This is an update of a randomized study comparing low-dose intravenous Cyclsporin A (CyA) (1 mg/kg/day) with high dose CyA (5 mg/kg/day).1 The results in 1991 suggested that disease-free survival was superior in patients receiving low-dose CyA, and this was mainly due to protection against leukemia relapse (Table 1). In that study 81 patients with acute leukemia (acute myeloid leukemia = 44; acute lymphocytic leukemia = 37; first complete remission [CR] = 53; >first CR = 28) were randomized to receive cyclosporin (CyA) 1 mg/kg intravenously (IV) or 5 mg/kg from day −1 to day +20. It is important to note that the average CyA serum levels were significantly different in the 2 arms only from day −1 to day +10 (295 ng/mL vs 686 ng/mL; P = .004), but not between day +11 and +20 (465 ng/mL vs 650 ng/mL, P = .1): this was due to the fact that patients in the CyA–1-mg arm had their dose increased beyond day +10 because of acute graft-versus-host diesase (GvHD) and patients in the CyA–5-mg arm had their dose decreased due to toxicity.
Comparative results in 1991 and in 2001
| . | Data analysis 1991 . | P . | Data analysis 2001 . | P . | ||
|---|---|---|---|---|---|---|
| Cyclosporin dose . | Cyclosporin dose . | |||||
| 1 mg . | 5 mg . | 1 mg . | 5 mg . | |||
| No. of patients randomized | 41 | 40 | 41 | 40 | ||
| Relapsed (%) | 6 (15) | 15 (38) | .01 | 6 (15) | 17 (43) | .005 |
| Alive (%) | 24 (59) | 15 (37) | .05 | 20 (49) | 11 (27) | .04 |
| Causes of death | ||||||
| Relapse (%) | 6 (15) | 15 (37) | .01 | 6 (15) | 17 (43) | .005 |
| TRM (%) | 1 (27) | 10 (25) | .5 | 15 (37) | 12 (30) | .3 |
| Median FU (y) | 2.7 | 1.7 | 13.1 | 12.1 | ||
| Range (y) | (0.9-3.8) | (0.7-3.6) | (11.4-14.2) | (11.0-14.0) | ||
| . | Data analysis 1991 . | P . | Data analysis 2001 . | P . | ||
|---|---|---|---|---|---|---|
| Cyclosporin dose . | Cyclosporin dose . | |||||
| 1 mg . | 5 mg . | 1 mg . | 5 mg . | |||
| No. of patients randomized | 41 | 40 | 41 | 40 | ||
| Relapsed (%) | 6 (15) | 15 (38) | .01 | 6 (15) | 17 (43) | .005 |
| Alive (%) | 24 (59) | 15 (37) | .05 | 20 (49) | 11 (27) | .04 |
| Causes of death | ||||||
| Relapse (%) | 6 (15) | 15 (37) | .01 | 6 (15) | 17 (43) | .005 |
| TRM (%) | 1 (27) | 10 (25) | .5 | 15 (37) | 12 (30) | .3 |
| Median FU (y) | 2.7 | 1.7 | 13.1 | 12.1 | ||
| Range (y) | (0.9-3.8) | (0.7-3.6) | (11.4-14.2) | (11.0-14.0) | ||
FU indicates follow-up in years; TRM, transplantation-related mortality; GvHD, graft-versus-host disease.
Updated follow-up.
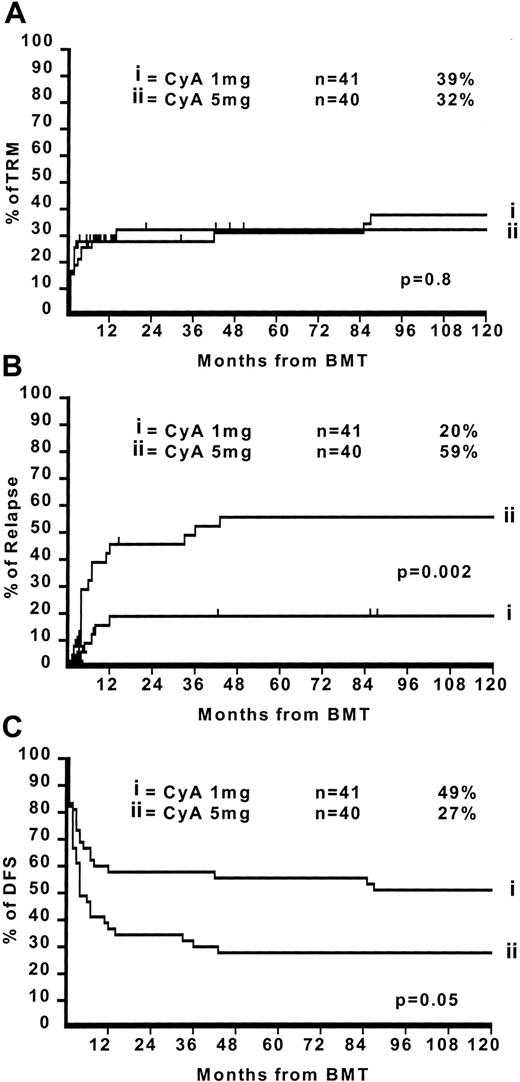
The median follow-up for surviving patients is now 11.7 years with a minimum follow-up of 10 years (range 10.2-13 years). There have been 8 additional deaths, 4 in both arms: in the CyA–1-mg arm they were all caused by transplantation-related complications (Table 1). This brings the crude transplantation-related mortality (TRM) in the CyA–1-mg arm from 27% to 38%. In the CyA–5-mg arm, the 4 additional deaths were caused by leukemia relapse in 2 patients and by transplantation complications in 2 patients. This brings the crude TRM in the CyA–5-mg arm from 25% to 30% and the relapse from 38% to 43%. Figure1 outlines the actuarial 10-year TRM (Figure 1A), relapse (Figure 1B), and disease-free survival (Figure 1C) in the CyA–1-mg/kg vs CyA–5-mg/kg, respectively: TRM 39% vs 32% (P = .8), relapse risk 20% vs 59% (P = .002), and disease-free survival 49% vs 27% (P = .05). For patients in first CR the figures are as follows: TRM 28% vs 21% (P = .3), relapse risk 10% vs 45% (P = .01), and disease-free survival 56% vs 44% (P = .3). For patients with advanced disease (beyond first CR) the figures are TRM 46% vs 60% (P = .6), relapse risk 43% vs 100% (P = .03), and disease-free survival 36% vs 0% (P = .05).
The 10-year TRM, relapse, and disease-free survival in the CyA–1-mg/kg vs CyA–5-mg/kg.
The 10-year TRM, relapse, and disease-free survival in the CyA–1-mg/kg vs CyA–5-mg/kg.
The effect of patient age.
TRM is significantly affected by patients age: 22% for patients aged 1 to 20 years, 21% for patients aged 21 to 30 years, and 71% for patients older than 31 (P = .02). There is no difference in TRM for patients receiving CyA 1 mg or CyA 5 mg in the 1 to 20 age group (27% vs 28%) or in the 21 to 30 age group (18% vs 24%) but this is not so in the group of patients older than 30 (89% vs 46%) (P = .02). As a consequence, the disease-free survival is superior for the CyA–1-mg/kg arm in patients younger than 30 years (60% vs 27%,P = .05) but not in patients older than 30 years (11% vs 27%, P = .8).
Conclusions.
We confirm in this updated analysis, with a minimum follow-up of 10 years, that low-dose (1 mg/kg) CyA and low CyA serum levels in the first 10 days after an allogeneic BMT, confers significant protection against leukemia relapse both in patients with early or advanced leukemia. This has been confirmed in a randomized trial performed in children, comparing CyA 1 mg/kg vs CyA 3 mg/kg.2 However, there is an increased risk of transplantation-related complications in the low-dose CyA arm, especially in patients older than 30 years. Therefore the increased antileukemic effect of GvHD is well tolerated by young patients but less so by older patients.
This work was supported by Associazione Italiana Ricerca contro il Cancro (A.I.R.C.) Milano grant to AB and Associazione Ricerca Trapianto Midollo Osseo (A.RI.T.M.O.) Genova. The great work of our nursing staff is gratefully acknowledged.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal