Abstract
Polymerization of hemoglobin S in sickle red cells, in deoxygenated conditions, is associated with K+ loss and cellular dehydration. It was previously reported that deoxygenation of sickle cells increases protein tyrosine kinase (PTK) activity and band 3 tyrosine phosphorylation and that PTK inhibitors reduce cell dehydration. Here, the study investigates which PTKs are involved and the mechanism of their activation. Deoxygenation of sickle cells induced a 2-fold increase in Syk activity, measured by autophosphorylation in immune complex assays, but had no effect on Lyn. Syk was not stimulated by deoxygenation of normal red cells, and stimulation was partly reversible on reoxygenation of sickle cells. Syk activation was independent of the increase in intracellular Ca++ and Mg2+ associated with deoxygenation. Lectins that promote glycophorin or band 3 aggregation did not activate Syk. In parallel to Syk stimulation, deoxygenation of sickle cells, but not of normal red cells, decreased the activity of both membrane-associated protein tyrosine phosphatase (PTPs) and membrane protein thiol content. In vitro pretreatment of Syk immune complexes with membrane PTP inhibited Syk autophosphorylation. It is suggested that Syk activation in vivo could be mediated by PTP inhibition, itself resulting from thiol oxidation, as PTPs are known to be inhibited by oxidants. Altogether these data indicate that Syk could be involved in the mechanisms leading to sickle cell dehydration.
Introduction
In sickle cell anemia red cells (SS cells), polymerization of hemoglobin S in deoxygenated conditions is associated with a marked increase in membrane cation permeability (sickling-induced pathway, or SIP), resulting in K+ loss and cell dehydration. K+ efflux is also mediated by the activation of K(Ca) channels and K/Cl cotransport.1 In a previous study, to characterize the cell signaling events that lead to the dehydration of deoxygenated SS cells, we have shown that deoxygenation increased protein tyrosine kinase (PTK) activity and that PTK inhibitors reduced SS cell dehydration by decreasing the K+ loss either in the presence or absence of Ca++ in the medium without affecting SIP.2These data provide evidence that either one or both K+transporters might be regulated by a pathway implicating a tyrosine phosphorylation. Unlike in SS cells, PTK activity in normal red cells (AA cells) was not affected by deoxygenation.
The purpose of the present study was to determine which PTKs are activated by deoxygenation of SS cells and to investigate the mechanism of their activation. Syk (p72syk) and Lyn (p53/56lyn) are nonreceptor PTKs belonging respectively to the Syk and Src family, expressed in human red cells in substantial amounts3,4 and in almost all hematopoietic cells.5 Syk has been implicated in a variety of hematopoietic cell responses, including immunoreceptor and integrin signaling.5,6 Syk activation through stimulation of immune response receptors results from the initial phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) by one or more Src family kinase leading to Syk engagement through its tandem amino-terminal SH2 domains. This binding results in Syk activation by increasing its autophosphorylating activity. In addition, Syk is activated by oxidative and osmotic stress7,8 or by cross-linking or aggregation of cell-surface glycoproteins by plant lectins, such as wheat germ agglutinin (WGA) or concanavalin A (Con A).9 10
In human red cells, Syk was shown to be responsible for the in vivo tyrosine phosphorylation of the cytoplasmic domain of band 3 (cdb3).3 An increase in band 3 tyrosine phosphorylation, which may reflect Syk activation or phosphotyrosine phosphatase (PTP) inhibition, was observed after vanadate or pervanadate treatment,3,11-13 cell shrinkage induced by ionophore/Ca++ treatment or hypertonic conditions,14,15 or elevation of intracellular Mg2+.12 Deoxygenation also results in an increase in band 3 tyrosine phosphorylation in SS cells2and to a much lower extent in AA cells.12 Evidence was provided recently that, on pervanadate treatment, band 3 is initially phosphorylated by Syk, providing docking sites (phosphotyrosines 8 and 21) for the binding of Lyn SH2 domain and subsequent phosphorylation of additional tyrosine residues (359 and 904).13 However, the mechanism for Syk activation in red cells is unknown, as well as the functional consequences of such an activation, except in skate red cells, in which Syk is involved in the activation of taurine efflux induced by hypotonic volume expansion.16
PTPs normally act to maintain a low level of phosphorylated tyrosines. Human red cells contain several PTPs, cytosolic acid PTP and membrane-associated neutral PTP.17,18 A neutral PTP, related to PTP1B, was found to be associated with and to dephosphorylate band 3 and to be inhibited by vanadate, diamide, and thiol-alkylating agents.19,20 The possibility that PTK activity could be regulated by PTP came from studies with PTP inhibitors, such as H2O2 and other oxidants.21,22 These agents are proposed to interact with a thiol group of a cysteine residue in the PTP reactive center, blocking the formation of a phosphoryl-cysteine intermediate, a critical step in dephosphorylation.22 Exposure of various cells to phenyl arsine oxide, diamide, H2O2, vanadate, or pervanadate induces a rapid tyrosine phosphorylation of proteins, including Src and Syk family PTK.7,23-25 Because H2O2 or vanadate does not induce tyrosine phosphorylation of isolated Lck (a Src kinase), or activation of immunoprecipitated Syk, it was proposed that these agents act on cellular PTPs that would regulate PTK activity.7,25Consistent with this hypothesis, pervanadate treatment in red cells increases Syk autophosphorylation activity.3 13
We report here that deoxygenation of SS cells, but not of AA cells, induces both an activation of Syk and an inhibition of membrane-associated PTP, without altering Lyn activity. Furthermore, the activity of Syk immune complexes from either SS or AA cells was inhibited by a pretreatment with membrane PTP. We suggest that deoxygenation-induced Syk activation in vivo could be mediated by PTP inhibition, itself resulting from an oxidative stress, as reflected by the decrease in membrane thiol content during deoxygenation of SS cells.
Materials and methods
Materials
[γ-32P]ATP was purchased from NEN Life Science (Paris, France). Percoll and protein A-Sepharose 4B were from Amersham Pharmacia Biotech (Saclay, France). Aprotinin (A1153), bovine serum albumin (BSA) (A7030), ConA (L7647), 5,5′-dithiobis-(2-nitrobenzoate) (DTNB) (D8130), ionophore A23187 (C7522), leupeptin (L2884), okadaic acid (O15606), pepstatin A (P4265), phenyl-methyl sulfonyl fluoride (PMSF) (P7626), p-nitrophenyl phosphate disodium salt (pNpp), 104-0 poly (E-Y) 4:1 (P0275), rabbit antimouse (A9044) and goat anti–rabbit immunoglobulin G (IgG) (A9169) linked to horseradish peroxidase, reduced glutathione (GSH) (G4251), and WGA (L9640) were obtained from Sigma-Aldrich-Chimie (St Quentin Fallavier, France). Immobilon P membranes were obtained from Millipore (Bedford, MA), and antiphosphotyrosine (antiPY) mouse monoclonal antibody (4G10) was obtained from UBI (Lake Placid, NY). Rabbit polyclonal anti-lyn (sc-15) and N-terminal anti-syk (NT sc-1077) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell preparation and incubation
After informed consent was obtained, blood was drawn into heparinized tubes either from healthy donors or from patients with sickle cell anemia homozygous for the hemoglobin S. All patients were at distance of crisis and blood transfusion. After centrifugation of SS blood, part of the plasma was removed to bring the suspension to about 40% hematocrit. This suspension was layered and centrifuged onto a discontinuous gradient of Percoll. The SS cell fraction between densities 1.076 and 1.106 (20%-30% reticulocytes and 70%-80% discocytes) was collected, washed 3 times, resuspended in solution A (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM NaH2PO4/Na2HPO4, 10 mM glucose, and 10 mM HEPES/Tris [pH 7.4 at 37°C and 295 mOsm/Kg]), and stored overnight at 4°C. Cells were washed 3 times in solution B (140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose, and 10 mM HEPES/Tris [pH 7.4 at 37°C and 295 mOsm/Kg]) and resuspended at 10% hematocrit. Cells were first preincubated at 37°C under oxygenated conditions for 30 minutes and for a further 15 to 60 minutes under either oxygenated or deoxygenated conditions. In this latter case, cell suspensions were flushed with humidified nitrogen at a flow rate of 8 to 10 mL/min in capped tubes and under continuous magnetic stirring. Under these conditions, the osmolarity of the medium did not change over 60 minutes of incubation. In the experiments designed to clamp or to increase intracellular Mg2+, cells were preincubated for 30 minutes in a solution containing 145 mM NaCl, 20 mM HEPES-Tris [pH 7.4], 0.1 mM EGTA, and 0.15 or 1 mM MgCl2. After addition of 3 μM A23187 for 5 minutes, cells were incubated for 15 minutes under either oxygenated or deoxygenated conditions. In experiments using the lectins, cells suspended in solution B (hematocrit 0.5%) were incubated at 20°C for 10 minutes with either WGA (20 μg/mL) or ConA (50 μg/mL).26
Immunoprecipitation and immune complex kinase assays
After incubation, cells (100 μL) were pelleted and lysed in 1 mL hypotonic solution C (10 mM HEPES/Tris [pH 7.7 at 4°C], 0.1 mM EDTA, 1 mM Na3VO4, 0.3 mM PMSF, and 10 μg/mL each aprotinin, pepstatin A, and leupeptin). Membranes were recovered by centrifugation and extracted under shaking for 60 minutes with 5 mL solution D (25 mM HEPES/Tris [pH 7.4], 10 mM NaCl, 0.1 mM EDTA, 1 mM Na3VO4, 1% Brij 96, and antiproteases). The extracts were collected after centrifugation (10 000g for 10 minutes) and incubated for 16 hours under gentle agitation with 3 μL anti-Lyn or anti-Syk NT antibodies and 20 μL protein A-Sepharose 4B (20% in solution D). After centrifugation, the beads were washed once with lysing solution containing 0.1% Brij 96, washed twice with the kinase assay solution (50 mM HEPES/Tris [pH 7.4], 10 mM MnCl2, 5 mM pNpp, and antiproteases), and resuspended in 50 μL of the same solution. All the preceding steps were performed at 4°C. Immune complex kinase (IC) assays were started by adding 2 μL of 250 μM [γ-32P]-ATP (37 kBq) and incubated for 15 minutes at 37°C (linear conditions). Reactions were stopped by addition of 25 μL sodium dodecyl sulfate (SDS) sample buffer. Proteins were separated by electrophoresis in 8.5% polyacrylamide gels, and Syk or Lyn autophosphorylation was detected by PhosphoImager (STORM 840; Molecular Dynamics) on alkali-treated gels and quantified by using the Image Quant Software.
Immunoblotting
After electrophoresis, gels were transferred to Immobilon P membranes for 12 hours at 0.8 mA/cm2. Membranes were incubated at 37°C for 60 minutes with a blocking solution (3% BSA, 0.1% Tween 20 in Tris buffered saline) for antiphosphotyrosine or with 5% skimmed dry milk, 0.2% Tween 20 in phosphate-buffered saline (PBS) for anti-Syk and anti-Lyn, washed with 2-fold dilution of the same solutions, and incubated overnight at 4°C with anti-Lyn (1:10 000), antiphosphotyrosine (1:5000), and anti-Syk NT (1:20 000). After another wash in PBS containing 2.5% skimmed dry milk and 0.1% Tween 20, membranes were incubated for 30 minutes at 20°C with horseradish peroxidase–conjugated secondary antibodies, antimouse (1:80 000) or antirabbit (1:15 000), for detection by enhanced chemiluminescence.
Membrane PTP activity
Cells were incubated under oxygenated or deoxygenated conditions and washed as described above. Membranes were prepared by hypotonic lysis of the cell pellets in 20 volumes of solution E (10 mM Tris/HCl [pH 7.4], 1 mM EDTA, and 0.1 mM PMSF), washed once in the same solution, once in the same solution containing 50 mM NaCl to remove bound hemoglobin, and resuspended in solution E. All these steps were performed at 4°C. Membrane protein content was determined by the Bradford method. PTP activity was estimated by using pNpp as substrate according to published procedures20 with some modifications. Membrane suspensions (2.5-3 mg protein/mL) were incubated at 37°C for 30 minutes in solution E containing 15 mM pNpp, 20 mM MgCl2, and 50 nM okadaic acid (to inhibit protein Ser/Thr phosphatases), either in the absence or presence of 0.1 mM Na3VO4 (to inhibit PTP). The reaction was stopped by adding 500 μL 0.1 M NaOH. Samples were centrifuged, and the release of p-nitrophenol, in the supernatant, was estimated from the absorbance at 410 nm against appropriate blanks.
Membrane thiol content and cellular GSH level
Cells were incubated in oxygenated or deoxygenated conditions, and membranes were prepared by hypotonic lysis as described above. One volume of membranes (2 mg protein/mL) was mixed with one volume of 500 mM Tris/HCl (pH 8.2), 1 mM EDTA, and 0.1 mM PMSF and boiled for 5 minutes. After cooling, DTNB was added to a final concentration of 0.2 mM (from a 10-mM stock solution in 1% NaHCO3) and incubated at 22°C for 30 minutes in the dark. The reaction product was quantified by measuring the absorbance of the supernatant at 412 nm against appropriate blanks. Thiol concentration was calculated from the molar extinction coefficient of the thiobenzoate ion (13 600 M−1cm−1) or from a standard curve derived from similar treatment of 10 to 100 μM glutathione.27 Cell GSH levels were determined as described previously.28
Modulation of Syk activity by membrane PTP
Syk immune complexes, from unincubated SS or AA cells, were given a final wash in solution E plus antiproteases without or with 0.1 mM Na3VO4. In the first set of experiments, complexes were incubated for 15 minutes at 37°C with membranes, suspended in solution E, and used as a source of PTP (50 μL, 3 mg protein/mL), in the presence of 50 nM okadaic acid and either 10 mM MgCl2 (active PTP) or 0.1 mM Na3VO4(inhibited PTP). In the second set of experiments, after preincubation of Syk immune complexes with active or inactive PTP as above, the complexes were washed twice at 4°C with the kinase assay solution and incubated for 15 minutes at 37°C with [γ-32P]-ATP for IC assays. In all these experiments, after electrophoresis and transfer, Syk phosphorylation state was detected by immunoblotting with antiphosphotyrosine antibody.
Statistics
Data are given as means ± SE. Differences between means were evaluated by the Student t test for paired samples, with P < .05 being taken as the level of significance.
Results
Deoxygenation of SS cells does not affect Lyn activity
We have previously observed that deoxygenation of SS cells induced an increase in PTK activity and in band 3 phosphorylation.2 To investigate the possible contribution of Lyn and Syk to this increase, the PTKs were immunoprecipitated from SS cells incubated under either oxygenated or deoxygenated conditions, and their autophosphorylation activity was determined in IC assays. This measurement was previously reported to correlate well with Lyn and Syk activity toward the exogenous substrate, cdb3.29 30
Analysis of IC assays with [γ-32P]-ATP of Lyn immune complexes by SDS–polyacrylamide gel electrophoresis and PhosphoImager showed that Lyn autophosphorylation was not affected by 30 minutes' deoxygenation of SS cells (Figure 1A). Immunoblots with anti-Lyn antibody indicated that a similar amount of Lyn was immunoprecipitated in each sample (Figure 1B). Other experiments confirmed that Lyn activity remained unchanged between 15 and 60 minutes of deoxygenation (Figure2A). In an independent experiment, Lyn activity was measured in anti-Lyn immunoprecipitates by phosphorylation of the exogenous substrate poly (E-Y) 4-1, and again no difference in Lyn activity was detected between oxygenated and deoxygenated samples (data not shown).
Effect of deoxygenation of SS cells on Lyn and Syk activity.
SS cells were incubated either under oxygenated (−) or deoxygenated conditions (+). After immunoprecipitation with anti-Lyn (A,B) or anti-Syk (C,D) antibody and IC assays with [γ-32P] ATP, the reaction products were separated by gel electrophoresis and either visualized by PhosphorImager (A,C) or transferred to Immobilon membranes for immunoblotting with anti-Lyn or anti-Syk antibody (B,D). In C, the lower band (38 kd), whose phosphorylation increased in deoxygenated samples, was generated by proteolysis of phosphorylated Syk (72 kd), as demonstrated by immunoblotting (data not shown).
Effect of deoxygenation of SS cells on Lyn and Syk activity.
SS cells were incubated either under oxygenated (−) or deoxygenated conditions (+). After immunoprecipitation with anti-Lyn (A,B) or anti-Syk (C,D) antibody and IC assays with [γ-32P] ATP, the reaction products were separated by gel electrophoresis and either visualized by PhosphorImager (A,C) or transferred to Immobilon membranes for immunoblotting with anti-Lyn or anti-Syk antibody (B,D). In C, the lower band (38 kd), whose phosphorylation increased in deoxygenated samples, was generated by proteolysis of phosphorylated Syk (72 kd), as demonstrated by immunoblotting (data not shown).
Time course of activation of Lyn and Syk in deoxygenated SS cells.
SS cells were incubated either under oxygenated (O2) or deoxygenated conditions (N2). After immunoprecipitation and IC assays with [γ-32P] ATP, the reaction products were separated by gel electrophoresis, and activities were estimated from the32P incorporation into Lyn (A) and Syk (B) and quantified by Image Quant Software. Data shown are means ± SE of 3 (Lyn) or 5 (Syk) independent experiments, carried out in duplicate. *Significantly different from oxygenated control.
Time course of activation of Lyn and Syk in deoxygenated SS cells.
SS cells were incubated either under oxygenated (O2) or deoxygenated conditions (N2). After immunoprecipitation and IC assays with [γ-32P] ATP, the reaction products were separated by gel electrophoresis, and activities were estimated from the32P incorporation into Lyn (A) and Syk (B) and quantified by Image Quant Software. Data shown are means ± SE of 3 (Lyn) or 5 (Syk) independent experiments, carried out in duplicate. *Significantly different from oxygenated control.
Deoxygenation of SS cells reversibly stimulates Syk activity
Similar analysis of IC assays of Syk immune complexes showed that deoxygenation of SS cells for 15 to 60 minutes increased Syk autophosphorylation (Figure 1C) and that a similar amount of Syk was immunoprecipitated in each sample (Figure 1D). The increase in Syk activity was about 2-fold after 15 minutes of deoxygenation and remained elevated for at least 60 minutes (Figure 2B). Deoxygenation-induced Syk activation was specific for SS cells, as it was not observed in AA cells (Figure 3A). Reoxygenation of SS cells for 60 minutes, after 15 minutes of deoxygenation, partly reversed the deoxygenation-induced Syk activation (Figure 3B).
Activation of Syk by deoxygenation is specific for SS cells, is reversed by reoxygenation, and is not mediated by increases in intracellular Mg2+ and Ca++.
(A) AA or SS cells (n = 5) were incubated for 30 minutes under oxygenated (O2) or deoxygenated conditions (N2). (B) SS cells (n = 3) were incubated for 15 minutes under oxygenated or deoxygenated conditions, and samples from deoxygenated SS cells were reoxygenated for 60 minutes (N2/O2). (C) SS cells (n = 4) were incubated for 15 minutes under oxygenated or deoxygenated conditions in solution B (−) or in a buffered isotonic medium containing 0.15 or 1 mM MgCl2, 0.1 mM EGTA, and 3 μM A23187 (+). In the latter conditions (A23187), the presence of EGTA and 0.15 mM MgCl2, although preventing any increase in [Ca++]i and [Mg2+]iassociated with deoxygenation of SS cells, did not abolish Syk activation. In the presence of A23187 and 1 mM MgCl2, [Mg2+]i increased to 1.7 mM33 in both oxygenated and deoxygenated samples, without affecting Syk activation. Syk activity was measured as indicated in the legend to Figure 2. Data shown are means ± SE. *Significantly different from oxygenated control; **significantly different from deoxygenated control. In C, the values in deoxygenated conditions without or with A23187 are not significantly different.
Activation of Syk by deoxygenation is specific for SS cells, is reversed by reoxygenation, and is not mediated by increases in intracellular Mg2+ and Ca++.
(A) AA or SS cells (n = 5) were incubated for 30 minutes under oxygenated (O2) or deoxygenated conditions (N2). (B) SS cells (n = 3) were incubated for 15 minutes under oxygenated or deoxygenated conditions, and samples from deoxygenated SS cells were reoxygenated for 60 minutes (N2/O2). (C) SS cells (n = 4) were incubated for 15 minutes under oxygenated or deoxygenated conditions in solution B (−) or in a buffered isotonic medium containing 0.15 or 1 mM MgCl2, 0.1 mM EGTA, and 3 μM A23187 (+). In the latter conditions (A23187), the presence of EGTA and 0.15 mM MgCl2, although preventing any increase in [Ca++]i and [Mg2+]iassociated with deoxygenation of SS cells, did not abolish Syk activation. In the presence of A23187 and 1 mM MgCl2, [Mg2+]i increased to 1.7 mM33 in both oxygenated and deoxygenated samples, without affecting Syk activation. Syk activity was measured as indicated in the legend to Figure 2. Data shown are means ± SE. *Significantly different from oxygenated control; **significantly different from deoxygenated control. In C, the values in deoxygenated conditions without or with A23187 are not significantly different.
Syk activation in SS cells does not result from an increase in intracellular Mg2+ or Ca++ associated with deoxygenation
Deoxygenation of SS cells is known to induce an elevation of intracellular ionized Mg2+([Mg2+]i) from 0.4 to 0.7 mM, as in normal red cells,31 and of intracellular Ca++([Ca++]i), specific for SS cells.32 To investigate whether these stimuli could be involved in Syk activation, SS cells were preincubated in a medium containing A23187 and 0.1 mM EGTA, to prevent the increase in [Ca++]i, and either 0.15 mM MgCl2, to clamp [Mg2+]i at its resting level, or 1 mM MgCl2 to increase [Mg2+]i to 1.7 mM,33 and incubated under either oxygenated or deoxygenated conditions for 15 minutes. Clamping [Mg2+]i at its resting level and preventing [Ca++]i increase had no significant effect on the deoxygenation-induced Syk activation (Figure3C), showing that it was independent of a rise in [Mg2+]i and in [Ca++]i. Mg2+ loading induced a small activation of Syk in oxygenated cells, whereas it did not affect significantly Syk activation in deoxygenated cells. In addition, the effect of deoxygenation alone on Syk stimulation was significantly greater than that induced by Mg2+ loading in oxygenated cells, although the increase in [Mg2+]i was smaller in the former case. Hence, activation of Syk in deoxygenated SS cells was not mediated by the changes in ionized divalent cation concentrations.
Aggregation of glycoproteins by lectins does not induce Syk activation
To test the hypothesis that Syk could be activated through a mechanism involving glycoprotein clustering in SS cells, Syk activity was determined after incubation of the cells with lectins (WGA or Con A) known to cause, in red cells, aggregation of glycophorin or band 3, respectively.26 The conditions of incubation were chosen to prevent cell agglutination (low lectin concentration and low hematocrit and incubation temperature).26 Treatment of AA or SS cells, with either WGA or Con A, was unable to affect significantly Syk activity (data not shown).
Deoxygenation of SS cells induces an inhibition of membrane PTP and a decrease in membrane thiol content
To explore the possibility that activation of Syk could involve an inhibition of a PTP, we first investigated whether deoxygenation could affect the activity of the membrane-associated PTP, identified in human red cells19 and the membrane thiol content, as the red cell membrane PTP is inhibited by thiol-oxidizing and thiol-alkylating agents.20 As illustrated in Figure4, deoxygenation of SS cells for 30 minutes caused a small but significant decrease (about 15%) in both membrane PTP activity and membrane SH content. In contrast, deoxygenation of AA cells had no significant effect either on PTP activity or SH content. Deoxygenation of SS cells for 30 minutes did not significantly affect GSH levels (−3.4% ± 2.5%, n = 4).
Effect of deoxygenation of AA and SS cells on membrane PTP activity and thiol (SH) content.
AA or SS cells were incubated for 30 minutes under either oxygenated (O2) or deoxygenated conditions (N2). Membranes were prepared by hypotonic lysis of the cells. PTP activity and thiol content were measured from the release of p-nitrophenol from pNpp and from the reduction of DTNB, respectively. Data are means ± SE of 4 to 6 independent experiments carried out in duplicate. *Significantly different from oxygenated control.
Effect of deoxygenation of AA and SS cells on membrane PTP activity and thiol (SH) content.
AA or SS cells were incubated for 30 minutes under either oxygenated (O2) or deoxygenated conditions (N2). Membranes were prepared by hypotonic lysis of the cells. PTP activity and thiol content were measured from the release of p-nitrophenol from pNpp and from the reduction of DTNB, respectively. Data are means ± SE of 4 to 6 independent experiments carried out in duplicate. *Significantly different from oxygenated control.
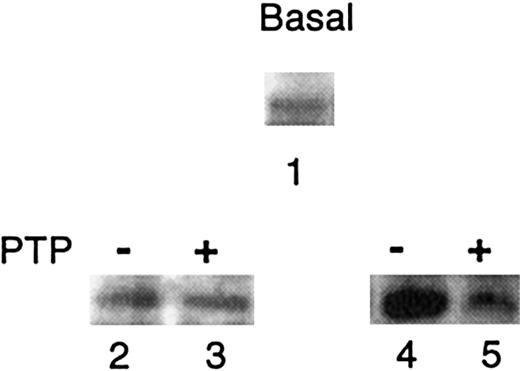
PTP regulates Syk activity in vitro
The phosphorylation state of Syk was analyzed by antiphosphotyrosine immunoblotting of Syk immune complexes from unstimulated cells, either at basal state or after different treatments (Figure 5). At basal state, Syk was found to be weakly tyrosine phosphorylated (Figure 5, lane 1), suggesting that its activity could be regulated by a membrane-associated PTP. In a first set of experiments (Figure 5, lanes 2 and 3), to investigate whether Syk could be a substrate for a membrane PTP, Syk immune complexes were incubated with membrane suspensions, as a source of PTP, either in the presence of vanadate (to inhibit PTP)19 21 or in its absence (active PTP). The treatment with active PTP had no apparent effect on the phosphorylation state of Syk, at least within the limits of the detection method with this antibody (see “Discussion”). However, the second set of experiments (Figure 5, lanes 4 and 5) demonstrates that a pretreatment with active PTP was able to inhibit Syk autophosphorylation activity. IC assays were performed after preincubation of the complexes with membrane suspensions containing either inactive or active PTP. Syk autophosphorylation was clearly observed when vanadate was added during preincubation to inactivate PTP (Figure 5, compare lanes 2 and 4), whereas autophosphorylation was prevented after preincubation with active PTP (Figure 5, compare lanes 3 and 5). This effect was observed in both AA and SS cell immune complexes, showing that this regulation is an intrinsic property of Syk. Preincubation of the complexes with or without vanadate, in the absence of membrane suspensions, had no effect on Syk autophosphorylation (data not shown), demonstrating that inhibition of Syk activity requires the presence of membrane PTP.
Effect of membrane PTP on Syk activity.
The phosphorylation state of Syk ICs was analyzed by antiphosphotyrosine immunoblotting under various conditions: IC from unincubated SS cells (lane 1); IC after incubation with membrane suspensions, either with Na3VO4 (inhibited PTP, −) (lane 2), or in its absence (active PTP, +) (lane 3); IC after preincubation with inhibited PTP (−) or active PTP (+) followed by IC assays (lanes 4 and 5, respectively). One representative experiment is shown. Similar data were obtained in 3 other experiments with SS cells and in 2 experiments with AA cells.
Effect of membrane PTP on Syk activity.
The phosphorylation state of Syk ICs was analyzed by antiphosphotyrosine immunoblotting under various conditions: IC from unincubated SS cells (lane 1); IC after incubation with membrane suspensions, either with Na3VO4 (inhibited PTP, −) (lane 2), or in its absence (active PTP, +) (lane 3); IC after preincubation with inhibited PTP (−) or active PTP (+) followed by IC assays (lanes 4 and 5, respectively). One representative experiment is shown. Similar data were obtained in 3 other experiments with SS cells and in 2 experiments with AA cells.
Discussion
The present study shows that deoxygenation of SS cells induced a 2-fold activation of Syk. In AA cells, deoxygenation had no significant effect on Syk activation, although under these conditions a small increase in band 3 phosphotyrosine has been previously reported and attributed to the increase in intracellular Mg2+ associated with deoxygenation.12 Our data show that, in deoxygenated SS cells, the increase in [Mg2+]i was not the trigger for Syk activation, because (1) activation occurred to the same extent when [Mg2+]i was clamped at its resting level and (2) activation was greater in deoxygenated cells than in Mg2+-loaded oxygenated cells. Additionally, stimulation of Syk by deoxygenation was shown to be independent of the increase in [Ca++]i associated with deoxygenation, because it was not prevented in the presence of ionophore and EGTA.
Total PTK activity is about 2- to 3-fold higher in SS cells than in mature AA cells.2,34 This difference cannot be attributed to the younger age of SS cells, as this activity was shown to be independent of cell age in red cells of both sickle cell disease patients and rabbits.34,35 The present study shows that deoxygenation of SS cells, but not of AA cells, induces Syk activation. However, these data do not compare age-matched AA and SS cells. The possibility that, in very young oxygenated AA reticulocytes, the level of Syk activity is comparable to that in deoxygenated SS cells remains to be investigated. In any case, the fact that activation of Syk was partly reversed by reoxygenation of SS cells argues in favor of a role of hemoglobin S in the activation process. It has been suggested that interactions between bundles of adjacent fibers of deoxygenated hemoglobin S polymers and cdb3 could form oligomeric aggregates of the band 3 dimers.36 A possible mechanism for Syk activation may involve its association with band 3. The cdb3 contains 2 consensus sequences specifically recognized by Syk (Y8EDM and Y21EDP) that, once phosphorylated, generate potential binding sites for Lyn molecules via their SH2 domain and further phosphorylation of Y359 and Y904 by Lyn.13,37 As Syk tandem SH2 domains bind with high specificity to ITAM sequences YpXXL,38 identical to that found downstream to Y359 (Y359KGL) in cdb3,39,40 Syk binding at this site could be expected. The requirement for biphosphorylated ITAM38 could imply clustering of band 3, allowing the binding of one Syk molecule to 2 ITAMs of adjacent cdb3 to enhance Syk activation. To test this hypothesis, we have used ConA and WGA, which, in red cells, bind to band 3 and glycophorin, respectively,26 and have been reported, in platelets or neutrophils, to cause cell surface glycoprotein oligomerization and to induce Syk activation.9 10 None of these lectins was able to activate Syk, at least when used at concentrations avoiding lectin-induced agglutination of the red cells. However, aggregation of band 3 by lectins may involve different types of interactions between band 3 molecules than those induced by deoxygenation. Consequently, the lack of Syk activation by lectins does not permit to eliminate the hypothesis of band 3 oligomerization in deoxygenated SS cells.
The mechanism of Syk activation in deoxygenated SS cells does not involve any change in Lyn activity and thus differs from that prevailing on immunoreceptor stimulation. Deoxygenation, by inhibiting a membrane PTP, could provide an alternative mechanism for the activation of Syk in the absence of receptor cross-linking. Such a mechanism was proposed to account for the increase in Syk and other PTK activity, induced by oxidative stress in various cells.7,24,25 Indeed, dephosphorylation of Lck in T-cell membranes, by a yet unidentified PTP, results in inactivation of its kinase activity,25 and expression of a dominant-negative SH2-containing protein tyrosine phosphatase-1 (SHP-1) in B cells causes an increase in Syk tyrosine phosphorylation and tyrosine kinase activity.41 Deoxygenation of SS cells was found to induce both Syk activation and inhibition of a membrane-associated PTP activity, whereas deoxygenation of AA cells had no effect on any of these activities. In addition, autophosphorylation of isolated Syk was inhibited in vitro following a pretreatment with membrane PTP. These data suggest that in vivo Syk activity could be regulated by a PTP-dependent mechanism. Tyr 130, located between the tandem SH2 domains of Syk, is a prominent and early site of Syk autophosphorylation42 and appears to be phosphorylated primarily in response to PTP inhibitors, such as pervanadate/H2O2, that can activate Syk in the absence of receptor aggregation.43 Replacement of Tyr 130 with Glu, which mimics a phosphorylated amino acid, increased the basal activity of Syk, whereas replacement with Phe decreased its activity.42 Likewise, in deoxygenated SS cells, phosphorylation of Tyr 130, mediated by inhibition of a membrane PTP, could provide a mechanism for the activation of Syk and the increase in tyrosine phosphorylation of band 3,2 its major substrate in red cells. This hypothesis would be consistent with the activation of Syk and, in band 3 phosphorylation, observed in red cells treated with pervanadate, a potent PTP inhibitor.3,13,16 However, a pretreatment with membrane PTP inhibited Syk autophosphorylation without detectable effect on Syk phosphorylation status. The lack of apparent Syk dephosphorylation could be linked to the detection method (the antiphosphotyrosine antibody 4G-10 that measures total phosphorylation) not sensitive enough to detect a change in the phosphorylation status of one Tyr residue, as reported recently in the case of Tyr 519-520 of Syk activation loop.44
The mechanism involved in the inhibition of PTP in deoxygenated SS cells remains speculative. All PTPs have a reactive cysteine residue in their active site, which must be in the reduced form for catalytic activity.22 Inhibition of a membrane PTP by diamide results from the formation of mixed disulfides between that PTP and band 3, a process considered to be an early cellular response to oxidative stress.20 We found that short-term deoxygenation in vitro led to a significant oxidation of membrane protein thiols in SS cells and not in AA cells. More substantial oxidation has been already reported after 22 hours of deoxygenation-reoxygenation cycles of SS cells.45 The enhanced rate of hemoglobin oxidation at reduced oxygen pressure, due to its relative instability and the resultant formation of superoxide, could be a possible source of hypoxic stress,46 a process accelerated in SS cells, because hemoglobin S is more susceptible to autoxidation than hemoglobin A.47 The present data suggest that enhancement of oxidative processes in deoxygenated SS cells, reflected by the decrease in membrane thiol content, would induce PTP inhibition and subsequent Syk activation.
Such a cascade could play a role in SS cell dehydration. Indeed, SS cell dehydration, induced by deoxygenation or a decrease in GSH level, is blocked by N-acetylcysteine, an antioxidant that increases the intracellular GSH.28,48 Dehydration of SS cells, under deoxygenated conditions, results from K+ loss mediated by KCa channels and/or K/Cl cotransport. As PTK inhibitors reduce deoxygenation-induced K+ loss even in the absence of external calcium, we have proposed that PTK could be involved in the activation of one or both of these K+transports.2 The present study shows that deoxygenation-induced activation of Syk is still observed in the absence of external calcium, suggesting that Syk would be the PTK involved in the activation of KCa channels and/or K/Cl cotransport. Activation of K/Cl cotransport is known to result from a stimulation of okadaic acid–sensitive phosphatases (PSPs).49 We have shown previously that deoxygenation stimulates these PSPs in both AA and SS cells50 but activates Syk (present data) and induces K+ loss only in SS cells, suggesting that both PSP and Syk activation would be required to activate K/Cl cotransport. A role for Syk in the activation of the transporter deserves to be investigated.
We thank Dr D. Mary for his gift of the anti-syk antibody used in initial experiments. We also thank Drs D. Bachir and F. Galactéros (Centre de la Drépanocytose et des Thalassémies, Hôpital Henri Mondor, Créteil, France) and Drs. J. P. Diara, L. Dumdo, C. Le Turdu-Chicot, and Y. Samuel (Centre Intégré de la Drépanocytose, Pointe-à-Pitre, Guadeloupe) for providing blood samples from their patients.
Supported by funds from the Centre National de la Recherche Scientifique (CNRS, UMR 8619), the Université Paris XI-Orsay, the Institut National de la Santé et de la Recherche Médicale (INSERM U-458), the Société Française d'Hématologie to P.M., and the Association pour la Recherche contre le Cancer to F. G. (ARC No. 9267).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Françoise Giraud, Biomembranes et Messagers Cellulaires, Bat 440, Université Paris XI-Orsay, 91405 Orsay Cedex, France; e-mail: francoise.giraud@ibaic.u-psud.fr.

![Fig. 1. Effect of deoxygenation of SS cells on Lyn and Syk activity. / SS cells were incubated either under oxygenated (−) or deoxygenated conditions (+). After immunoprecipitation with anti-Lyn (A,B) or anti-Syk (C,D) antibody and IC assays with [γ-32P] ATP, the reaction products were separated by gel electrophoresis and either visualized by PhosphorImager (A,C) or transferred to Immobilon membranes for immunoblotting with anti-Lyn or anti-Syk antibody (B,D). In C, the lower band (38 kd), whose phosphorylation increased in deoxygenated samples, was generated by proteolysis of phosphorylated Syk (72 kd), as demonstrated by immunoblotting (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/10/10.1182_blood.v98.10.3121/5/m_h82211738001.jpeg?Expires=1765884857&Signature=zZ5GD87JULm4VbDaT4snV2-5BdJplzQJPyLhJUc8TlBN5LSbCfpQlbJ~Rpt8QyHinrUe90tUyfYMYe~1zK90pLvpinbl8noRFKCOuYPaOj1fYZ496VHatGyq0iA0h9VJ~gBqxhz2appdNoIGe-pOg68UZeYR2nZL45oK3hiuVud4~NdIpb2QRiYARG1j2ewGRayKNA8y6HoTTa1fHYDPUtwcZhIwZakMiDu1UsCDK1bRa4H8fOyTYI6NWj4rbbcdzAizrwZFMezrzmdMN75dcJo0K8vcwUsWyp4MGZwMxHlzca64tUlShrC6yMOLJDousZniYpWdcGRv61ahwCvl9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Time course of activation of Lyn and Syk in deoxygenated SS cells. / SS cells were incubated either under oxygenated (O2) or deoxygenated conditions (N2). After immunoprecipitation and IC assays with [γ-32P] ATP, the reaction products were separated by gel electrophoresis, and activities were estimated from the32P incorporation into Lyn (A) and Syk (B) and quantified by Image Quant Software. Data shown are means ± SE of 3 (Lyn) or 5 (Syk) independent experiments, carried out in duplicate. *Significantly different from oxygenated control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/10/10.1182_blood.v98.10.3121/5/m_h82211738002.jpeg?Expires=1765884857&Signature=r~OpjSkosMM-mIghsNpuGXGE~QZloyN4MmGeJ4vZp8Z8YLs2eqs4vxZZr5LGe-k6zbXz4oK8bkCEGp~RZHjvWzAcGgEp41NYEDlDGE8A4PCQHywXMFACJull8Zs3rSSc3WXUSqGEkMJc~Ze4A8TNeFb4Y~gqdU~Ahr~xg7l-pwTIm-vFgbonBRH5n8b3Ilsr~g6aCkILQWgjIr1jGrGZoz-MgcOHLgiY61poUbXN~UJV9URFxQx7TmmqH7Oaq8B6DmtDTiMYONFnOCi9CuReVzLUyo81RHvIJ~KLZptR9FHjIzIacjK~MOWEhnN5JzsZBAkA2NW~mK26uBr3fWCjXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Activation of Syk by deoxygenation is specific for SS cells, is reversed by reoxygenation, and is not mediated by increases in intracellular Mg2+ and Ca++. / (A) AA or SS cells (n = 5) were incubated for 30 minutes under oxygenated (O2) or deoxygenated conditions (N2). (B) SS cells (n = 3) were incubated for 15 minutes under oxygenated or deoxygenated conditions, and samples from deoxygenated SS cells were reoxygenated for 60 minutes (N2/O2). (C) SS cells (n = 4) were incubated for 15 minutes under oxygenated or deoxygenated conditions in solution B (−) or in a buffered isotonic medium containing 0.15 or 1 mM MgCl2, 0.1 mM EGTA, and 3 μM A23187 (+). In the latter conditions (A23187), the presence of EGTA and 0.15 mM MgCl2, although preventing any increase in [Ca++]i and [Mg2+]iassociated with deoxygenation of SS cells, did not abolish Syk activation. In the presence of A23187 and 1 mM MgCl2, [Mg2+]i increased to 1.7 mM33 in both oxygenated and deoxygenated samples, without affecting Syk activation. Syk activity was measured as indicated in the legend to Figure 2. Data shown are means ± SE. *Significantly different from oxygenated control; **significantly different from deoxygenated control. In C, the values in deoxygenated conditions without or with A23187 are not significantly different.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/10/10.1182_blood.v98.10.3121/5/m_h82211738003.jpeg?Expires=1765884857&Signature=RFJ7lz4IMtnV3oTevWwYNupPkvIB-3hXFbArpy9YfrElKOcRie8tCZwwo~-1yOHkibRfj7UV1JtlRouQnBBl1U6Q6DfquVjwWwbF0KVltp2796GDnRGJL3j6vj1cOyG3KIMT9TOUtWXESTjk23Tz~-PvXTzttku3N-n15OAgQVKqsfC0j2hAh2IaMoAOzmC-AFY3m7bcnKjEgq2-~Qfh1RjL4bXuMFO2vTzs4hsss4CGf4uMOasT2XFqU-brE01D-6qN5ry1xYR5pRB5RU1THqsLvaK6tlWnMW46YBr3gbyhBTXIcvB68f5xSUHcfVfKUDpZeVYANGHgswEh4ywyiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal