Abstract
Recently, it has been postulated that the beneficial effect of intravenous immunoglobulins (IVIGs) in antibody-mediated autoimmune disorders is based on accelerated catabolism of autoantibodies. In the current study, in vivo experiments were performed with mice in which autoantibody production was mimicked by continuous infusion of monoclonal antibodies. In this model, a single dose of IVIG reduced the plasma concentrations of the infused immunoglobulin (Ig)G1 monoclonal antibody (mAb) by approximately 40% after 3 days, whereas the concentration of an IgA mAb was not affected. To extrapolate these findings to humans, a computational model for IgG clearance was established that accurately predicted the time course and magnitude of the decrease in IgG plasma levels observed in mice. Adapted for humans, this model predicted a gradually occurring decrease in autoantibody levels after IVIG administration (2 g/kg), with a maximum reduction of approximately 25% after 3 to 4 weeks and a continued decrease of several months. In conclusion, a single high dose of IVIG induces a relatively small but long-lasting reduction of autoantibody levels by accelerated IgG clearance. This mechanism has clinical relevance in the sense that it can fully explain, as the sole mechanism, the gradual decrease in autoantibody levels observed in several patient studies. However, in some clinical studies, larger or more rapid effects have been observed that cannot be explained by accelerated clearance. Hence, IVIG can also reduce autoantibody levels through mechanisms such as down-regulation of antibody production or neutralization by anti-idiotypic antibodies.
Introduction
Immunoglobulin preparations, originally developed for the treatment of patients with agammaglobulinemia, have also been successfully applied in a number of inflammatory and autoimmune diseases, such as immune thrombocytopenic purpura, Kawasaki disease, and Guillain-Barré syndrome. Several mechanisms of action of intravenous immune globulin (IVIG) therapy in the latter disorders have been proposed—blocking of Fc receptors on phagocytes, inhibition of complement deposition, modulation of cytokine production, neutralization of circulating autoantibodies by anti-idiotypic antibodies, and down-regulation of autoantibody production by anti-idiotypic antibodies interacting with B cells.1-3 No conclusive evidence exists, however, for any of these mechanisms, and other modes of action are still being proposed. In recent years, several investigators have suggested that the therapeutic effect of IVIG may be caused by an effect on immunoglobulin (Ig)G catabolism, leading to a reduction of autoantibody titers.4 5
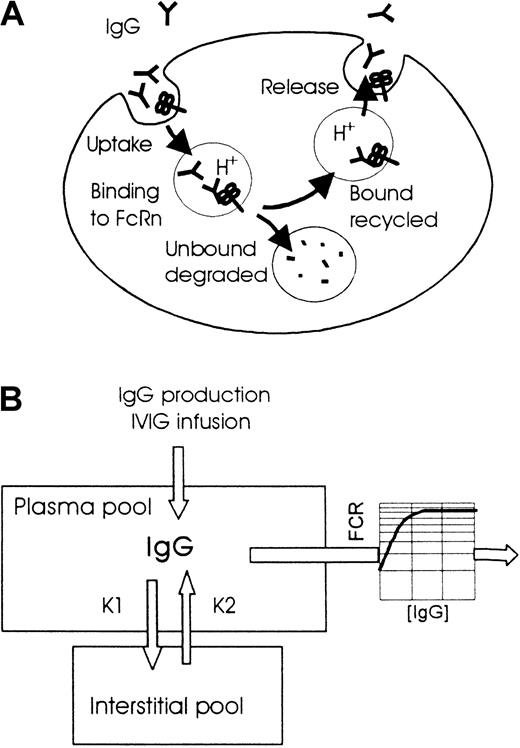
More than 30 years ago, it was found in several species that the clearance rate of IgG greatly depends on its plasma concentration.6 At low concentrations, the plasma half-life is approximately 10 times longer than at high concentrations. Brambell et al7 proposed in 1964 a hypothesis to explain this phenomenon, stating that IgG is endocytosed in an aspecific way. Part of this endocytosed IgG then binds to receptors in the wall of the endocytotic vesicles to be protected from degradation and is returned to the circulation (Figure1). In this model, the protecting receptors become saturated at high plasma concentration, resulting in the degradation of a larger proportion of the endocytosed IgG. Recently, experiments by several investigators with β2-microglobulin knock-out mice provided solid support for the existence of such a protecting receptor.8-10 Because β2-microglobulin is part of the neonatal Fc receptor (FcRn), these mice lacked functional FcRn. It was observed that the knockout mice had low IgG levels in combination with a normal synthesis rate and a shortened plasma half-life, whereas IgM and IgA levels were unaffected. These findings indicated that, in addition to a role in the transfer of maternal IgG across the rodent neonatal gut, the fetal yolk sac, and the human placental barrier, FcRn plays a role in IgG catabolism and probably is the protecting receptor in the Brambell7 model.
Models of the role of FcRn in the degradation of IgG and of the 2-compartment pharmacokinetics used for the simulations.
(A) IgG is taken up by aspecific pinocytosis into endosomes. At decreasing pH (approximately pH 6), IgG binds to FcRn in the wall of the endosomes, after which the IgG-FcRn complexes are recycled to the cell surface, where IgG is released because of higher pH. IgG not bound to FcRn is delivered to lysosomes and degraded. At higher IgG concentrations FcRn will become saturated, resulting in a smaller proportion of the endocytosed IgG rescued by FcRn from degradation in the lysosomes. This results in a shorter plasma half-life (or higher fractional clearance rate) with a minimum value determined by the pinocytosis rate when all FcRn is saturated. (B) Elimination from the plasma compartment, consisting of cellular uptake with saturable return as depicted above, is a nonlinear process described by a concentration-dependent FCR.
Models of the role of FcRn in the degradation of IgG and of the 2-compartment pharmacokinetics used for the simulations.
(A) IgG is taken up by aspecific pinocytosis into endosomes. At decreasing pH (approximately pH 6), IgG binds to FcRn in the wall of the endosomes, after which the IgG-FcRn complexes are recycled to the cell surface, where IgG is released because of higher pH. IgG not bound to FcRn is delivered to lysosomes and degraded. At higher IgG concentrations FcRn will become saturated, resulting in a smaller proportion of the endocytosed IgG rescued by FcRn from degradation in the lysosomes. This results in a shorter plasma half-life (or higher fractional clearance rate) with a minimum value determined by the pinocytosis rate when all FcRn is saturated. (B) Elimination from the plasma compartment, consisting of cellular uptake with saturable return as depicted above, is a nonlinear process described by a concentration-dependent FCR.
The identification of FcRn as a protecting receptor has renewed interest in the mechanism of IgG clearance and, as mentioned above, has led to the hypothesis that the effect of high-dose IVIG in autoantibody-mediated disorders is based on the saturation of FcRn, leading to increased catabolism of IgG, including that of autoantibodies.4 5 However, without knowing the extent of reduction of autoantibody levels affected by this mechanism, it is difficult to decide whether this mechanism has any clinical significance in relation to the overall therapeutic effects of IVIG therapy.
The aim of the current study was to determine the time course and the magnitude of the decrease of autoantibody levels by IVIG therapy. This was done first by studying the IVIG effect on immunoglobulin plasma concentrations in a mouse model, in which autoantibody production was simulated by a continuous infusion of monoclonal antibodies (mAbs). Next, we established a computational model for IgG pharmacokinetics using data from the literature on the concentration dependency of IgG clearance in mice and humans. The model was validated by comparing simulations with in vivo data from mouse experiments and was used to predict the effects in humans. By comparing our results with those from clinical studies, we concluded that accelerated clearance can explain some, but not all, clinical observations on the reduction of autoantibody levels.
Materials and methods
In vivo clearance studies in mice
In the first series, we determined the effect of high-dose IVIG on endogenous IgG1, IgG2a, IgG3, IgM, and albumin plasma concentrations. Experiments were performed in C57BL/6 mice, with body weights between 25 and 35 g (Harlan CPB, Zeist, The Netherlands). Mice were anesthetized with halothane for intravenous injections and blood sampling. IVIG was injected into the tail vein at a volume of 30 mL/kg in approximately 1 minute, resulting in a dose of 1.8 g/kg body weight IgG. Control mice received an equivalent volume of saline. Fifty-microliter blood samples were taken 5 minutes before, 5 minutes after, and 5, 24, 48, and 72 hours after injection. Blood samples were collected from the orbital plexus using heparinized capillary tubes. Plasma was separated by centrifugation and stored at −20°C.
In the second series, we created a steady-state plasma level of specific mouse IgG1 and IgA mAbs (without affinity for mouse antigens) by continuous infusion and determined the effect of subsequent IVIG administration on their plasma concentrations as described above. Monoclonal antibodies were continuously infused using an osmotic pump implanted in the peritoneal cavity (Alzet micro-osmotic pump, model 1002; Alza, Palo Alto, CA). Pumps with a pumping rate of 0.24 μL/h for a duration of 14 days were filled with 100 μL mixture containing 0.3 mg/mL mouse IgG1 mAb to human C1 inhibitor and approximately 0.5 mg/mL mouse IgA mAb (in saline) to human interleukin-6 (IL-6). An intravenous bolus dose of 35 μL infusate per mouse was given at the beginning of infusion to obtain a steady-state plasma concentration more quickly.
IVIG was intravenously administered at day 4 as a bolus dose of 1.8 g/kg body weight IgG. Animal experiments were approved by the local ethics committee and were governed by the pertinent national legislation.
Immunoglobulin preparations
IVIG, a solution of purified human IgG (60 mg/mL), was prepared from pooled human donor plasma at the Center Laboratory of the Blood Transfusion Service (CLB) (Immunoglobuline I.V.; CLB, Amsterdam, The Netherlands). Mouse IgG1 mAb to human C1 inhibitor (RII) was affinity purified using protein G-Sepharose. A mouse IgA mAb to human IL-6 (mAb 8-α) was purified from culture supernatant using size exclusion chromatography to isolate monomeric IgA. Both monoclonal antibodies have been characterized and produced in our department.
Assays for immunoglobulin (sub)classes and albumin in plasma
Mouse IgG1, IgG2a, IgG3, and IgM plasma concentrations were measured using enzyme-linked immunosorbent assay (ELISA). Capturing rat monoclonal antibodies to mouse IgG1, IgG2a, IgG3, or IgM (LO-MG1, LO-MG2a, LO-MG3, and LO-MM; Caltag, Burlingame, CA) were coated to 96-well Nunc Maxisorp plates (Nunc Brand Products, Roskilde, Denmark) by overnight incubation at room temperature at a concentration of 1 to 2 μg/mL in 0.1 M carbonate–bicarbonate, pH 9.6. Plates were washed twice in phosphate-buffered saline (PBS)–0.02% (wt/vol) Tween 20 (PBS-Tween). Plasma samples were appropriately diluted in PBS containing 2% (vol/vol) cow milk. One hundred microliters of each dilution was incubated for 1 hour at 4°C, and plates were gently shaken. Plates were washed 5 times in PBS-Tween and were incubated with biotinylated rat monoclonal anti–mouse κ light chain (226-BT; CLB) as the detecting antibody, diluted 1:2000 in PBS containing 2% (vol/vol) cow milk, for 1 hour at room temperature. Plates were then washed 5 times in PBS-Tween and incubated with streptavidin–horseradish peroxidase (HRP; Amersham Life Science, Buckinghamshire, United Kingdom), diluted 1:1000 in PBS containing 2% cow milk, for 30 minutes at room temperature. Finally, the plates were developed with 3,3', 5,5'-tetramethylbenzidine (0.1 mg/mL in 0.11 M sodium acetate, pH 5.5, 0.003% H2O2), and the reaction was stopped by the addition of H2SO4. Absorbance was measured at 450 nm. Concentrations were expressed as percentages of those in normal mouse serum (CLB).
Total mouse IgG concentrations in plasma were measured in a similar ELISA with monoclonal anti–mouse κ light chain 226 as the capturing antibody and biotinylated anti–mouse κ light chain (226-BT) as the detecting antibody, using purified mouse IgG (Sigma, St Louis, MO) as reference.
Plasma concentrations of mouse IgG1 mAb to human C1 inhibitor (RII) was measured in an ELISA with purified human C1 inhibitor (CLB) as the capturing protein. Biotinylated rat mAb to mouse IgG1 (Caltag) was used as conjugate; this was followed by incubation with streptavidin–poly-HRP (CLB). Plasma concentrations of mouse IgA mAb to human IL-6 (mAb 8-α) was measured in a similar ELISA with recombinant human IL-6 (CLB) as the capturing protein. Biotinylated rat mAb to mouse IgA (Caltag) was used as conjugate. Plasma concentrations of both mAbs were expressed as percentage of concentration in the infused antibody mix.
All above-mentioned ELISAs for mouse immunoglobulins were unaffected by the presence of human IVIG in the samples, ruling out the presence of anti-idiotypic antibodies to the RII and mAb 8-α in IVIG. Human IgG concentrations in mouse plasma were determined in an ELISA with mouse mAb anti–human IgG (MoHu16; CLB) as the capturing antibody and a phosphatase-labeled mouse mAb anti–human IgG (GG-5-AP; Sigma) for detection. p-Nitrophenyl phosphate was used as substrate. IVIG was used as reference. Plasma albumin was quantified using a colorimetric assay based on reaction with bromcresol purple (Sigma).
Computational model
Figure 1B shows the 2-compartment pharmacokinetic model adopted for the simulation. The following assumptions were made: (1) produced and infused IgG are immediately mixed in the plasma compartment and redistributed by approximately 50% into the interstitial space11; (2) the plasma volume is 40 mL/kg body weight; (3) the exchange between the plasma and the interstitial pool is a linear process with rate constants (k1 = k2) of −0.087 for mice and −0.014 for humans; and (4) the elimination of IgG occurs from the plasma compartment according to a nonlinear process, with rate constants depending on the plasma concentration. For mice, the relation between plasma IgG concentration and fractional clearance rate (FCR) was derived from data published by several investigators who measured the disappearance of tracer doses of radiolabeled IgG in mice with IgG plasma concentrations ranging from 0.12 mg/mL to 50 mg/mL.12-15 Notably, these studies include experiments in germ-free and low-pathogen mice with low IgG plasma concentrations and total body half-lives for IgG up to 9 days and in mice with plasma cell tumors or receiving intraperitoneal injections of human IVIG and with high IgG plasma concentrations and half-lives as brief as 1.5 days. Figure 2 shows the sigmoid curve fitted to the data points: FCRivp, mouse = 0.055 + 0.79/{1 + e[(8.9 − [IgG]pl)/5.9]}. IgG plasma concentration ([IgG]pl) is expressed in milligrams per milliliter mg/mL. FCRivp is the fraction of the intravascular pool eliminated in 24 hours; it relates to the FCR of the total body pool as FCRivP = FCRtbp/(fraction of IgG intravascular). FCRtbP = ln2/t½, wheret½ is the total body half-life, or elimination half-life, in days.
Relation between the IgG plasma concentration and FRC for mice (upper curve) and humans (lower curve) used in the corresponding computational models.
Both curves were fitted to data published by Humphrey and Fahey,12 Fahey and Robinson,13 Sell and Fahey,14 and Junghans and Anderson15 for mice and by Waldmann and Strober6 for humans.
Relation between the IgG plasma concentration and FRC for mice (upper curve) and humans (lower curve) used in the corresponding computational models.
Both curves were fitted to data published by Humphrey and Fahey,12 Fahey and Robinson,13 Sell and Fahey,14 and Junghans and Anderson15 for mice and by Waldmann and Strober6 for humans.
For humans, the relation between IgG plasma concentration and FCRivp was derived from data published by Waldmann and Strober,6 who reviewed several studies in humans with widely varying IgG plasma concentrations, including patients with hypogammaglobulinemia and myeloma, in whom IgG catabolism was determined by measuring the disappearance of radiolabeled IgG from plasma. Figure 2 shows the sigmoid curve fitted to the data points: FCRivp,human = −0.3 + 0.43/{1 + e[(−19 − [IgG]pl)/18]}. For humans, the elimination half-life increases to values between 30 and 70 days at very low IgG plasma concentrations, whereas at concentrations greater than 30 mg/mL, the half-life reaches a lower limit of approximately 11 days. For the simulations, all IgG transfers and subsequent concentration changes were calculated in discrete time steps using an Excel (Microsoft) worksheet. For mice and humans the time steps were 0.1 and 1 hour, respectively. The fate of endogenous and infused IgG was followed separately, and the sum of both determined the FCR. For each time step, a fraction from the plasma pool according to k1 and the duration of the time interval was transferred to the interstitial pool, and a fraction from the interstitial pool according to k2 was transferred to the plasma pool. Furthermore, the FCR at the current IgG concentration was calculated, and a fraction of the plasma pool according to that FCR and the duration of the time interval were eliminated. IgG production rates, expressed as mg/kg−1 per interval, were chosen in accordance with the desired plasma concentration. IVIG was ‘infused’ as a bolus within a single time interval.
Statistical analysis
In vivo data are presented as mean ± SD. Results were compared with an unpaired or a paired Student ttest, as indicated, using GraphPad Prism (GraphPad Software).
Results
In vivo experiments in mice: effect of IVIG on endogenous immunoglobulin concentrations
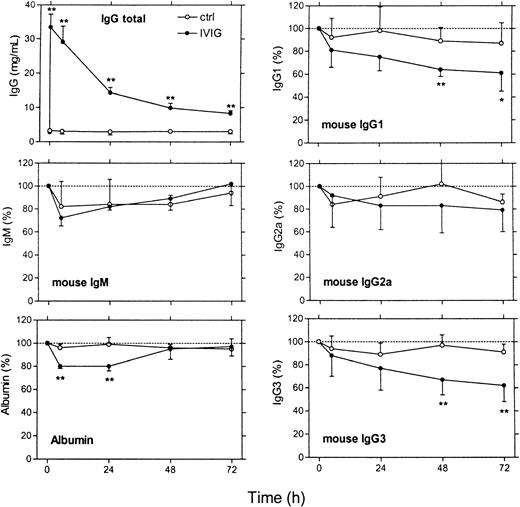
Administration of 1.8 g/kg IVIG to mice resulted in an increase of the total IgG plasma concentration (human plus mouse IgG) from approximately 3 to 33 mg/mL (Figure 3). Plasma concentrations of mouse IgG1 and IgG3 showed a gradual decrease to approximately 60% of baseline after 3 days (Figure 3). For IgG2a, the decrease was not significant because of a large standard deviation in the results. IgM and albumin concentrations showed a transient decrease after IVIG administration but returned to normal values after 3 days. Decreased albumin concentration indicated that transient plasma dilution occurred in the IVIG-treated mice, which could, at least partly, have accounted for the decreased IgG concentrations in the first 24 hours. This dilution effect was not significant in the control mice receiving saline, which has no oncotic effect. After 48 hours, albumin concentrations were back to baseline, indicating that the 40% decrease in endogenous IgG1 and IgG3 concentrations, 3 days after IVIG, was unrelated to dilution (Figure 3).
In vivo effects of IVIG infusion on endogenous immunoglobulin levels in mice.
IVIG was given at a dose of 1.8 g/kg body weight (black dots). Control animals (open circles) received an equivalent volume of saline. The 6 panels show total IgG plasma concentration (in mg/mL), endogenous levels of mouse IgG1, IgG2a, IgG3, and IgM, and albumin (expressed as a percentage of the pre-infusion levels), respectively. The time scale is indicated below the lower 2 panels. Data represent mean ± SD (n = 4). **P < .01 and *P < .05 for difference IVIG and control group (t test).
In vivo effects of IVIG infusion on endogenous immunoglobulin levels in mice.
IVIG was given at a dose of 1.8 g/kg body weight (black dots). Control animals (open circles) received an equivalent volume of saline. The 6 panels show total IgG plasma concentration (in mg/mL), endogenous levels of mouse IgG1, IgG2a, IgG3, and IgM, and albumin (expressed as a percentage of the pre-infusion levels), respectively. The time scale is indicated below the lower 2 panels. Data represent mean ± SD (n = 4). **P < .01 and *P < .05 for difference IVIG and control group (t test).
In vivo experiments in mice: effect of IVIG on plasma levels of infused monoclonal antibodies
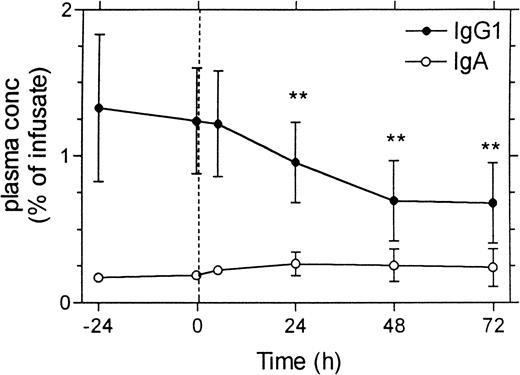
To obtain an experimental model that mimics production of autoantibodies in vivo, we continuously infused a mixture of IgG1 and IgA mAbs in mice by means of an implanted osmotic pump. We choose mAbs without affinity for mouse antigens to avoid binding to epitopes in vivo, which could make interpretation of the results difficult. An intravenous bolus dose (35 μL), followed by continuous infusion of the mAbs at a rate of 0.24 μL/hour, resulted after 3 days in steady-state plasma concentrations for IgG1 and IgA of 1.2% and 0.2%, respectively, of the concentration in the infusate. This difference in relative concentration is compatible with the difference in plasma half-life, which is reported to be much shorter for IgA.8Figure 4 shows the effect of a single intravenous dose of 1.8 g/kg IVIG on the plasma levels of the mAbs, 4 days after the start of the infusion. The plasma concentration of the IgG1 mAb decreased by approximately 40%, whereas, as expected, the concentration of the simultaneously infused IgA mAb remained unchanged.
In vivo effects in mice of IVIG infusion on plasma levels of monoclonal antibodies that were continuously infused at a fixed rate.
A mixture of a mouse monoclonal IgG1 antibody to human C1 inhibitor (RII) and a mouse monoclonal IgA antibody to human IL-6 was continuously infused at a rate of 0.25 μL/h using an implanted osmotic pump. Infusion was started 4 days before IVIG administration, and at the beginning of the infusion an intravenous bolus dose of 35 μL per mouse was given to obtain a steady state plasma concentration more quickly. IVIG was given at a dose of 1.8 g/kg body weight at time zero. Plasma concentrations of the monoclonal antibodies are expressed as a percentage of their concentration in the infusate. Data represent mean ± SD (n = 6). **P < .01 compared with values at time zero (paired t test).
In vivo effects in mice of IVIG infusion on plasma levels of monoclonal antibodies that were continuously infused at a fixed rate.
A mixture of a mouse monoclonal IgG1 antibody to human C1 inhibitor (RII) and a mouse monoclonal IgA antibody to human IL-6 was continuously infused at a rate of 0.25 μL/h using an implanted osmotic pump. Infusion was started 4 days before IVIG administration, and at the beginning of the infusion an intravenous bolus dose of 35 μL per mouse was given to obtain a steady state plasma concentration more quickly. IVIG was given at a dose of 1.8 g/kg body weight at time zero. Plasma concentrations of the monoclonal antibodies are expressed as a percentage of their concentration in the infusate. Data represent mean ± SD (n = 6). **P < .01 compared with values at time zero (paired t test).
Control simulations
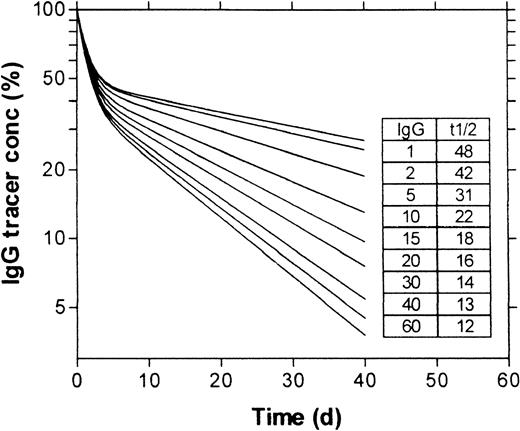
To check whether the method of calculation was accurate, we first simulated for mice and humans the clearance of tracer doses of IgG at different endogenous IgG plasma concentrations (ranging from 1 to 60 mg/mL). Figure 5 shows the curves generated for humans. The t½ calculated from terminal parts of the double exponential curves ranged from approximately 50 days at an IgG concentration of 1 mg/mL to approximately 11 days at concentrations greater than 60 mg/mL, exactly as was expected from the relation between FCR and IgG plasma concentration used in this model.6 Furthermore, the intercepts of the terminal parts of the curves with the y-axis (time 0) were at plasma concentrations between 40% and 50% of the initial value after administration, indicating the expected redistribution of 50% to 60% of the dose into the interstitial space. For mice, the terminal parts of the double exponential curves (not shown) also showed the expected t½, ranging from 7 days at an IgG concentration of 1 mg/mL to 1.8 days at concentrations greater than 30 mg/mL.
Simulation of the clearance of intravenously injected tracer doses of IgG at different IgG plasma concentrations in humans.
Simulation concerns the administration of IgG at time zero, at a dose that did not increase the plasma IgG concentration. Plasma concentration of tracer IgG is expressed as a percentage of the concentration immediately after administration. The inset table shows the basal IgG plasma concentrations in milligrams per milliliter (IgG) used in the simulations shown and the half-lives in days (t½) of the tracer IgG, calculated from the terminal parts of the generated curves.
Simulation of the clearance of intravenously injected tracer doses of IgG at different IgG plasma concentrations in humans.
Simulation concerns the administration of IgG at time zero, at a dose that did not increase the plasma IgG concentration. Plasma concentration of tracer IgG is expressed as a percentage of the concentration immediately after administration. The inset table shows the basal IgG plasma concentrations in milligrams per milliliter (IgG) used in the simulations shown and the half-lives in days (t½) of the tracer IgG, calculated from the terminal parts of the generated curves.
Simulation of the IVIG effect in mice
Figure 6 shows the simulated effect of intravenous administration of a single dose of IVIG on endogenous immunoglobulin concentrations in mice. In this simulation, the IgG production was set at 50 mg/kg per day, giving a basal IgG plasma concentration of 4.2 mg/mL, which is a normal concentration for laboratory mice.14 Administration of a single dose of 1.8 g/kg IVIG caused an increase in total IgG plasma concentration to 50 mg/mL, followed by a biphasic decline. The FCR increased more than 2-fold for several days, resulting in a gradual decrease in endogenous IgG concentration over several days and reaching a minimum after 3 to 4 days at approximately 65% of control, which corresponded well to the in vivo effects observed in mice (Figures 3, 4).
Simulation of the effect of IVIG infusion (1.8 g/kg body weight) in mice.
The figure shows the effect on the total IgG (endogenous plus exogenous) plasma concentration (lower curve), the FRC (percentage of intravascular pool per day, fine line) and the endogenous IgG concentration (percentage of control, upper bold line).
Simulation of the effect of IVIG infusion (1.8 g/kg body weight) in mice.
The figure shows the effect on the total IgG (endogenous plus exogenous) plasma concentration (lower curve), the FRC (percentage of intravascular pool per day, fine line) and the endogenous IgG concentration (percentage of control, upper bold line).
As expected, the magnitude of the effect of IVIG on endogenous IgG depended to some degree on the basal IgG concentration because at higher basal concentrations the FCR was already closer to the maximum value. For example, in simulations with a basal level of 1 mg/mL (production rate, 9 mg/kg per day), a dose of 1.8 g/kg induced a 40% decrease in endogenous IgG, whereas at 10 mg/mL (production rate, 190 mg/kg per day), the decrease was only 20%.
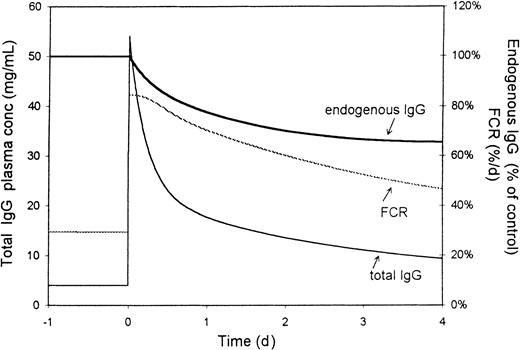
Simulation of the IVIG effect in humans
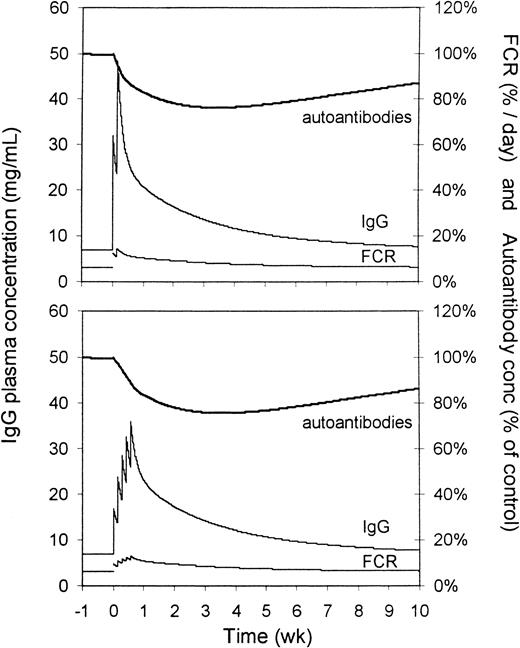
Figure 7 shows the simulated effect of intravenous administration of 2 g/kg IVIG to humans on IgG plasma concentration, FCR, and relative autoantibody levels. In this simulation, the values for k1, k2, and FCR are adapted for humans. Basal IgG production was set at 17 mg/kg per day, giving a basal IgG plasma concentration of 7 mg/mL. Autoantibody production is assumed to remain unchanged by IVIG administration. Administration of 2 daily doses of 1 g/kg IVIG (upper panel) caused a rise in plasma concentration to approximately 40 mg/mL, followed by a biphasic decline. FCR showed a sustained increase for several weeks, with initial doubling of the clearance rate. Autoantibody levels (endogenous IgG concentration) gradually decreased over several weeks, reaching a minimum after 3 to 4 days at approximately 75% of the pre-infusion level. The effect lasted more than 7 weeks. When IVIG was simulated to be given as 5 daily doses of 0.4 g/kg (lower panel), initial IgG concentrations were somewhat lower, but the magnitude of the effect on autoantibody levels was comparable.
Simulation of the effect of IVIG administration to humans on IgG autoantibody levels in the plasma.
For the upper panel, the dose was 2 × 1 g/kg body weight on 2 consecutive days; for the lower panel, it was 5 × 0.4 g/kg body weight on 5 consecutive days. Upper bold lines represent autoantibody levels (percentage of control), middle lines represent the total IgG plasma concentration (mg/mL), and lower lines represent the FCRs (percentage of the intravascular pool per day).
Simulation of the effect of IVIG administration to humans on IgG autoantibody levels in the plasma.
For the upper panel, the dose was 2 × 1 g/kg body weight on 2 consecutive days; for the lower panel, it was 5 × 0.4 g/kg body weight on 5 consecutive days. Upper bold lines represent autoantibody levels (percentage of control), middle lines represent the total IgG plasma concentration (mg/mL), and lower lines represent the FCRs (percentage of the intravascular pool per day).
Discussion
In the in vivo experiments in mice, we first studied the effect of IVIG administration on endogenous immunoglobulin concentrations. We used human IVIG because it already had been observed by other investigators that human IgG has the same clearance characteristics in mice as murine IgG.12,15 After a single high dose of IVIG, we observed a reduction of 40% in endogenous IgG1 and IgG3 levels that occurred gradually and could not be ascribed to plasma dilution after day 2. Only minimal changes were observed in mouse IgG2a, which is in accordance with the findings of Israel et al,9 who investigated subclass differences in the concentration dependency of clearance. To better mimic the condition of a patient producing autoantibodies, we next continuously infused mAbs and investigated the effect of IVIG on their plasma levels. We added an IgA mAb to the infusate as an internal control, because Ghetie et al8 found that FcRn plays no protecting role in its clearance. They also observed that IgA has a similar half-life in both β2-microglobulin knockout mice and wild-type mice—ie, 1 day—whereas the half-life of IgG was 0.8 days in β2-microglobulin knock-out mice and 4 days in wild-type mice. In other words, the clearance rates of IgG1 and IgA are the same after complete elimination of FcRn function. In our experiments, the steady-state plasma concentration of IgA was much lower than that of IgG1, which is in accordance with the reported difference in clearance rates. After a single high dose of IVIG, the IgG1 mAb levels showed a reduction of approximately 40%, whereas, as expected, the IgA levels were unaffected.
Simulations for mice accurately predicted the time course and magnitude of observed in vivo effects, both regarding the endogenous IgG (Figure3) and the infused IgG1 mAb (Figure 4), which supported the validity of our computational model. It should be noted that in our in vivo experiments, plasma levels of endogenously produced IgG were reduced to the same extent as IgG1 mAb infused at a fixed rate. This supports the idea that there is no immunoregulatory feedback on IgG synthesis,16 and it justifies the assumption in our pharmacokinetic model that IgG production is constant for a patient, independent of IgG concentration. A conspicuous finding was the extended time course of the IgG reduction after a single dose of IVIG. Further analysis of our model revealed that the critical element in this respect is the FCR–concentration relation, which was based on data from the literature.6 12-15 Other elements, such as the rate of equilibration between plasma and interstitial pool, had only a minor influence on the simulated effects. The slow kinetics of the effect prevents steady-state conditions from being reached after a single IVIG dose and may explain why the effect is smaller than might be intuitively expected.
Because IgG autoantibodies are expected to have the same clearance behavior as all other plasma IgG, our simulations also predict the effect of IVIG on the level of freely circulating IgG autoantibodies. We do not want to speculate about whether a 25% decrease in autoantibody level could have pathophysiological significance and will limit the discussion to whether the mechanism of accelerated clearance can explain the IVIG-induced reduction in autoantibody titers observed in clinical patients. We compared the predictions from our simulation with clinical data from patient studies. Bain et al17performed a randomized, placebo-controlled crossover trial on IVIG therapy in 9 patients with Lambert-Eaton myasthenic syndrome. The patients received IVIG on 2 consecutive days at a dose of 1 g/kg body weight per day or an equivalent volume of 0.3% albumin. They measured calcium-channel antibodies using an immunoprecipitation assay. Their observations closely corresponded to the effects predicted by our model (Figure 7): after IVIG infusion, total IgG plasma concentrations increased from 7 to approximately 40 g/L and gradually decreased toward normal values over a period of more than 6 weeks. During this period, autoantibody levels gradually decreased by approximately 30% after 3 weeks, remaining below pre-infusion levels for at least 8 weeks. No changes were observed after albumin administration. Notably, in vitro, using the same immunoassay, the investigators found no evidence for anti-idiotype autoantibody neutralization by IVIG, ruling out the possibility that the lower autoantibody levels were caused by neutralization of antibody activity by IVIG. Hence, in these patients, the IVIG-induced decrease in autoantibody titers can be fully explained by accelerated IgG clearance as the sole mechanism. Similar reductions in autoantibody levels have been described by Jayne et al18 after high-dose IVIG treatment of 8 patients with systemic vasculitis. They observed a gradual reduction in antineutrophil cytoplasm autoantibodies (ANCA) by approximately 30% after 5 weeks that lasted for at least 10 weeks. These authors suggest that this reduction is caused by the suppression of ANCA production by ANCA anti-idiotype antibodies in IVIG. However, our simulation reveals that a reduction of this magnitude can entirely be explained by enhanced catabolism.
Because the effects on autoantibody levels in the above-mentioned studies are completely covered by the effect of an accelerated clearance as predicted by our model, the question arises whether other proposed mechanisms of IVIG therapy may also play a role in decreasing autoantibody levels in patients. On the one hand, some observations suggest that this may not be the case; on the other hand, findings from several clinical reports on large and rapid changes in autoantibody levels cannot be explained by accelerated clearance. For example, assuming other mechanisms of reduction in antibody levels, such as neutralization by anti-idiotypes or down-regulation of production, one should expect that IgM autoantibodies would also be reduced by IVIG therapy. Yet, Hammerström et al19 used an autoimmunity model based on SCID mice reconstituted with peripheral blood lymphocytes from a patient with primary biliary cirrhosis, which is associated with anti-M2 autoantibodies. In this model, they observed that mice treated with repeated dosages of IVIG after reconstitution showed markedly lower anti-M2 IgG plasma levels, whereas anti-M2 IgM levels were not influenced. Consistent with this, Dalakas et al20 observed, in a selected series of 11 patients with demyelinating polyneuropathy associated with monoclonal IgM antibodies against myelin-associated glycoprotein and sphingoglycolipids, neither an appreciable change in antibody titers nor a clear clinical benefit after IVIG therapy.
In contrast to the studies discussed above, several clinical observations on rapid reductions in antibody levels favor a role for other mechanisms. Levy et al21 studied the effect of IVIG on autoantibody levels before and after 5-day treatment courses in 3 patients with systemic vasculitis. Unlike an earlier study by Jayne et al,18 they observed erratic changes in anti-myeloperoxidase and anti-PR3 levels (5-fold increases or decreases or no change at all in 2-week intervals). Sultan et al22 observed, in 2 patients with hemophilia, a more than 10-fold reduction in anti-VIIIc activity within 5 days of IVIG therapy. Because IVIG also inhibited anti-VIIIc activity in patient plasma in vitro, they found evidence that the effect of IVIG was based on the presence of anti-idiotypic antibodies. In a prospective study on IVIG treatment of acquired factor VIII autoantibodies, Schwartz et al23 measured inhibitor titers in 8 of 16 assessable patients. In 6 patients the inhibitor completely disappeared after IVIG therapy, and in 3 of them a strong decline already occurred within 4 days. It should be noted that in these studies, factor VIII inhibitor levels were measured using a functional assay (Bethesda units); the results are difficult to translate into free antibody concentrations. Nevertheless, the observed changes seem to be too rapid and too large to be explained by accelerated clearance, which strongly suggests that IVIG may also reduce antibody levels by other mechanisms. A rapid decrease in autoantibodies is compatible with direct neutralization through binding to anti-idiotypic antibodies in IVIG, after which they will escape detection in an assay. Gradually occurring, long-lasting, complete disappearance may point to a down-regulation of antibody production, such as, for example, an effect on B cells that could possibly be affected by Fas-mediated induction of apoptosis,24 interaction of anti-idiotypic antibodies in IVIG with inhibitory FcγRIIb-receptor,25 or in some way through up-regulation of this inhibiting Fcγ receptor on different cell types.26 Because the different proposed mechanisms for IVIG-induced reduction of autoantibody levels do not seem to be mutually exclusive, we conclude that FcRn saturation will contribute as an independent mechanism in all cases of IgG autoantibodies.
In summary, our study shows that IVIG therapy induces a relatively long-lasting, but modest, reduction of autoantibody levels by accelerated IgG clearance. This mechanism has clinical relevance in the sense that it can explain, as the sole mechanism, the gradual 20% to 40% decrease in autoantibody levels observed in several patient studies. However, larger or more rapid effects observed in some other clinical studies cannot be explained by accelerated clearance, suggesting that IVIG can also reduce autoantibody levels through other mechanisms.
We thank Mr Theo Jansen-Hendriks for excellent technical assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wim K. Bleeker, Department of Immunopathology, Central Laboratory of the Blood Transfusion Service, Dept PDH, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail:e_hack@clb.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal