range of 10 to 20 mU/mL.22 We found that, whereas wild-type and Stat5a−/−5b−/− mice with near-normal hematocrits had a serum Epo concentration of less than 100 mU/mL, anemic Stat5a−/−5b−/− mice had serum Epo levels that were grossly elevated, ranging between 360 and 12 000 mU/mL (Table 2). In the mouse, increased erythropoiesis in response to elevated Epo occurs principally in the spleen,23 24 stimulating expansion of the spleen red pulp and an increase in spleen size. We therefore examined spleen size in Stat5a−/−5b−/− mice. We found that the spleen was enlarged up to 10 times the normal size in anemic Stat5a−/−5b−/− mice. Histologic examination of enlarged spleens in anemic Stat5a−/−5b−/− mice shows marked expansion of red pulp with predominantly erythroid extramedullary erythropoiesis. There was also follicular hyperplasia of white pulp (Figure3A). We found an inverse correlation between spleen size and hematocrit or serum hemoglobin concentration (Figure 3B). It is therefore likely that the increase in spleen size is primarily a compensatory response to anemia. Consistent with this, we also found that enlarged spleens in Stat5a−/−5b−/− mice were first seen at 3.5 weeks of age (Figure 3B). This correlates with the developmental stage at which growth rate in the mouse begins to slow down, erythropoietic reserve becomes available, and some of the Stat5a−/−5b−/− mice begin to recover from their neonatal anemia.
Raised serum Epo in anemic Stat5a−/−5b−/− mice
| Mouse no. . | Genotype . | Hematocrit (%) . | Hemoglobin (g/dL) . | Serum Epo (mU/mL) . |
|---|---|---|---|---|
| 435 | 5a−/−5b−/− | 23 | 9.5 | 360 |
| 431 | 5a−/−5b−/− | 20 | 8.4 | 720 |
| 181 | 5a−/−5b−/− | 16 | ND | 4 000 |
| 436 | 5a−/−5b−/− | 4 | 1.0 | 12 000 |
| 573 | 5a−/−5b−/− | 36 | 11.9 | < 100 |
| 575 | 5a−/−5b−/− | 45 | 13.6 | < 100 |
| 449 | Wild-type | 45 | 17.9 | < 100 |
| 557 | Wild-type | 46 | 15 | < 100 |
| Mouse no. . | Genotype . | Hematocrit (%) . | Hemoglobin (g/dL) . | Serum Epo (mU/mL) . |
|---|---|---|---|---|
| 435 | 5a−/−5b−/− | 23 | 9.5 | 360 |
| 431 | 5a−/−5b−/− | 20 | 8.4 | 720 |
| 181 | 5a−/−5b−/− | 16 | ND | 4 000 |
| 436 | 5a−/−5b−/− | 4 | 1.0 | 12 000 |
| 573 | 5a−/−5b−/− | 36 | 11.9 | < 100 |
| 575 | 5a−/−5b−/− | 45 | 13.6 | < 100 |
| 449 | Wild-type | 45 | 17.9 | < 100 |
| 557 | Wild-type | 46 | 15 | < 100 |
Epo was measured using a sandwich ELISA in several anemic and nonanemic Stat5a−/−5b−/− mice and in wild-type littermate controls. Serum Epo below 100 mU/mL could not be reliably measured by this assay. Normal mouse serum Epo is in the range of 10 to 20 mU/mL.22
ND indicates not done.
Splenomegaly in Stat5a−/−5b−/− mice correlates with their degree of anemia.
(A) Hematoxylin and eosin-stained sections of adult spleen from either wild-type or Stat5a−/−5b−/− mice. Thick arrow points at a region of red pulp. Thin arrow points at a region of white pulp. Photographs were taken at an original magnification of × 25. (B) Splenomegaly in Stat5a−/−5b−/−mice inversely correlates with both the hematocrit and hemoglobin concentration. Correlation curve was fitted using Microsoft Excel Trendline tool to the Stat5a−/−5b−/− adult data, excluding one data point from an extremely anemic mouse (in brackets). The curves drawn fit the equations y = 3487 × −2.4 (R2 = 0.91) for the hemoglobin data, and y = 12 089 × −2.0(R2 = 0.72) for the hematocrit data.
Splenomegaly in Stat5a−/−5b−/− mice correlates with their degree of anemia.
(A) Hematoxylin and eosin-stained sections of adult spleen from either wild-type or Stat5a−/−5b−/− mice. Thick arrow points at a region of red pulp. Thin arrow points at a region of white pulp. Photographs were taken at an original magnification of × 25. (B) Splenomegaly in Stat5a−/−5b−/−mice inversely correlates with both the hematocrit and hemoglobin concentration. Correlation curve was fitted using Microsoft Excel Trendline tool to the Stat5a−/−5b−/− adult data, excluding one data point from an extremely anemic mouse (in brackets). The curves drawn fit the equations y = 3487 × −2.4 (R2 = 0.91) for the hemoglobin data, and y = 12 089 × −2.0(R2 = 0.72) for the hematocrit data.
A block to erythroid differentiation at the early erythroblast stage in Stat5a−/−5b−/−mice
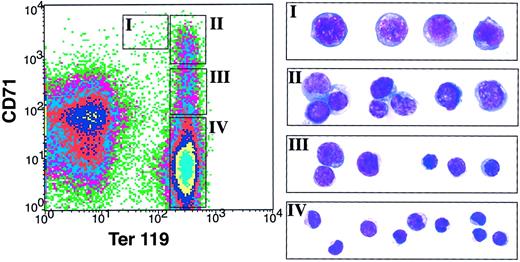
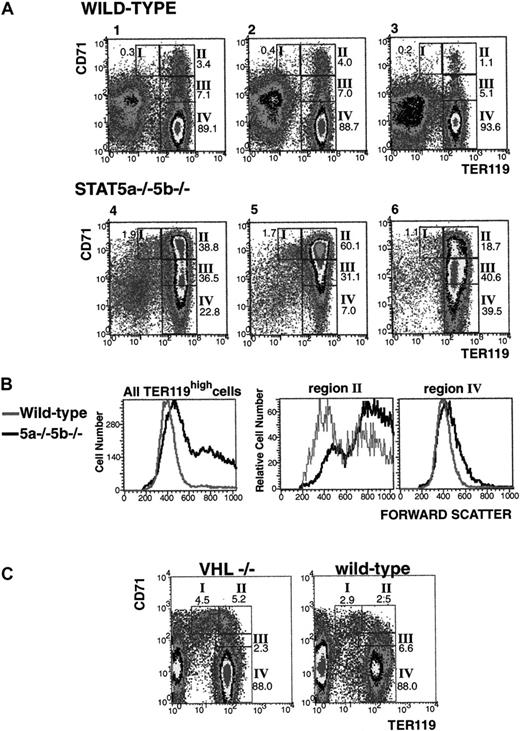
Despite raised serum Epo levels (Table 2) and vastly expanded extramedullary erythropoiesis in the spleen, anemia persists in a significant fraction of the Stat5a−/−5b−/−mice. This raised the possibility that erythropoiesis in these mice, although expanded, is ineffective. To examine this, we developed a flow cytometry assay that allows quantitative assessment of the maturation stage of differentiating erythroblasts in hematopoietic tissue (Figure4). Epo signaling becomes essential during terminal red cell differentiation. Several erythroid precursor differentiation stages have been recognized and defined morphologically, based on a gradual decrease in cell volume, increasing chromatin condensation and increasing hemoglobinization as terminal differentiation proceeds.25 These are, from least to most differentiated, the proerythroblasts, the basophilic and polychromatophilic erythroblasts, and the orthochromatophilic erythroblasts, which give rise to reticulocytes by enucleation (Figure4). The cell-surface erythroid-specific Ter119 antigen is expressed by terminally differentiating erythroblasts26,27 and is closely associated with murine glycophorin A.27,28 We found that it is first expressed at intermediate levels at the proerythroblast stage. All erythroid precursors subsequent to the proerythroblast stage express Ter119 at high levels. Conversely, the transferrin receptor (CD71), though not erythroid-specific, is expressed at very high levels by early erythroid precursors, principally proerythroblasts and early basophilic erythroblasts,29 and its levels decrease with erythroid maturation. We immunostained cells from spleen or bone marrow simultaneously for Ter119 and CD71. Using flow cytometry, we defined 4 cell populations with specific staining characteristics: Ter119medCD71high, Ter119highCD71high, Ter119highCD71med, and Ter119highCD71low (Figure 4, regions I-IV, respectively). Cells from each of these populations were sorted and analyzed morphologically. The morphologic characteristics broadly corresponded to proerythroblasts in the Ter119medCD71high cell population (Figure 4B, region I), basophilic erythroblasts in the Ter119highCD71high cell population (Figure 4B, region II), late basophilic and polychromatophilic erythroblasts in the Ter119highCD71med cell population (Figure 4B, region III), and orthochromatophilic erythroblasts in the Ter119highCD71low cell population (Figure 4B, region IV). Erythroblasts sorted from regions III and IV are all positive for hemoglobin expression, as judged by positive staining for diaminobenzidine (data not shown). Therefore, simultaneous immunostaining for Ter119 and CD71 allows flow cytometric identification of erythroblasts as they proceed through terminal differentiation in hematopoietic tissue. Although the relative number of cells in each of these populations varied somewhat between mice, it was very similar for a given mouse, regardless of whether hematopoietic cells were derived from bone marrow or from spleen (data not shown).
Flow cytometry assessment of spleen erythroblast maturation.
Freshly dissociated wild-type mouse spleen cells were labeled with a biotin-conjugated monoclonal antibody (mAb) to CD71 and a PE-conjugated anti-Ter119 mAb, followed by APC-conjugated streptavidin. Dead cells (staining positive with propidium iodide) and anucleated red cells (with low forward scatter) were excluded from analysis. The left-hand panel illustrates a density plot of all viable spleen cells; axes indicate relative fluorescence units for PE (x-axis) and APC (y-axis). Regions I to IV were selected as indicated. The right-hand panels show May-Grunwald Giemsa–stained cytospin preparations of cells sorted from each of regions I to IV. Representative cells from 2 to 3 fields are shown for each region. These are predominantly proerythroblasts in region I, basophilic erythroblasts in region II, late basophilic and chromatophilic erythroblasts in region III, and orthochromatophilic erythroblasts in region IV. The photographs were taken at an original magnification of × 400.
Flow cytometry assessment of spleen erythroblast maturation.
Freshly dissociated wild-type mouse spleen cells were labeled with a biotin-conjugated monoclonal antibody (mAb) to CD71 and a PE-conjugated anti-Ter119 mAb, followed by APC-conjugated streptavidin. Dead cells (staining positive with propidium iodide) and anucleated red cells (with low forward scatter) were excluded from analysis. The left-hand panel illustrates a density plot of all viable spleen cells; axes indicate relative fluorescence units for PE (x-axis) and APC (y-axis). Regions I to IV were selected as indicated. The right-hand panels show May-Grunwald Giemsa–stained cytospin preparations of cells sorted from each of regions I to IV. Representative cells from 2 to 3 fields are shown for each region. These are predominantly proerythroblasts in region I, basophilic erythroblasts in region II, late basophilic and chromatophilic erythroblasts in region III, and orthochromatophilic erythroblasts in region IV. The photographs were taken at an original magnification of × 400.
We used this assay to quantitatively analyze erythroid precursors in anemic Stat5a−/−5b−/− mice (Figure5). In wild-type mice, the vast majority (>90%) of erythroid, Ter119high cells in spleen or bone marrow are late erythroblasts (region IV, Ter119highCD71low). All other, less differentiated erythroid cells typically constitute 10% or less of all erythroid precursors (Figure 5, mice 1-3). The ratio of early (Ter119highCD71high, region II) to late (Ter119highCD71low, region IV) erythroblasts varies from mouse to mouse but is in the range of 1:10 to 1:80. In contrast, the erythroid progenitor profile in hematopoietic tissue of anemic Stat5a−/−5b−/− mice is strikingly different (Figure 5, mice 4-6). The ratio of early (Ter119highCD71high, region II) to late (Ter119highCD71 low, region IV) erythroblasts is often inverted and is in the range of 1:2 to 6:1. This therefore represents a massive increase in the ratio of early to late erythroblasts in anemic Stat5a−/−5b−/−hematopoietic tissue, of as much as 2 orders of magnitude when compared with the wild-type tissue. This marked increase in early to late erythroblasts in Stat5a−/− 5b−/− mice was accompanied by a substantial increase in the absolute number of the less differentiated, early progenitors (regions I and II). The flow cytometry profiles for the anemic Stat5a−/−5b−/− mice shows an average expansion of 10-fold and 25-fold, respectively, in the number of cells in regions I and II when compared with wild-type mice. These anemic Stat5a−/−5b−/− mice had a mean 10-fold increase in spleen size. This therefore suggests an absolute increase of cell numbers in regions I and II of 100- and 250-fold, respectively. The number of erythroblasts begins to decline sharply, however, in the progression from region II to regions III and IV. The substantial increase in early erythroid progenitors presumably reflects a compensatory response to anemia, driven in part by raised serum Epo.
Massive increase in the ratio of early to late erythroblasts in spleens of anemic Stat5a−/−5b−/− mice.
(A) Flow cytometry density plots of spleen cells from 3 representative wild-type mice (numbers 1-3, mean hematocrit = 48.5%) and 3 anemic Stat5a−/−5b−/− mice (numbers 4-6, mean heamtocrit = 23%) from the same mouse colony. Cells were labeled for Ter119 and CD71 as described in Figure 4. The relative number of cells in each of regions I to IV as a percent of all viable, nucleated erythroid cells is indicated on each plot. Erythroid cells (defined as all cells in regions I to IV) in each spleen constituted 61.7%, 53%, and 37.4% of all spleen cells for the wild-type mice (numbers 1-3, respectively); and 87.7%, 93.2% and 91.1% of all spleen cells for Stat5a−/− 5b−/− mice (numbers 4-6, respectively). (B) Flow cytometry forward-scatter distribution histograms of wild-type and Stat5a−/−5b−/−mice (corresponding to mice numbers 1 and 4, respectively, in panel A). Left-hand panel shows data for all Ter119high cells. Middle and right-hand panels show data for Ter119highCD71high (region II) cells and Ter119highCD71low (region IV) cells, respectively. (C) Flow cytometry density plots of spleen cells from a representative wild-type mouse (right-hand panel, hematocrit = 42%) and a representative mouse with a tissue-specific deletion of VHL (VHL−/−, left-hand panel; see “Materials and methods”). The VHL−/− mouse shown had an enlarged spleen and a hematocrit of 84%.
Massive increase in the ratio of early to late erythroblasts in spleens of anemic Stat5a−/−5b−/− mice.
(A) Flow cytometry density plots of spleen cells from 3 representative wild-type mice (numbers 1-3, mean hematocrit = 48.5%) and 3 anemic Stat5a−/−5b−/− mice (numbers 4-6, mean heamtocrit = 23%) from the same mouse colony. Cells were labeled for Ter119 and CD71 as described in Figure 4. The relative number of cells in each of regions I to IV as a percent of all viable, nucleated erythroid cells is indicated on each plot. Erythroid cells (defined as all cells in regions I to IV) in each spleen constituted 61.7%, 53%, and 37.4% of all spleen cells for the wild-type mice (numbers 1-3, respectively); and 87.7%, 93.2% and 91.1% of all spleen cells for Stat5a−/− 5b−/− mice (numbers 4-6, respectively). (B) Flow cytometry forward-scatter distribution histograms of wild-type and Stat5a−/−5b−/−mice (corresponding to mice numbers 1 and 4, respectively, in panel A). Left-hand panel shows data for all Ter119high cells. Middle and right-hand panels show data for Ter119highCD71high (region II) cells and Ter119highCD71low (region IV) cells, respectively. (C) Flow cytometry density plots of spleen cells from a representative wild-type mouse (right-hand panel, hematocrit = 42%) and a representative mouse with a tissue-specific deletion of VHL (VHL−/−, left-hand panel; see “Materials and methods”). The VHL−/− mouse shown had an enlarged spleen and a hematocrit of 84%.
We looked at whether the altered Ter119/CD71 FACS profile in Stat5a−/−5b−/− cells (Figure 5A) genuinely reflects an increase in early erythroblasts, by morphologically examining Stat5a−/−5b−/−cells sorted from each of regions I to IV, in the way shown for wild-type mice in Figure 4. We found that erythroblast morphology in each of those regions was similar to that found for wild-type mice (data not shown). We further examined this issue quantitatively, by using FACS forward scatter as an independent parameter that does not rely on CD71 expression (Figure 5B). FACS forward scatter is a function of cell size and has been previously used as an indicator of erythroblast maturation.30 The left-hand panel in Figure5B shows the FACS forward scatter distribution for Ter119high erythroblasts in wild-type and Stat5a−/−5b−/− spleens. There is a clear increase in the number of high forward-scatter (large) cells in Stat5a−/−5b−/− spleen erythroblasts compared with wild-type controls. Therefore, the predominance of large, early erythroblasts detected in Figure 5A is also found when looking at cell size, independent of CD71 expression. The middle and right-hand panels of Figure 5B look at cell size distribution for either CD71high Ter119high (region II) or CD71low Ter119high (region IV) cells. These show that cell size distribution, and therefore erythroblast size and stage of maturation, for a given CD71 expression level, is comparable between wild-type and Stat5a−/− 5b−/−erythroblasts. Therefore, the relation between CD71 expression and erythroblast maturation identified for wild-type mice also holds for Stat5a−/−5b−/− mice.
We next examined whether the dramatic increase in ratio of early to late erythroblasts in Stat5a−/−5b−/− mice is simply a result of increased spleen erythropoiesis. We used for comparison a mouse model in which Epo levels are constitutively elevated, as a result of a tissue-specific deletion of the VHL tumor suppressor in the proximal kidney tubules and a subgroup of periportal hepatocytes (VHL−/− mice, Figure 5C).20Despite expanded erythropoiesis, increased spleen size, and erythrocytosis (hematocrit = 84%), the VHL−/− mice had a near-proportional increase in each of their erythroblast populations, with late erythroblasts comprising 88% of all Ter119 cells. Therefore, increased erythropoiesis per se does not result in a relative reduction of late erythroblasts.
Thus, our findings show that there is a significant delay in progression of early to late erythroblasts in Stat5a−/−5b−/− mice, suggesting that erythropoiesis in Stat5a−/−5b−/− mice is ineffective. Specifically, efficient progression of erythroid precursors through differentiation is blocked at the early erythroblast stage (region II).
Increased apoptosis in early erythroblasts of anemic Stat5a−/−5b−/− mice
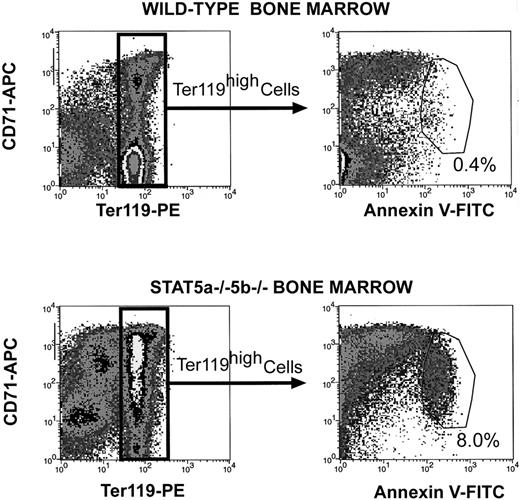
We previously showed that the Epo-activated Stat5 mediates an antiapoptotic effect in fetal liver erythroid progenitors.11 We therefore looked at whether decreased survival of early erythroblasts may account for the relative block in their differentiation into late erythroblasts in anemic adult Stat5a−/−5b−/− mice. We immunolabeled bone marrow and spleen cells for CD71 and Ter119 and then incubated the cells with annexin V and propidium iodide (Figure6). Dead cells with leaky plasma membranes label positive for propidium iodide and were excluded from further analysis. Cells that have entered the apoptotic program label positive for annexin V but remain negative for propidium iodide. We found a 20-fold increase in the total number of cells positive for annexin V in Stat5a−/−5b−/−bone marrow: 8% of Stat5a−/−5b−/−Ter119-positive bone marrow cells were annexin V–positive, compared with 0.4% of wild-type Ter119-positive bone marrow cells (Figure 6). Further, increased apoptosis was principally seen in the early erythroblast population (Ter119highCD71highcells). Therefore, decreased survival of early erythroblasts may account, at least in part, for the apparent block in their differentiation into late erythroblasts. A similar examination of apoptotic rates in bone marrow erythroblasts of the tissue-specific VHL−/− mice did not show increased apoptosis (data not shown). Therefore, the increased rate of apoptosis in Stat5a−/−5b−/− erythroblasts is not a result of splenomegaly or of increased erythropoiesis per se. Despite a clear increase in apoptosis of Stat5a−/−5b−/− bone marrow erythroblasts, we could not detect annexin V binding in spleen erythroblasts. This may be due to more efficient clearance of apoptotic cells in adult spleen.31 Significant levels of apoptosis were detected in neonatal spleens from Stat5a−/−5b−/− mice (see below).
Increased apoptosis in Stat5a−/−5b−/− bone marrow erythroblasts.
Bone marrow from either wild-type or Stat5a−/−5b−/− mice was labeled with CD71 (APC channel) and Ter119 (PE channel) as described in Figure 4. Cells were then incubated with FITC-conjugated annexin V and propidium iodide and analyzed by flow cytometry. Cells positive for propidium iodide were excluded from analysis. The top and bottom left-hand panels show flow cytometry density plots for CD71 and Ter119 similar to those shown for spleen cells in Figures 4 and 5. The right-hand panels show density plots for annexin V binding on Ter119high cells only. The region of increased annexin V binding is indicated and contains 0.4% of wild-type Ter119high cells (top right-hand panel) and 8% of Stat5a−/−5b−/−Ter119high cells (bottom right-hand panel). Data shown are representative of 2 experiments.
Increased apoptosis in Stat5a−/−5b−/− bone marrow erythroblasts.
Bone marrow from either wild-type or Stat5a−/−5b−/− mice was labeled with CD71 (APC channel) and Ter119 (PE channel) as described in Figure 4. Cells were then incubated with FITC-conjugated annexin V and propidium iodide and analyzed by flow cytometry. Cells positive for propidium iodide were excluded from analysis. The top and bottom left-hand panels show flow cytometry density plots for CD71 and Ter119 similar to those shown for spleen cells in Figures 4 and 5. The right-hand panels show density plots for annexin V binding on Ter119high cells only. The region of increased annexin V binding is indicated and contains 0.4% of wild-type Ter119high cells (top right-hand panel) and 8% of Stat5a−/−5b−/−Ter119high cells (bottom right-hand panel). Data shown are representative of 2 experiments.
Decreased bcl-xL expression in Stat5a−/−5b−/− early erythroblasts correlates with anemia in adult Stat5a−/−5b−/− mice
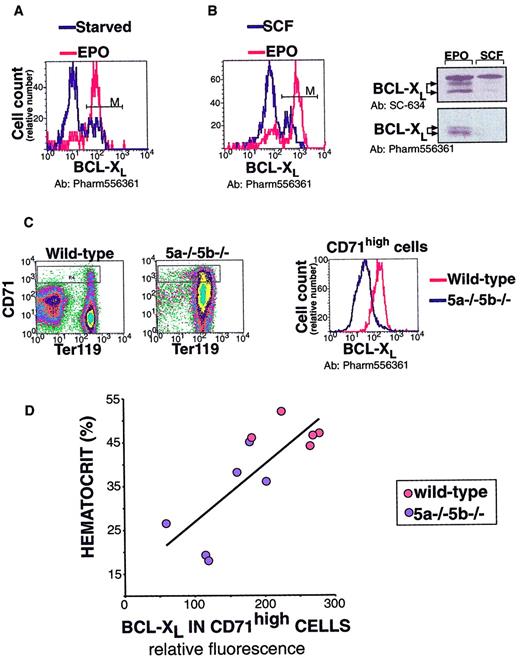
In the cultured, Epo-dependent cell line HCD-57, Stat5 mediates an antiapoptotic effect by directly binding to the gene encoding the antiapoptotic bcl-xL protein and inducing its immediate early expression.11 We examined whether this pathway was physiologically relevant to the Stat5-mediated survival of early erythroblasts. We first evaluated a flow cytometric assay for bcl-xL expression (Figure7A,B). HCD-57 cells were cultured in Epo, or starved of Epo for 18 hours. As expected from Western blot analysis,11 flow cytometry analysis indicated that cells starved of Epo were depleted of bcl-xL (Figure 7A). In a separate experiment, we looked at HCD-57 cells that were cultured either in Epo or washed and cultured in stem cell factor (SCF) for 72 hours. SCF does not activate Stat5 in these cells, and Western blot analysis indicates that it does not support expression of bcl-xL (Figure 7B). Similarly, flow cytometry shows that HCD-57 cells growing in SCF are depleted of bcl-xL(Figure 7B).
Decreased bcl-xLexpression in Stat5a−/−5b−/− early erythroblasts correlates with anemia.
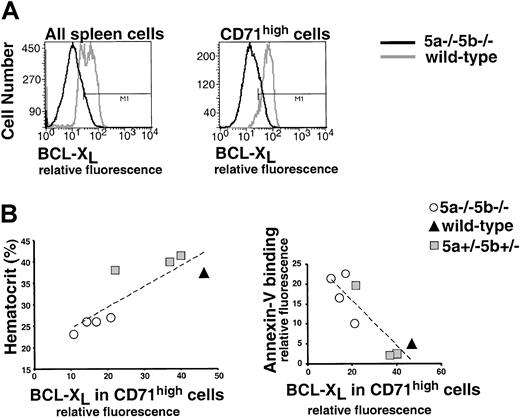
Panels A-B show evaluation of a flow cytometry assay for bcl-xL. (A) Histogram of HCD-57 cells labeled for bcl-xL (x-axis: relative fluorescence). HCD-57 cells were cultured in Epo (1 U/mL) or starved of Epo for 18 hours in the presence of serum. Cells were then fixed, permeabilized, and labeled for bcl-xL using a rabbit polyclonal antiserum (Pharmingen, see “Materials and methods,” here labeled as Pharm556361) and Alexa Fluor 488 goat anti–rabbit IgG. In the presence of Epo, 83% of cells label positive for bcl-xL (marked by “M”); after 18 hours of starvation, this declines to 24%. (B) Bcl-xLlevels in HCD-57 cells growing either in Epo (1 U/mL) or in SCF (100 ng/mL) assessed by flow cytometry or by Western blot analysis. Flow cytometry as described in panel A; 75% of cells are positive for bcl-xL when cultured in Epo, but only 20% of cells express bcl-xL when cultured in SCF. Western blot analysis was carried out either with a polyclonal rabbit IgG bcl-xL(SC-634, Santa-Cruz Biotechnology, Santa Cruz, CA) or with the Pharm556361 antibody, as indicated. (C) A representative flow cytometry measurement of bcl-xL in spleen erythroblasts. Spleen cells from one wild-type and one anemic Stat5a−/−5b−/− mouse were labeled with CD71 (APC channel) and Ter119 (PE channel). Cells were then fixed, permeabilized, and labeled for bcl-xL (Alexa 488 channel). Bcl-xL expression in CD71high cells (which are >90% Ter119+ erythroblasts) is clearly reduced in Stat5a−/−5b−/− cells when compared with wild-type cells (right-hand panel). (D) Bcl-xL expression in CD71high cells was measured as described in panel C, in 5 wild-type mice and 6 Stat5a−/−5b−/− mice in the same experiment. Correlation line was fitted to the Stat5a−/−5b−/− data points (R2 = 0.64). Representative of 3 similar experiments.
Decreased bcl-xLexpression in Stat5a−/−5b−/− early erythroblasts correlates with anemia.
Panels A-B show evaluation of a flow cytometry assay for bcl-xL. (A) Histogram of HCD-57 cells labeled for bcl-xL (x-axis: relative fluorescence). HCD-57 cells were cultured in Epo (1 U/mL) or starved of Epo for 18 hours in the presence of serum. Cells were then fixed, permeabilized, and labeled for bcl-xL using a rabbit polyclonal antiserum (Pharmingen, see “Materials and methods,” here labeled as Pharm556361) and Alexa Fluor 488 goat anti–rabbit IgG. In the presence of Epo, 83% of cells label positive for bcl-xL (marked by “M”); after 18 hours of starvation, this declines to 24%. (B) Bcl-xLlevels in HCD-57 cells growing either in Epo (1 U/mL) or in SCF (100 ng/mL) assessed by flow cytometry or by Western blot analysis. Flow cytometry as described in panel A; 75% of cells are positive for bcl-xL when cultured in Epo, but only 20% of cells express bcl-xL when cultured in SCF. Western blot analysis was carried out either with a polyclonal rabbit IgG bcl-xL(SC-634, Santa-Cruz Biotechnology, Santa Cruz, CA) or with the Pharm556361 antibody, as indicated. (C) A representative flow cytometry measurement of bcl-xL in spleen erythroblasts. Spleen cells from one wild-type and one anemic Stat5a−/−5b−/− mouse were labeled with CD71 (APC channel) and Ter119 (PE channel). Cells were then fixed, permeabilized, and labeled for bcl-xL (Alexa 488 channel). Bcl-xL expression in CD71high cells (which are >90% Ter119+ erythroblasts) is clearly reduced in Stat5a−/−5b−/− cells when compared with wild-type cells (right-hand panel). (D) Bcl-xL expression in CD71high cells was measured as described in panel C, in 5 wild-type mice and 6 Stat5a−/−5b−/− mice in the same experiment. Correlation line was fitted to the Stat5a−/−5b−/− data points (R2 = 0.64). Representative of 3 similar experiments.
We next used the flow cytometric measurement of bcl-xL to look at its expression in adult spleen early erythroblasts. We found a clear reduction of bcl-xL levels in early erythroblasts of anemic adult Stat5a−/−5b−/− mice compared with wild-type mice (Figure 7C). Specifically, bcl-xL levels correlated with the hematocrit of Stat5a−/−5b−/− mice. Figure 7D shows a representative sample of 5 wild-type and 6 Stat5a−/−5b−/− mice analyzed in the same experiment. The highest levels of bcl-xL were seen in wild-type mice. Stat5a−/−5b−/− mice with near-normal hematocrit expressed near-normal levels of bcl-xL. Three of the Stat5a−/−5b−/− mice are anemic with hematocrits in the range of 20% to 25%. These anemic Stat5a−/−5b−/− mice expressed substantially lower levels of bcl-xL than wild-type controls (Figure 7D). This suggests that Stat5 regulates the level of bcl-xL in early erythroblasts. Further, the absence of the Stat5-bcl-xL pathway may contribute directly to reduced survival of early erythroblasts and to the observed anemia in adult Stat5a−/−5b−/− mice.
Neonatal spleen erythropoiesis: apoptosis and reduced bcl-xL expression in Stat5a−/−5b−/− erythroblasts correlates with the severity of anemia
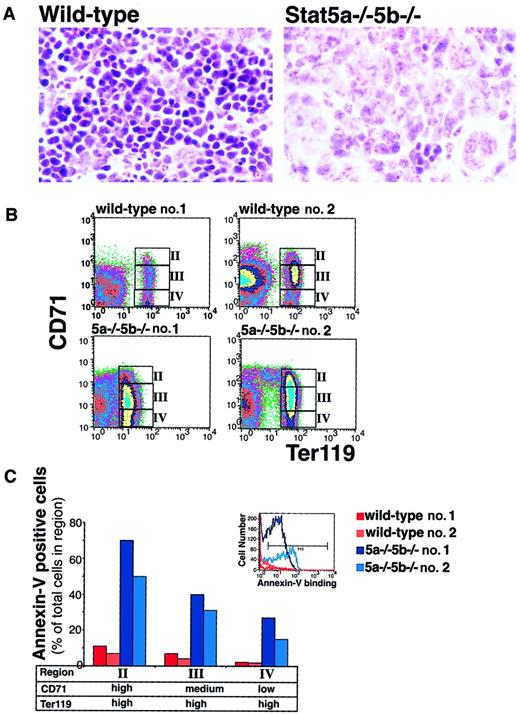
The spleen is a major site of erythropoiesis during early neonatal development. Neonatal spleens from Stat5a−/−5b−/− mice showed an increase in the number of cells with immature morphology (Figure8A). On flow cytometry analysis, we found that wild-type fetal and neonatal hematopoietic tissues contained relatively larger pools of proerythroblasts and early erythroblasts compared with adult hematopoietic tissue (Figure 8B and data not shown). Figure 8 shows representative flow cytometry analysis on neonatal spleen cells. The ratio of early to late erythroblasts in the Stat5a−/−5b−/− neonatal spleen is elevated compared to that of wild-type neonates, but not as dramatically as in adult Stat5a−/−5b−/− hematopoietic tissue. Presumably this is, in part, due to decreased ability to mount a compensatory increase in early erythroblast numbers. The most striking abnormality in the neonatal spleens is a substantial increase in annexin V binding in Stat5a−/−5b−/−erythroblasts throughout terminal differentiation (Figure 8C). The largest increase in apoptosis is seen in early Stat5a−/−5b−/− erythroblasts (region II, Ter119highCD71high), where there is a 13-fold increase in annexin V+ cells compared with control. Apoptosis is also clearly increased at later stages of differentiation (regions III and IV). The increased apoptosis throughout terminal differentiation in Stat5a−/−5b−/− neonatal erythroblasts may also account for the relatively preserved ratio of early to late erythroblasts when compared with the adult, where apoptosis was primarily confined to early Stat5a−/−5b−/− erythroblasts.
Increased apoptosis in Stat5a−/−5b−/−neonatal spleens.
(A) Hematoxylin and eosin-stained sections of neonatal spleen from either wild-type or Stat5a−/−5b−/− mice. Original magnification at the time of photography was × 250. (B) Flow cytometry density plots of wild-type and Stat5a−/−5b−/−neonatal spleen cells simultaneously labeled for Ter119, CD71, and annexin V binding. Regions II to IV are indicated and correspond to the analysis of annexin V binding on these cells shown in panel C. Ter119 expression was variably reduced in Stat5a−/−5b−/− spleens. In the representative plots shown, the geometric mean fluorescence for Ter119 in the Ter119-positive populations is 73 and 68 for wild-type spleens 1 and 2, and 14 and 50 for Stat5a−/−5b−/−spleens 1 and 2, respectively. (C) Annexin V binding of Ter119high erythroblasts in regions II to IV for the spleen cells analyzed in panel B. Inset shows an example of annexin V binding histogram for region II. Cells within the marker M1′ were considered annexin V–postiive.
Increased apoptosis in Stat5a−/−5b−/−neonatal spleens.
(A) Hematoxylin and eosin-stained sections of neonatal spleen from either wild-type or Stat5a−/−5b−/− mice. Original magnification at the time of photography was × 250. (B) Flow cytometry density plots of wild-type and Stat5a−/−5b−/−neonatal spleen cells simultaneously labeled for Ter119, CD71, and annexin V binding. Regions II to IV are indicated and correspond to the analysis of annexin V binding on these cells shown in panel C. Ter119 expression was variably reduced in Stat5a−/−5b−/− spleens. In the representative plots shown, the geometric mean fluorescence for Ter119 in the Ter119-positive populations is 73 and 68 for wild-type spleens 1 and 2, and 14 and 50 for Stat5a−/−5b−/−spleens 1 and 2, respectively. (C) Annexin V binding of Ter119high erythroblasts in regions II to IV for the spleen cells analyzed in panel B. Inset shows an example of annexin V binding histogram for region II. Cells within the marker M1′ were considered annexin V–postiive.
In addition to increased apoptosis, expression of the erythroid differentiation marker Ter119 is also reduced, substantially in some neonates (Figure 8B, Stat5a−/−5b−/− no. 1).
We measured the level of bcl-xL expression in representative 3-day-old neonates from 2 litters analyzed in the same experiment (Figure 9). This showed that expression of bcl-xL is reduced in neonatal spleen from Stat5a−/−5b−/− mice. The difference in bcl-xL expression between Stat5a−/−5b−/− and wild-type mice is largest in early erythroblasts (Figure 9A). Further, analysis of a group of neonates shows a clear correlation between neonatal hematocrit, bcl-xL expression in early erythroblasts, and early erythroblast apoptosis (Figure 9B).
Decreased expression of bcl-xL and increased erythroblast apoptosis correlate with anemia in Stat5a−/−5b−/− neonates.
(A) Flow cytometry histograms for bcl-xL expression in neonatal spleens. The left-hand histogram contains data from all spleen cells of one representative wild-type neonate and one Stat5a−/−5b−/− neonate. The right-hand histogram contains data from CD71high (early erythroblasts) cells only for the same spleens. In wild-type spleens, 65% of all cells and 94% of CD71high cells express bcl-xL(within the marker “M1”). In Stat5a−/−5b−/−spleens, 11% of all cells and 9% of CD71high cells express bcl-xL. (B) Summary of bcl-xL and annexin V binding data on representative neonates from 2 litters analyzed in the same experiment. Bcl-xL was measured as in panel A; annexin V was measured as described in Figure 8. Correlation lines were fitted to all the data points.
Decreased expression of bcl-xL and increased erythroblast apoptosis correlate with anemia in Stat5a−/−5b−/− neonates.
(A) Flow cytometry histograms for bcl-xL expression in neonatal spleens. The left-hand histogram contains data from all spleen cells of one representative wild-type neonate and one Stat5a−/−5b−/− neonate. The right-hand histogram contains data from CD71high (early erythroblasts) cells only for the same spleens. In wild-type spleens, 65% of all cells and 94% of CD71high cells express bcl-xL(within the marker “M1”). In Stat5a−/−5b−/−spleens, 11% of all cells and 9% of CD71high cells express bcl-xL. (B) Summary of bcl-xL and annexin V binding data on representative neonates from 2 litters analyzed in the same experiment. Bcl-xL was measured as in panel A; annexin V was measured as described in Figure 8. Correlation lines were fitted to all the data points.
Discussion
Stimulation of EpoR leads to the rapid activation of Stat5, one of several signals emanating from the EpoR.32 We evaluated the role of Stat5 in EpoR function in vivo by studying Stat5a−/−5b−/− mice. Previously, we identified anemia and apoptosis of erythroid progenitors in Stat5a−/−5b−/− embryos.11 Here we found that Stat5a−/− 5b−/− mice are born anemic and remain so throughout development. Anemia resolves in approximately half of adult Stat5a−/− 5b−/− mice, but persists in the remainder. Anemic adult Stat5a−/− 5b−/−mice show ineffective erythropoiesis with markedly expanded erythropoietic tissue. Further, the subset of adult Stat5a−/− 5b−/− mice with normal basal hematocrit are found to be deficient in generating elevated erythropoietic rates in response to stress. Therefore, the loss of Stat5 function gives rise to significant impairment of erythropoiesis.
We investigated the cellular deficit underlying the erythropoietic phenotype of Stat5a−/−5b−/− mice. To this end, we developed a novel flow cytometric assay allowing analysis of erythroblasts at different maturation stages in hematopoietic tissue. With this assay, we identified the early erythroblast as a critical target of Stat5 action. In anemic Stat5a−/−5b−/− adults and neonates, early erythroblasts show markedly increased apoptosis and reduced levels of bcl-xL expression. Therefore, Stat5 modulates erythropoietic rate by regulating early erythroblast survival.
Quantitative analysis by flow cytometry of hematopoietic tissue from anemic adult Stat5a−/−5b−/− mice showed a marked increase, by up to 2 orders of magnitude, in the relative number of early to late erythroblasts. This suggests that early Stat5a−/−5b−/− erythroblasts fail to differentiate into late erythroblasts. Therefore, the increased apoptosis we found in early erythroblasts in Stat5a−/−5b−/− mice poses a severe impediment to erythroblast differentiation. In vitro culture experiments have shown that Epo is required for survival of erythroid progenitors during terminal differentiation.33 34Here we find that, in vivo, in the absence of Stat5, there is a quantitative deficit in the antiapoptotic effect of EpoR. This quantitative deficit, though not absolutely preventing the formation of red cells, clearly decreases the rate at which they are produced, resulting in a range of phenotypes, from severe anemia to a defect unmasked during response to erythropoietic stress.
The decreased expression of bcl-xL in Stat5a−/−5b−/− early erythroblasts is likely to be a principal cause of their decreased survival. Bcl-xL is essential for the formation of red cells; bcl-xL mutant mice die in utero at embryonic day 13.5, and are severely anemic.35 The regulation of bcl-xL in erythroblasts is likely to be multifactorial and involves the synergistic interaction of both EpoR-dependent with EpoR-independent factors such as GATA-1.36 We recently showed that Stat5 mediates the Epo-dependent “immediate-early” induction of bcl-xL in erythroid HCD-57 cells through canonical Stat5 sites in the bcl-x gene.11Stat5 was similarly found to induce bcl-xL expression in other hematopoietic cell lines.12-14 Here we find that Stat5 absence leads to decreased expression of bcl-xL in differentiating erythroblasts in vivo. Remarkably, the level of bcl-xL expression in early erythroblasts in both Stat5a−/−5b−/− adults and neonates correlates with the degree of anemia. This suggests that bcl-xL is a principal determinant of early erythroblast survival and that it is a principal target of Stat5 action in vivo. Further, the extent to which alternative pathways can compensate for Stat5 absence and maintain sufficient bcl-xL expression in early erythroblasts appears to determine the severity of the anemic phenotype (Figure 10). Heterogeneity in the ability of mice to compensate for the loss of the Stat5-bcl-xL pathway may account, at least in part, for the heterogeneity in erythropoietic phenotype of adult Stat5a−/−5b−/− mice. Erythroid progenitors, even within the same animal, are heterogeneous in their sensitivity to apoptosis, because some Stat5a−/−5b−/−erythroblasts are always able to survive and differentiate. The ability of some Stat5a−/−5b−/− mice to recover from neonatal anemia and attain a near-normal adult hematocrit may be due to in vivo selection and expansion of progenitors with reduced sensitivity to apoptosis. Similar in vivo selection of progenitors was proposed to explain recovery from neonatal anemia in GATA-1 heterozygote mice.37 Heterogeneity between mice may also be due to random segregation of potential genetic modifiers in the mixed 129xC57BL/6 Stat5a−/−5b−/− colony. Despite compensatory pathways, however, approximately 50% of adult Stat5a−/−5b−/− mice are unable to maintain sufficient levels of bcl-xL and are chronically anemic.
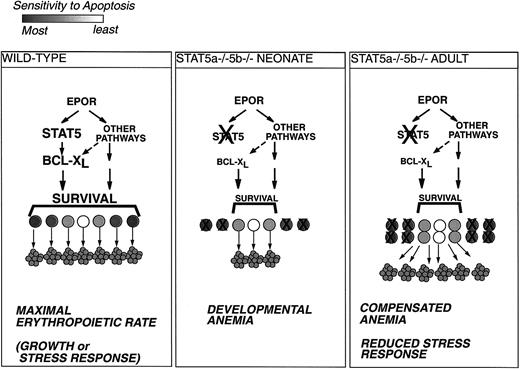
Model for Stat5a−/−5b−/− erythropoiesis.
Erythroid progenitors vary in their sensitivity to apoptosis and in their requirements for antiapoptotic signaling from EpoR.34 Under conditions of maximal erythropoietic rate, during rapid growth or in response to stress, all erythroid progenitors are rescued from apoptosis and give rise to red cells (left-hand panel). Impairment of EpoR antiapoptotic signaling in Stat5a−/−5b−/− neonates under rapid growth conditions will lead to apoptosis of the more sensitive progenitors and result in developmental anemia (middle panel). Surviving Stat5a−/−5b−/− adults are able to partly compensate for the deficit in EpoR antiapoptotic signaling by increasing the size of the early erythroblast population. This may result in improved rates of red cell production at the expense of expanded erythropoietic tissue and increased apoptosis (right-hand panel).
Model for Stat5a−/−5b−/− erythropoiesis.
Erythroid progenitors vary in their sensitivity to apoptosis and in their requirements for antiapoptotic signaling from EpoR.34 Under conditions of maximal erythropoietic rate, during rapid growth or in response to stress, all erythroid progenitors are rescued from apoptosis and give rise to red cells (left-hand panel). Impairment of EpoR antiapoptotic signaling in Stat5a−/−5b−/− neonates under rapid growth conditions will lead to apoptosis of the more sensitive progenitors and result in developmental anemia (middle panel). Surviving Stat5a−/−5b−/− adults are able to partly compensate for the deficit in EpoR antiapoptotic signaling by increasing the size of the early erythroblast population. This may result in improved rates of red cell production at the expense of expanded erythropoietic tissue and increased apoptosis (right-hand panel).
Although a subgroup of adult Stat5a−/−5b−/−mice recover from neonatal anemia, they have a persistent deficit in generating elevated erythropoietic rates in response to stress. This is consistent with our finding that Stat5 fulfills a similar role in adult and in fetal/neonatal erythropoiesis. Despite a persistent erythropoietic defect, anemia in the neonate may resolve into a normal basal hematocrit in the adult because of the large erythropoietic reserve in the adult. Erythropoiesis during development is analogous to stress erythropoiesis, in that the fetus and neonate need to sustain high rates of red cell production. The fetus must generate its entire red cell mass and achieve a near-adult hematocrit level in only a few days (approximately between embryonic days 9 and 1538). The continuing rapid growth during development needs to be matched by an equally rapid expansion in red cell mass. This leaves little erythropoietic reserve, and a deficit in erythropoietic rate due to Stat5 absence results in anemia. In contrast, the adult has a remarkable erythropoietic reserve. In the steady state, the rate of red cell production need only match the rate of senescent red cell loss, approximately 2.5% of the total red cell mass per day in the mouse. In response to erythropoietic stress, however, erythropoietic rate may rise up to 10-fold2 (Figure 2). Under these conditions, the Stat5a−/−5b−/− deficit is unmasked, preventing maximal erythropoietic rate. Fetal anemia resolving into a normal steady-state adult hematocrit is also seen in the recessive mouse mutation “flexed-tail” (f). Similar to our finding in Stat5a−/−5b−/− mice, an erythropoietic deficit in adult f/f mice is revealed under conditions of rapid expansion of the erythropoietic system.39,40 Of interest, mice expressing a glucocorticoid receptor defective for transactivation were recently found to be deficient in the erythropoietic response to hypoxic stress, despite normal steady-state hematocrit.41Cooperation between the glucocorticoid receptor and Stat5 is required for optimal induction of milk proteins.42 A similar cooperation between the glucocorticoid receptor and Stat5 may occur during stress erythropoiesis.
Stress erythropoiesis occurs in the mouse spleen. We found that anemic Stat5a−/−5b−/− mice had increased spleen size due to expansion of erythropoietic tissue. Spleen enlargement correlated with the severity of anemia, suggesting it is largely a compensatory response. However, because erythropoiesis is ineffective, anemia does not resolve and the enlarged spleen persists. This interpretation was supported by the raised Epo levels in these mice, in part responsible for driving the compensatory expansion of erythropoietic tissue. Flow cytometry analysis showed that the spleen erythroid expansion is largely a result of 100- to 200-fold increase in the absolute size of the less differentiated proerythroblast and early erythroblast pools (Ter119med/high CD71high). Apoptosis of the Stat5a−/−5b−/− early erythroblasts, however, impedes their further differentiation. The anemia of Stat5a−/−5b−/− mice, therefore, resembles other anemias caused by ineffective erythropoiesis, such as megaloblastic anemia and myelodysplastic syndromes, characterized by expanded erythropoietic tissue and persisting, partially compensated anemia.21
Enlarged spleens in Stat5a−/−5b−/− mice were previously interpreted as a myeloproliferative disorder of the erythroid lineage, secondary to T-cell autoimmunity.43However, the hematocrit of affected mice was not reported. No autoreactive antibodies were found, and no evidence for autoreactive T cells was provided. An autoimmune etiology for ineffective erythropoiesis in Stat5a−/− 5b−/− mice is unlikely, because reduced survival of Stat5a−/−5b−/− early erythroblasts results from an intrinsic reduction in their expression of the bcl-xL protein. Further, anemia as well as the early erythroblast defect are seen in the Stat5a−/−5b−/− neonate, when autoimmunity is unlikely.
Our findings identify the early erythroblast (CD71highTer119high) as a principal target of EpoR and Stat5 signaling. EpoR is first expressed by very early erythroid progenitors (erythroid burst-forming unit), and its expression is down-regulated following the late basophilic erythroblast stage.44,45 This suggests it may potentially act during multiple stages of erythroid differentiation. The principal target cell thought to be regulated by Epo is the erythroid colony-forming unit (CFU-E), a cell giving rise to differentiated red cells within 5 to 6 generations.46,47 The EpoR−/− mouse, which lacks red cells, contains normal numbers of fetal liver CFU-E cells.1 This suggests that Epo regulates an essential function either at, or subsequent to, the CFU-E stage. Landschulz and coworkers48 showed in vitro that CFU-E cells giving rise to 5 generations, required Epo for survival during the S phase of the second generation. This is consistent with our finding here in vivo, that it is the early erythroblast, a cell 2 generations subsequent to CFU-E cells, that is primarily the target of the survival function of Stat5. These findings also suggest that other potential syndromes of ineffective erythropoiesis, if due to deficits in EpoR signaling, may manifest as apoptosis in early erythroblasts. This contrasts with ineffective erythropoiesis syndromes secondary to abnormalities in hemoglobin synthesis, which may be expected to manifest as apoptosis in late erythroblasts.30
The mechanisms allowing EpoR to modulate erythropoietic rate in response to stress are largely unknown. A long-standing hypothesis suggests that erythropoietic rate may depend on the fraction of erythroid progenitors rescued from apoptosis by EpoR.23,33,47 The close correlation between early erythroblast survival and erythropoietic rate in Stat5a−/−5b−/− are consistent with this hypothesis. Further, the quantitative deficit in erythropoietic rate seen in Stat5a−/−5b−/− mice suggests that additional antiapoptotic pathways may emanate from EpoR. Analysis of EpoR signaling in vitro indicates that multiple EpoR pathways support similar red cell differentiation and survival functions, including pathways downstream of phosphoinositide-3 kinase.49-51These multiple signals may represent the molecular mechanism through which erythropoietic reserve capacity is recruited during stress. Presumably, higher Epo levels lead to recruitment of multiple antiapoptotic pathways additively or synergistically, rescuing maximal numbers of progenitors from apoptosis and supporting a high erythropoietic rate.
The work presented here suggests that in vivo analysis of EpoR signaling may shed light on the homeostatic mechanisms regulating stress erythropoiesis, as well as on possible deficits in syndromes of disrupted red blood cell production.
We thank Drs Stefan Constantinescu and Lily Huang for helpful discussion, Glen Pardis for help with flow cytometry, Jeanne Reis for technical help and Dr J. N. Ihle (St Jude Children's Research Hospital, Memphis, TN) for permission to use the Stat5a−/−5b−/− mice.
Supported by grant HL 32262 from the National Institutes of Health and by a grant from Amgen (H.F.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Harvey F. Lodish, Whitehead Institute for Biomedical Research, 9 Cambridge Ctr, Cambridge, MA 02142; e-mail:lodish@wi.mit.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal