Abstract
The receptor-associated protein tyrosine kinase janus-kinase 2 (JAK2) is essential for normal red cell development and for erythropoietin receptor (EpoR) signaling. JAK2−/− embryos are severely deficient in erythropoiesis and die at an early stage of development from fetal anemia. The binding of erythropoietin (Epo) to the EpoR triggers the activation of JAK2, the phosphorylation of the EpoR, and the initiation of the EpoR signaling cascade. In addition to Epo binding to its receptor, signaling pathways downstream of the EpoR can also be stimulated by the BCR-ABL oncoprotein. This study explored whether JAK2 is required for BCR-ABL–mediated stimulation of erythropoiesis. Here, it is shown that JAK2 is constitutively tyrosine phosphorylated in cultured and primary erythroid cells expressing BCR-ABL. However, BCR-ABL effectively supports normal erythroid proliferation, differentiation, and maturation in JAK2-deficient fetal liver cells. Using mutants of BCR-ABL, this study shows that certain signaling pathways activated by BCR-ABL segments distinct from its tyrosine kinase domain are essential for rescue of erythropoiesis in JAK2−/− progenitors. The consequences of these multiple signaling pathways for normal erythroid development are discussed.
Introduction
Erythropoietin (Epo) is essential for the proliferation, differentiation, and survival of erythroid cells as demonstrated definitively by the properties of Epo and erythropoietin receptor (EpoR)−/− mice.1-3 These mice die during embryogenesis from severe anemia, due to failure of erythroid colony-forming unit (CFU-E) progenitors to undergo terminal proliferation, differentiation, and red cell maturation. EpoR itself lacks any intrinsic catalytic activity4; binding of Epo to the EpoR results in receptor dimerization and conformational changes, and phosphorylation and activation of receptor-associated JAK2.
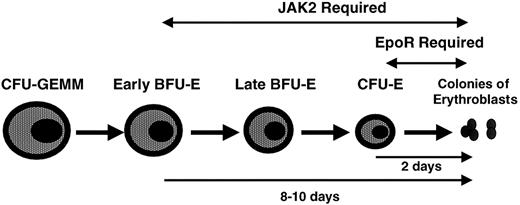
Janus-kinase 2 (JAK2), a member of the JAK family of protein tyrosine kinases, is constitutively bound to cell surface EpoR and is crucial for EpoR signaling. JAK2−/− embryos die from profound anemia around day 11 to 13 of embryogenesis.5,6 Erythroid maturation is arrested earlier and more severely in JAK2−/− embryos compared with EpoR−/−embryos (Figure 1), because the absence of JAK2 abrogates the signaling not only of the EpoR but also of numerous additional cytokine receptors that are important for proliferation and survival of primitive erythroid progenitors.5 6
Activated JAK2 phosphorylates several tyrosine residues on the EpoR; these phosphotyrosine residues on the EpoR provide docking sites for SH2 (src-homology-2) domain-containing signal transduction proteins including STAT5 that bind to the receptor and are phosphorylated by JAK2, thereby initiating EpoR signaling cascades including activation of STAT5, Ras, mitogen-activated protein kinase (MAPK), JNK, P38, PI3-kinase–AKT, SHP1, SHP2, SHIP, and BCL-xL(reviewed in Constantinescu et al7). JAK2 may also directly phosphorylate and activate signaling proteins such as Shc in a receptor tyrosine-independent fashion8; as another example, an EpoR devoid of any cytosolic tyrosines activates STAT5 to a level approximately 10% that of the wild-type receptor.9The relative contribution of the different EpoR-activated signaling pathways to erythroid development, specifically proliferation, differentiation, and survival, of erythroid progenitors is not well understood.
JAK2 is the major JAK associated with and activated by a number of cytokine receptors besides the EpoR, including the growth hormone receptor, the type 2 interferon receptor, the thrombopoietin receptor Mpl, and the β common containing receptors that bind granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 3 (IL-3), IL-5, and IL-12 (reviewed in Yeh and Pellegrini10). Moreover, JAK2 is also associated with and activated along with other JAKs in response to the cardiotrophin receptor, the prolactin receptor, the granulocyte colony-stimulating factor receptor (G-CSF-R), and cytokine receptors containing gp130, such as IL-6R, ciliary neurotrophic factor-receptor (CNTF-R), leukemia-inhibitory factor-receptor (LIF-R), and oncostatin M-receptor (OSM-R) (reviewed in Yeh and Pellegrini10).
Many of the signaling pathways activated by JAK2 in response to Epo are also activated by the BCR-ABL oncoprotein (reviewed in Constantinescu et al7 and Ghaffari et al11), the molecular hallmark of chronic myeloid leukemia (CML). P210BCR-ABL(P210) and a related fusion protein P185BCR-ABL(P185)12,13 are constitutively active protein tyrosine kinases whose activity is significantly more potent than their normal c-ABL counterpart.14,15 These chimeric BCR-ABL proteins result from the fusion of the N-terminal segment of BCR (902 amino acids in P210 and 426 amino acids in P185) to most of the c-ABL protein.16 Despite a mild anemia which affects most patients at the chronic phase, erythroid progenitors are Epo-independent in culture and their number is increased in CML patients.17 In addition, BCR-ABL complements EpoR signaling and supports the proliferation, differentiation, and maturation of red cell progenitors when expressed in EpoR−/− fetal liver cells.18 Furthermore, BCR-ABL partially complements signals provided by IL-3, IL-6, and Steel factor (SF) required for proliferation and survival of primitive erythroid burst-forming unit (BFU-E) progenitors.18
Since JAK2 is crucial for EpoR signaling and red cell development, we investigated whether JAK2 is required for BCR-ABL complementation of EpoR signaling. Although JAK2 was constitutively tyrosine phosphorylated in cultured and primary erythroid cells expressing BCR-ABL, erythropoiesis proceeded normally in JAK2−/−fetal liver cells transduced with BCR-ABL. This demonstrates that JAK2 is not required for BCR-ABL complementation of EpoR signaling.
In addition to the tyrosine kinase domain, several distinct sequences within BCR-ABL, either alone or in concert, contribute to BCR-ABL transforming potential by activating signaling pathways that are also activated by many cytokine receptors. Signal transduction pathways generated by BCR-ABL and its individual domains (Table1) that promote proliferation and survival have been studied extensively both in vitro and in vivo.19 Thus, we also expressed several BCR-ABL mutants that exhibit impaired signaling (summarized in Table 1) in JAK2−/− fetal liver cells. Two of these mutants were defective in rescuing JAK2−/− progenitors but were able to rescue EpoR−/− progenitors.18 Thus, there exist signaling pathways activated directly by JAK2, independent of the EpoR (ie, in EpoR−/− cells), which are also activated by BCR-ABL and which are important for erythropoiesis. We discuss the potential importance of these findings for understanding of EpoR signaling.
Signaling and transforming properties of BCR-ABL mutants
| BCR-ABL mutant . | Signaling protein binding/ pathways abrogated . | Growth factor independence of hematopoietic cells . | In vitro transformation* . | CML-like disease in mice . |
|---|---|---|---|---|
| P210ΔSH2 | Tyrosine phosphorylated proteins55,56 PI3-kinase/AKT (?)42,53,54 57 | +30 60 | −55 56 | −53 54 |
| P210 Y177F | Grb-2/Sos/Ras31 | +30 31 | −31 | −59 |
| P185Δ176-427 | Grb-2/Sos/Ras31/14-3-336,58/Raf143/ PI3-kinase/AKT42 | +30 | −31 36 | ND |
| P185 TM† (Y177F, R552L, Y793F) | Grb-2/Sos/Ras31 tyrosine phosphorylated proteins55 56 | +/−30 31‡ | −31 | ND |
| BCR-ABL mutant . | Signaling protein binding/ pathways abrogated . | Growth factor independence of hematopoietic cells . | In vitro transformation* . | CML-like disease in mice . |
|---|---|---|---|---|
| P210ΔSH2 | Tyrosine phosphorylated proteins55,56 PI3-kinase/AKT (?)42,53,54 57 | +30 60 | −55 56 | −53 54 |
| P210 Y177F | Grb-2/Sos/Ras31 | +30 31 | −31 | −59 |
| P185Δ176-427 | Grb-2/Sos/Ras31/14-3-336,58/Raf143/ PI3-kinase/AKT42 | +30 | −31 36 | ND |
| P185 TM† (Y177F, R552L, Y793F) | Grb-2/Sos/Ras31 tyrosine phosphorylated proteins55 56 | +/−30 31‡ | −31 | ND |
In vitro transformation by individual mutants assessed by suppression of contact-dependent growth in a Rat-1 focus formation assay, abrogation of anchorage-dependent growth in a Rat-1 soft agar colony formation assay, or propagation of lymphoid cells in long-term bone marrow cultures.
P185 triple mutant (P185 TM) contains a mutation in the Grb-2 binding site (Y177F), the phosphotyrosine-binding site within the SH2 domain (R552L), and an autophosphorylation site (Y793F).
P185 TM confers IL-3 independence to BaF3 but not to 32D cells.30 ND indicates not determined.
Materials and methods
Cells
Heterozygote JAK2+/− (129J × C57BL/6J background) mice were screened by polymerase chain reaction (PCR) as previously described,5 and bred and maintained at the Whitehead Institute animal facility. Individual E12.5 JAK2−/− fetal livers were isolated according to their distinct pale white color. They were dissected and disaggregated into single cell suspensions in alpha-modified minimum essential media (α-MEM, Stem Cell Technologies, Vancouver, BC, Canada) containing 15% fetal calf serum (FCS), passed through a 21-gauge needle, and washed 2 times in the same medium. A portion of the cells was diluted in 2% acetic acid to lyse mature erythrocytes and counted. Wild-type fetal liver cells were isolated from 14-day BALB/c embryos (Jackson Laboratories, Bar Harbor, ME).
The erythroleukemic cell line HCD57 was maintained in Iscoves modified Dulbecco medium (IMDM) containing 20% FCS, 0.01 M β-mercaptoethanol and erythropoietin (2 units/mL). HCD57 cells stably expressing P210 or the vector control were generated by electroporation of the murine stem cell virus (MSCV)-P210-pac or MSCV-pac retroviral vectors and selection of puromycin-resistant cells. To isolate Epo-independent HCDP210 cells, puromycin-resistant cells were cultured in the absence of Epo for 10 days and the resultant surviving cells were collected.
Phoenix packaging cells (kindly provided by Dr Gary Nolan, Stanford University, CA) were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% FCS.
Retroviral constructs
cDNAs were cloned upstream of the internal ribosomal entry site (IRES) in the bicistronic retroviral MSCV-IRES-(green fluorescent protein) GFP (MIG) vector, a gift of Dr Luk Van Parijs (Massachusetts Institute of Technology, Cambridge, MA).20The translation of the complementary DNA (cDNA)–encoded protein and GFP is tightly linked in that the expression of GFP is proportional over a 100-fold range to the level of expression of the protein encoded by the cDNA placed upstream of the IRES.21 To construct the vectors MSCV-P210-IRES-GFP, MSCV-P210 Y177F-IRES-GFP, MSCV-P210 ΔSH2-IRES-GFP, MSCV-P185-IRES-GFP, and MSCV-P185 Δ176-427-IRES-GFP, the desired inserts were flanked by EcoRI sites and cloned into the corresponding site in the MIG vector. The BCR-ABL P185 triple mutant (P185 TM) and JAK2 cDNA were inserted by blunt-end ligation into the HpaI site of the MIG vector. MSCV-P210-pac was described previously.18
Retroviral supernatant production and infection procedure
High titer replication-free retroviral supernatants were generated as follows: 10 μg retroviral plasmids together with the pCL-Eco vector22 were cotransfected using calcium phosphate (Invitrogen kit) into 106 cells Phoenix packaging cells plated on 60-mm dishes 18 hours prior to transfection. The resulting retroviral supernatant was collected 48 hours later and was used to infect fetal liver cells. Titers of 2 × 106 to 8 × 106 were routinely obtained. A 1-to-5 dilution of the packaged virus was used to infect NIH 3T3 cells and protein expression was analyzed by Western blot using the appropriate antibody (anti-ABL or anti-JAK2 antibodies). JAK2−/− fetal liver cells (2 × 105 cells/mL) were resuspended in viral supernatants at a multiplicity of infection of 5 to 10 and plated in 60 mm retronectin (Takara Biomedicals)-coated dishes in the presence of 100 ng/mL each of IL-6 and SF (PeproTech) for 36 hours. Cells were then washed once and resuspended in α-MEM containing 15% FCS and the same growth factors for another 24 hours. Wild-type fetal liver cells were incubated with IL-3 (6 ng/mL) (PeproTech), IL-6 (10 ng/mL), and SF (100 ng/mL) for 24 hours prior to infection. Cells were resuspended in the appropriate viral supernatant in the presence of the same growth factors for 48 hours. Cells were then washed and resuspended in media containing the same growth factors for an additional 24 hours.
Flow cytometry and immunostaining
Retrovirally infected cells were washed twice in phosphate buffer saline (PBS) solution containing 2% FCS. Cells were then incubated with control rat serum at room temperature for 15 minutes, followed by incubation with 1 μg/mL Ter119-PE antibody (BD PharMingen) for 30 minutes on ice. Afterward, the cells were washed once with cold PBS 2% FCS, and once with PBS 2% FCS containing 1 μg/mL propidium iodide (PI), resuspended in 500 μL PBS containing 2% FCS prior to a FACS sort (Becton Dickinson). GFP+ or GFP+ Ter119− cells were selected and FACS-sorted for further analysis by cytospin or colony assays. CELLQuest (Becton Dickinson) was used for FACS analysis.
Colony assays
Retrovirally transduced cells were washed once in α-MEM containing 15% FCS and plated in duplicate in semisolid medium containing 0.9% methylcellulose in IMDM containing 15% FCS, 1% bovine serum albumine (BSA), 10 μg/mL bovine insulin, 200 μg/mL human transferrin, 10−4 M 2-mercaptoethanol, 2 mM L-glutamine (MethoCult M3234; StemCell Technologies), to measure colony formation as previously described.18 23 CFU-E formation was carried in methylcellulose cultures containing SF (100 ng/mL; PeproTech) with or without Epo (3 units/mL; Amgen, Thousand Oaks, CA), while BFU-E formation was assayed in methylcellulose cultures in the presence or absence of IL-6 (10 ng/mL) and SF (100 ng/mL), with or without Epo (3 u/mL). The number of CFU-E colonies was determined after diaminobenzidine staining of hemoglobin and counted 2 days after plating. BFU-E colonies of hemoglobinized erythroblasts were counted after 9 days. Colonies were individually aspirated for reverse transcriptase (RT)–PCR or cytospin, and analysis of their erythroblast morphology determined after Wright Giemsa staining.
Cytospin and cytoplasmic staining
Cells were washed in PBS with 2% FCS, and resuspended in PBS containing 1% BSA at a concentration of 3 × 105cells/mL. Cells (100 μL per slide) were subjected to a cytospin for 2 minutes at 600 rpm (Cytospin 3; Shandon) and air dried. Cells were then stained with Wright Giemsa (Harleco) according to the manufacturer's recommendations.
PolyA RT-PCR from single erythroid colonies
To analyze the gene expression profile of transduced erythroid colonies, we performed RT-PCR using oligo-dT–based primers and a polyA tailing strategy as previously described.24,25 Briefly, single BFU-E colonies were aspirated (2 μL to 10 μL) from methylcellulose plates and lysed directly in a 5 M guanidinium isothyocyanate solution containing 20 mM dithiothreitol. Nucleic acids were precipitated and the entire sample was reverse transcribed using an oligo-dT primer (1 μg/μL) (5′-CAT-GTC-GTC-CAG-GCC-GCT-CTG-GAC-AAA-ATA-TGA-ATT-C[T]24-3′), tailed, and subjected to PCR25 containing the oligo-dT primer described above. Total cDNA was amplified using 5 units of Taq polymerase. One-fourth of the total amplified product from each colony was separated by electrophoresis through 1% agarose and transferred to a Zeta-probe GT membrane (Bio-rad) and probed with either β-major globin,26 GATA-1,24,27 or L-32.24 Probes (see Figure 5D)were prepared as previously described.24 27
Immunoprecipitation and Western blot analysis
Populations of HCD57 or HCDP210 cells or of infected wild-type fetal liver cells were washed 4 times in serum-free media and starved overnight in IMDM containing 0.1% FCS. HCD57 and HCDP210 (5 × 107 cells), and fetal liver cells (2 × 106 cells) were stimulated 18 hours later with or without Epo (100 u/mL) for 5 minutes at 37°C. Cells were then washed twice with cold PBS and extracts were prepared by the addition of 1 mL lysis buffer, 50 mM Tris-HCl (pH 7.5 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid [EDTA], 2 mM Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 1% Brij-96, 1 mM dithiothreitol (DTT). For immunoprecipitations, cell extracts were incubated overnight at 4°C with 5 μL anti-JAK2 polyclonal anti-sera (Upstate Biotechnology, Lake Placid, NY) or with 1 μg mouse anti-c-ABL monoclonal antibody (sc-23; Santa Cruz). Immune complexes were recovered by binding to protein A-Sepharose or protein G-Sepharose beads (Roche-Boehringer). The captured immunocomplexes were washed 3 times with lysis buffer and once with PBS and were then eluted by boiling in sodium dodecyl sulfate (SDS) sample buffer. Samples were fractionated through SDS-polyacrylamide gels, transferred electrophoretically to nitrocellulose membranes, and incubated with the indicated antiserum: (1) antiphosphotyrosine monoclonal antibodies (4G10, Upstate Biotechnology) (1:1000); (2) anti-JAK2 polyclonal antibodies (1:1000); and (3) anti-ABL monoclonal antibodies (1:1000). Bound antibodies were detected by enhanced-chemiluminescence system (DuPont-NEN).
Results
JAK2 is constitutively phosphorylated in cultured erythroleukemia cells and primary erythroid cells expressing BCR-ABL
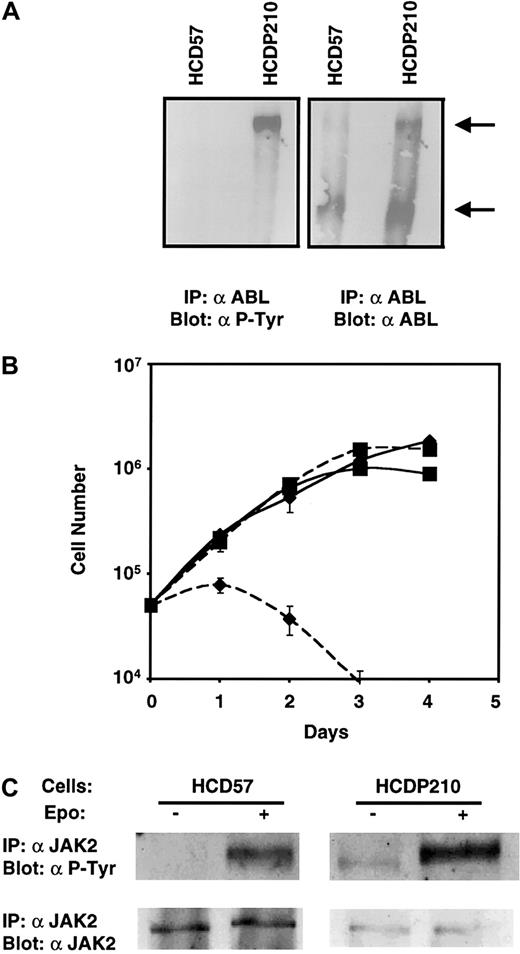
We first examined the tyrosine phosphorylation status of JAK2 in an erythroid cell line expressing BCR-ABL. We expressed P210 in the Epo-dependent erythroleukemic HCD57 cells (Figure2A) and derived a population that proliferated in the absence of Epo (HCDP210 cells, Figure 2B). The Western blot in Figure 2A shows that P210 is expressed in the Epo-independent HCDP210 cells (right panel) and as expected, P210 BCR-ABL but not c-ABL (left panel) was tyrosine phosphorylated in HCDP210 cells starved of all growth factors. As shown in Figure 2C, JAK2 is tyrosine phosphorylated and thus activated in the presence but not the absence of Epo in HCD57 cells. In contrast, HCDP210 cells exhibited a low level of JAK2 tyrosine phosphorylation in the absence of Epo, and Epo stimulation induced a further increase of JAK2 tyrosine phosphorylation (Figure 2C, upper panel). In HCDP210 cells, Epo addition also caused the mobility of the tyrosine phosphorylated JAK2 to decrease, suggesting that after Epo addition JAK2 becomes phosphorylated on tyrosine residues additional to those phosphorylated as a result of BCR-ABL expression, and that causes a slower gel migration.
Epo-independent HCDP210 cells.
(A) Expression and tyrosine phosphorylation of P210 in Epo-independent HCDP210 cells: Fifty million serum-starved HCD57 and HCDP210 cells were lysed and ABL immunoprecipitates were fractionated on a 6% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and probed with antiphosphotyrosine (α P-Tyr) (4G10, left panel) or anti-ABL antibodies (right panel). Arrows show BCR-ABL P210 (top) and the endogenous c-ABL (bottom) proteins. IP indicates immunoprecipitation. (B) Growth curve of HCD57 (diamond) and HCDP210 (square) cells in the presence (solid lines) and the absence (dotted lines) of 2 u/mL Epo. (C) Constitutive JAK2 tyrosine phosphorylation in HCDP210 cells: serum starved cells were stimulated with or without Epo (100 u/mL) for 5 minutes. JAK2 immunocomplexes were fractionated on an 8% SDS-PAGE, transferred to nitrocellulose, and probed with 4G10 (upper panel) or anti-JAK2 antisera (lower panel).
Epo-independent HCDP210 cells.
(A) Expression and tyrosine phosphorylation of P210 in Epo-independent HCDP210 cells: Fifty million serum-starved HCD57 and HCDP210 cells were lysed and ABL immunoprecipitates were fractionated on a 6% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and probed with antiphosphotyrosine (α P-Tyr) (4G10, left panel) or anti-ABL antibodies (right panel). Arrows show BCR-ABL P210 (top) and the endogenous c-ABL (bottom) proteins. IP indicates immunoprecipitation. (B) Growth curve of HCD57 (diamond) and HCDP210 (square) cells in the presence (solid lines) and the absence (dotted lines) of 2 u/mL Epo. (C) Constitutive JAK2 tyrosine phosphorylation in HCDP210 cells: serum starved cells were stimulated with or without Epo (100 u/mL) for 5 minutes. JAK2 immunocomplexes were fractionated on an 8% SDS-PAGE, transferred to nitrocellulose, and probed with 4G10 (upper panel) or anti-JAK2 antisera (lower panel).
To examine the status of JAK2 tyrosine phosphorylation in primary erythroid cells expressing P210 we chose wild-type E14 fetal liver cells since this population of cells is mostly erythroid. We used a bicistronic retroviral vector to express P210 and GFP together (MSCV-P210-IRES-GFP) or, as a control, GFP alone (MSCV-IRES-GFP), and we used GFP expression as a marker of retroviral transduction. In these bicistronic retroviral vectors, the expression of GFP and P210 is under the transcriptional control of the retroviral long-terminal repeat and the translational control of IRES of the encephalomyocarditis virus, resulting in the expression of both GFP and P210 in the same cell (see “Materials and methods”).
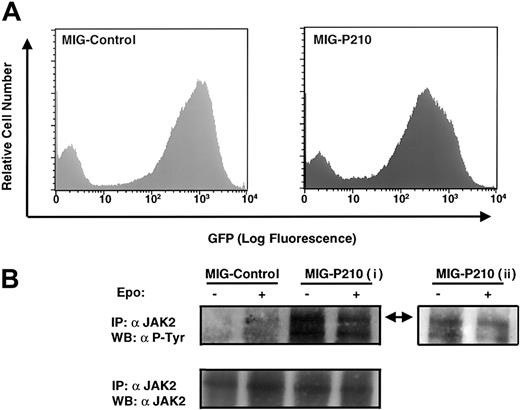
Wild-type E14 fetal liver cells were retrovirally transduced with either MSCV-P210-IRES-GFP or the control vector and analyzed by FACS for GFP expression. Approximately 50% of P210-infected and 70% of control vector–infected fetal liver cells expressed the GFP marker (Figure 3A). To avoid cellular loss, we subjected the totality of these populations, without further FACS selection, to serum starvation and examined the tyrosine phosphorylation status of JAK2 in response to Epo stimulation. A low level of JAK2 tyrosine phosphorylation in response to Epo stimulation was detected in primary fetal liver cells infected with control vector but, as expected, none was detected in the absence of Epo (Figure 3B, upper panel). In contrast, tyrosine phosphorylation of JAK2 was easily detectable in P210-transduced fetal liver cells starved in the absence of serum and Epo, and Epo stimulation did not affect the level of JAK2 tyrosine phosphorylation (Figure 3B, upper panel, i). A shorter exposure of the Western blot confirmed the conclusion that the level of JAK2 tyrosine phosphorylation in BCR-ABL–transduced cells is unaffected by addition of Epo (Figure 3B, upper panel, ii). As a control, we showed that JAK2 protein was present at equal levels in P210-transduced and control fetal liver cells (Figure 3B, lower panel).
Tyrosine phosphorylation of JAK2 in primary fetal liver cells retrovirally expressing BCR-ABL.
(A) Flow cytometry analysis of GFP expression of wild-type fetal liver cells infected with either MSCV-P210-IRES-GFP or the control vector MSCV-IRES-GFP. Live cells were gated. (B) Equal number of serum-starved cells from A were stimulated with or without Epo (100 u/mL). JAK2 immunoprecipitates were run on an 8% SDS-PAGE and probed with antiphosphotyrosine antibody (4G10) (upper panel, i: 2 hours exposure; ii: 10 minutes exposure) or anti-JAK2 antisera (lower panel). The arrow indicates JAK2; IP, immunoprecipitation; WB, Western blot.
Tyrosine phosphorylation of JAK2 in primary fetal liver cells retrovirally expressing BCR-ABL.
(A) Flow cytometry analysis of GFP expression of wild-type fetal liver cells infected with either MSCV-P210-IRES-GFP or the control vector MSCV-IRES-GFP. Live cells were gated. (B) Equal number of serum-starved cells from A were stimulated with or without Epo (100 u/mL). JAK2 immunoprecipitates were run on an 8% SDS-PAGE and probed with antiphosphotyrosine antibody (4G10) (upper panel, i: 2 hours exposure; ii: 10 minutes exposure) or anti-JAK2 antisera (lower panel). The arrow indicates JAK2; IP, immunoprecipitation; WB, Western blot.
Thus, JAK2 becomes constitutively tyrosine phosphorylated and presumably active, as a result of BCR-ABL expression both in HCD57 erythroleukemia and in fetal liver erythroid cells.
JAK2 is not required for red cell formation by BCR-ABL
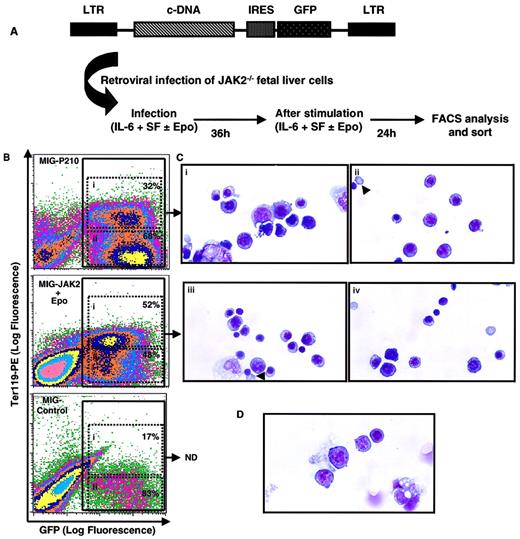
To assess whether tyrosine phosphorylation of JAK2 and activation of JAK2 signaling pathways is required for BCR-ABL–induced maturation of erythroid progenitors, we examined the potential of P210 to support red cell formation in the absence of JAK2 by using primary JAK2−/− fetal liver cells. To this end, E12.5 JAK2−/− fetal liver cells were retrovirally transduced with either P210, JAK2, or vector control and analyzed for GFP expression as a measure of infection efficiency (Figure4B; see “Materials and methods”). We established optimum conditions (Figure 4A) for retroviral transduction of JAK2−/− fetal liver cells; this also allowed for a significant expansion of transduced erythroid progenitors. Furthermore, this system provided reproducible conditions for direct comparison of the effects of transduced P210 and JAK2 on JAK2−/−fetal liver cells.
JAK2 is not required for red cell formation by BCR-ABL.
(A) Retroviral infection of JAK2−/− fetal liver cells: E12.5 JAK2−/− fetal livers contain very few cells (< 2 × 105 cells/embryo)5 and are unresponsive to many cytokines used in retroviral infection.6 The protocol detailed here provided optimum conditions for retroviral infection of JAK2−/− fetal liver cells. (B) Flow cytometry analysis of transduced JAK2−/− fetal liver cells, a representative analysis of 10 independent experiments performed. E12.5 JAK2−/− fetal liver cells were infected with the control vector MIG-control or with the bicistronic retroviral vectors MIG-P210 or MIG-JAK2 in the presence of SF and IL-6 alone (MIG-control and MIG-P210) or together with Epo added 12 hours after initiation of infection (MIG-JAK2). Cells were analyzed 60 hours later for their expression of GFP and the erythroid marker Ter119-PE; 100 000 live events were collected. The percentage of Ter119+ (i) and Ter119− (ii) cells within the population of GFP+ cells are indicated in each case. (C) GFP-positive cells (panel B, populations i and ii) of JAK2−/− fetal liver cells transduced with MIG-P210 and cultured in the absence of Epo (Ci, ii) or with MIG-JAK2 and cultured in the presence of Epo (iii, iv) were isolated by FACS and analyzed for their morphology by Wright Giemsa staining. Representative fields are depicted (× 1000). Arrows (Cii, iii) show an enucleated erythrocyte derived from a P210-transduced cell (Cii), or an orthochromatic erythroblast derived from a JAK2-transduced cell cultured with Epo, in the process of exonucleation (Ciii). Control MIG-transduced cells did not generate enough cells to perform morphologic analyses routinely. (D) A representative field of Wright Giemsa analysis of freshly isolated JAK2−/− fetal liver cells (× 1000). ND indicates not determined.
JAK2 is not required for red cell formation by BCR-ABL.
(A) Retroviral infection of JAK2−/− fetal liver cells: E12.5 JAK2−/− fetal livers contain very few cells (< 2 × 105 cells/embryo)5 and are unresponsive to many cytokines used in retroviral infection.6 The protocol detailed here provided optimum conditions for retroviral infection of JAK2−/− fetal liver cells. (B) Flow cytometry analysis of transduced JAK2−/− fetal liver cells, a representative analysis of 10 independent experiments performed. E12.5 JAK2−/− fetal liver cells were infected with the control vector MIG-control or with the bicistronic retroviral vectors MIG-P210 or MIG-JAK2 in the presence of SF and IL-6 alone (MIG-control and MIG-P210) or together with Epo added 12 hours after initiation of infection (MIG-JAK2). Cells were analyzed 60 hours later for their expression of GFP and the erythroid marker Ter119-PE; 100 000 live events were collected. The percentage of Ter119+ (i) and Ter119− (ii) cells within the population of GFP+ cells are indicated in each case. (C) GFP-positive cells (panel B, populations i and ii) of JAK2−/− fetal liver cells transduced with MIG-P210 and cultured in the absence of Epo (Ci, ii) or with MIG-JAK2 and cultured in the presence of Epo (iii, iv) were isolated by FACS and analyzed for their morphology by Wright Giemsa staining. Representative fields are depicted (× 1000). Arrows (Cii, iii) show an enucleated erythrocyte derived from a P210-transduced cell (Cii), or an orthochromatic erythroblast derived from a JAK2-transduced cell cultured with Epo, in the process of exonucleation (Ciii). Control MIG-transduced cells did not generate enough cells to perform morphologic analyses routinely. (D) A representative field of Wright Giemsa analysis of freshly isolated JAK2−/− fetal liver cells (× 1000). ND indicates not determined.
Ter119 is a marker of erythroid differentiation28,29expressed on the cell surface of most (80%-90%) wild-type E14 fetal liver cells (data not shown). In contrast, Ter119 is present only on 10% to 20% of JAK2−/− fetal liver cells, consistent with the notion that these cells are blocked in an early stage of erythroid differentiation5 (and data not shown). We therefore used Ter119 as a marker of erythroid differentiation and analyzed its expression two and a half days postinfection by flow cytometry of retrovirally transduced GFP-positive cells. In contrast to control-infected cells, which exhibited less than 20% Ter119 positivity within the GFP-positive population, (these “Ter119 positive cells” clearly include many autofluorescent cells within the chosen window [Figure 4B, bottom panel]), in P210- and JAK2-infected populations 30% to 50% of GFP-positive cells were Ter119-positive (Figure 4B). Thus, P210 expression induced a significant proportion of Ter119-positive cells even in the absence of JAK2.
To confirm this key point, we used the FACS to isolate all retrovirally transduced (ie, GFP-positive) cells from JAK2 and P210-infected populations, regardless of their Ter119 expression (Figure 4B, populations i and ii) and analyzed their morphology (Figure 4C). A majority of GFP-positive cells transduced with P210 were morphologically erythroid, at different stages of differentiation, as were a majority of JAK2-transduced cells that were cultured in the presence of Epo (Figure 4C). In contrast, freshly isolated JAK2−/− fetal liver cells, prior to infection, exhibited only a few erythroid cells (Figure 4D). Specifically, expression either of JAK2 (in the presence of Epo) or P210 (in the absence of Epo) induced terminal erythroid differentiation and maturation of some of JAK2−/− fetal liver cells (Figure 4Cii,iii). In contrast, freshly isolated JAK2−/− fetal liver cells did not exhibit any mature enucleated erythrocytes. Interestingly, JAK2-transduced cells cultured in the presence of Epo were found mostly at a slightly later stage of differentiation (60% orthochromatic erythroblasts and 33% polychromatophilic erythroblasts) as compared with P210-transduced cells (which contained only 25% orthochromatic erythroblasts and 58% polychromatophilic erythroblasts). Thus, JAK2 and BCR-ABL are similar, but not identical, in their abilities to support induction of erythroid differentiation and maturation from JAK2−/− fetal liver cells.
Ter119 positive cells are composed of erythroblasts at different stages of differentiation and do not give rise to BFU-E or CFU-E erythroid colonies29 (and data not shown). Thus, they do not contain any committed erythroid progenitors. Consequently, the population of GFP+Ter119− cells encompasses all retrovirally transduced cells including all transduced progenitors of erythroid and other hematopoietic cell type origin. Thus, GFP+Ter119− cells, the population relatively enriched in transduced erythroid progenitor cells, were selectively FACS-sorted from infected JAK2−/− fetal liver cells (population ii in Figure 4B) and plated in semisolid cultures in the presence of SF. CFU-E–derived colonies that stained positively with diaminobenzidine, a hemoglobin marker, were counted 2 days later. JAK2−/− fetal liver cells transduced with P210 generated as many mature erythroid CFU-E progenitors in vitro as did cells transduced with JAK2 and cultured in the presence of Epo (Figure5A). The size of clusters and the degree of hemoglobinization were comparable between the 2 groups (data not shown). No CFU-E colonies were formed from JAK2−/− fetal liver cells infected with the control vector (Figure 5A).
BCR-ABL rescues JAK2−/− erythroid progenitors.
The population of GFP+Ter119− cells encompasses all retrovirally transduced erythroid progenitors and, as detailed in the legend to Figure 4B, this population was FACS sorted from JAK2−/− fetal liver cells transduced with either JAK2 or P210 BCR-ABL. From these, CFU-E (A) and BFU-E (B) colonies were generated. Total numbers of diaminobenzidine-positive CFU-Es cultured in the presence or absence of 2 u/mL Epo and 100 ng/mL SF (A) and BFU-Es cultured in the presence or absence of 2 u/mL Epo, 100 ng/mL SF, and 10 ng/mL IL-6 (B) were counted after 2 (A) and 9 (B) days, respectively. Results are presented as percentage of colonies formed in the JAK2-infected populations and cultured under optimum conditions (Epo and SF for CFUE [A] and Epo, SF, and IL-6 for BFU-E [B]) shown by the hatched bars. Graphs are averages from duplicate cultures of 4 (A) and 5 (B) independent experiments. Under optimum conditions, the average absolute number of JAK2-transduced colonies derived from JAK2−/− fetal liver cells is 1248 ± 263 CFU-E and 96 ± 9 BFU-E colonies per 105GFP+Ter119− FACS-sorted cells. (C) Representative BFU-E–derived colonies from GFP+Ter119− JAK2−/− fetal liver cells transduced with either P210 or JAK2 and cultured in the presence of SF and IL-6, with (JAK2-transduced cells) or without (P210-transduced cells) Epo (× 100). (D) PolyA RT-PCR from single P210- or JAK2-generated BFU-E colonies of similar size were analyzed by southern blotting probed with β-major globin, GATA-1, and L-32. Shown are representative results from 3 independent experiments. Controls consist of HCD57 (104 cells) in lane 1 and PCR reagents with no cells in lane 2.
BCR-ABL rescues JAK2−/− erythroid progenitors.
The population of GFP+Ter119− cells encompasses all retrovirally transduced erythroid progenitors and, as detailed in the legend to Figure 4B, this population was FACS sorted from JAK2−/− fetal liver cells transduced with either JAK2 or P210 BCR-ABL. From these, CFU-E (A) and BFU-E (B) colonies were generated. Total numbers of diaminobenzidine-positive CFU-Es cultured in the presence or absence of 2 u/mL Epo and 100 ng/mL SF (A) and BFU-Es cultured in the presence or absence of 2 u/mL Epo, 100 ng/mL SF, and 10 ng/mL IL-6 (B) were counted after 2 (A) and 9 (B) days, respectively. Results are presented as percentage of colonies formed in the JAK2-infected populations and cultured under optimum conditions (Epo and SF for CFUE [A] and Epo, SF, and IL-6 for BFU-E [B]) shown by the hatched bars. Graphs are averages from duplicate cultures of 4 (A) and 5 (B) independent experiments. Under optimum conditions, the average absolute number of JAK2-transduced colonies derived from JAK2−/− fetal liver cells is 1248 ± 263 CFU-E and 96 ± 9 BFU-E colonies per 105GFP+Ter119− FACS-sorted cells. (C) Representative BFU-E–derived colonies from GFP+Ter119− JAK2−/− fetal liver cells transduced with either P210 or JAK2 and cultured in the presence of SF and IL-6, with (JAK2-transduced cells) or without (P210-transduced cells) Epo (× 100). (D) PolyA RT-PCR from single P210- or JAK2-generated BFU-E colonies of similar size were analyzed by southern blotting probed with β-major globin, GATA-1, and L-32. Shown are representative results from 3 independent experiments. Controls consist of HCD57 (104 cells) in lane 1 and PCR reagents with no cells in lane 2.
Similarly, P210 expression in JAK2−/− fetal liver cells, cultured in the presence of SF and IL-6 but in the absence of Epo, induced the generation of primitive BFU-E–derived colonies (Figure5B). P210-induced BFU-E colonies were similar in number to those induced by the expression of JAK2 in JAK2−/− fetal liver cells and then cultured with Epo in addition to SF and IL-6. P210- and JAK2-generated BFU-E colonies were comparable in size, morphology, and degree of hemoglobinization (Figure 5C), and contained morphologically normal erythroblasts (data not shown). In addition, these colonies expressed erythroid genes such as β-major globin and GATA-1 (Figure5D). Taken together, these data demonstrate that P210-generated BFU-E colonies from JAK2−/− fetal liver cells were in all aspects similar to JAK2-rescued erythroid colonies cultured with Epo. Thus, P210 and JAK2 are equivalent in their abilities to support BFU-E and CFU-E formation in fetal liver cells lacking JAK2.
In addition to Epo, for their optimum proliferation and survival in in vitro culture assays, primitive erythroid progenitors require cytokines and growth factors such as IL-3, IL-6, GM-CSF, and SF. Previously, we showed that P210-transduced EpoR−/− fetal liver cells generate a significant numbers of BFU-E–derived colonies in the absence of any added growth factors, indicating that in EpoR−/− cells, BCR-ABL partially complements cytokine signaling pathways normally induced by IL-3, IL-6, and SF.18 This is significantly different from our current findings with P210-infected JAK2−/− fetal liver cells, which did not generate any BFU-E–derived colonies above the background when cultured in the absence of SF and IL-6 (Figure 5B). Thus, some critical signals induced by IL-6 or SF in EpoR−/− cells require JAK2, and BCR-ABL cannot complement these signals unless JAK2 is present.
JAK2 is required for red cell formation by certain BCR-ABL mutants expressed in fetal liver cells
We previously demonstrated that the P210 mutants P210 ΔSH2 and P210 Y117F, whose transforming potential and capacity in activating certain cytokine signaling pathways are compromised (Table 1), still can induce red cell differentiation to the same extent as wild-type P210 in EpoR−/− as well as wild-type fetal liver cells.17 Here, by transducing these mutants into JAK2−/− fetal liver cells, we determined their ability to support erythropoiesis in the absence of JAK2 (Table2).
Erythroid colony-forming units in fetal liver cells retrovirally transduced with BCR-ABL mutants
| BCR-ABL constructs . | Wild type (%) . | JAK2−/−(%) . |
|---|---|---|
| P210 | ND | 100(2) |
| P210 ΔSH2 | ND | 32 ± 4* |
| P210 Y177F | ND | 68 ± 7† |
| P185 | 100(1) | 399 ± 98† |
| P185Δ176-427 | 5.5 ± 2* | 15 ± 5* |
| P185 TM | 19.5 ± 4* | ND |
| BCR-ABL constructs . | Wild type (%) . | JAK2−/−(%) . |
|---|---|---|
| P210 | ND | 100(2) |
| P210 ΔSH2 | ND | 32 ± 4* |
| P210 Y177F | ND | 68 ± 7† |
| P185 | 100(1) | 399 ± 98† |
| P185Δ176-427 | 5.5 ± 2* | 15 ± 5* |
| P185 TM | 19.5 ± 4* | ND |
Results are obtained in the absence of Epo and are shown as a percentage of control ± se; the 100% control is determined by (1) expression of P185 in wild-type fetal liver resulting in generation of 5230 ± 1050 CFU-E per 105GFP+Ter119− FACS-sorted cells or (2) expression of P210 in JAK2−/− fetal liver resulting in generation of 1268 ± 221 per 105GFP+Ter119− FACS-sorted cells.
P < .001.
P < .01.
The P value for each mutant is determined by comparing numbers obtained for the mutant to the ones obtained for P185, in wild-type fetal liver cells, and to the numbers obtained for P210, in JAK2−/− fetal liver cells. ND indicates not determined.
Depending on the BCR-ABL mutant (Table 1) studied, we used either wild-type P210 or P185 as the control. As expected, the ability of P185 to induce CFU-E formation from JAK2−/− fetal liver cells was higher than that of P210 (Table 2), most likely due to the elevated protein tyrosine kinase activity of P185.14,15 The ability of BCR-ABL mutants P210 Y177F and P210 ΔSH2 to support erythroid differentiation, as monitored by the number of CFU-Es, was significantly impaired in JAK2−/− fetal liver cells (Table 2). This is in contrast to the normal ability of these mutants to support erythropoiesis in EpoR−/− fetal liver cells.18 Although we are not certain of the precise signaling pathways “missing” in these BCR-ABL mutants but required for full erythropoietic support by BCR-ABL in JAK2−/−fetal liver cells, we conclude that there exists at least one signaling pathway that is activated by BCR-ABL but not by these mutants, and that is essential for erythropoiesis in JAK2−/− but not in EpoR−/− fetal liver cells.18 These results suggest that JAK2 directly (ie, independent of EpoR phosphotyrosines) activates downstream signal transduction pathways that support normal erythropoiesis and that are activated by P210 but not by these mutants (see “Discussion”).
Among the 3 BCR-ABL mutants tested (Table 2), P185 Δ176-427 was noticeably deficient in supporting erythropoiesis in both JAK2−/− and wild-type fetal liver cells (Table 2). The difference from wild-type BCR-ABL was not due to differences in the amount of proteins produced in retrovirally transduced cells (Figure6). In wild-type fetal liver cells (Table2) the so-called P185 triple mutant (P185 TM)30 supported little erythropoiesis, compared with P185. This mutant, which contains a mutation in the Grb-2 binding site (Y177F), the phosphotyrosine-binding site within the SH2 domain (R552L) and the autophosphorylation site (Y793F), is severely deficient in its transforming potential.30 However, the ability of P185 TM to rescue erythropoiesis in wild-type fetal liver cells was constantly and reproducibly better than that of P185 Δ176-427 (Table 2). This was surprising, given that transforming potential and ability to confer growth factor independence are more severely compromised by the P185 TM mutation than by P185 Δ176-427 in all cell types and assays tested.30-32 Taken together, these results suggest an important role in supporting erythropoiesis for signaling pathways downstream of EpoR that overlap with signaling pathways activated specifically by BCR-ABL amino acids 176-427.
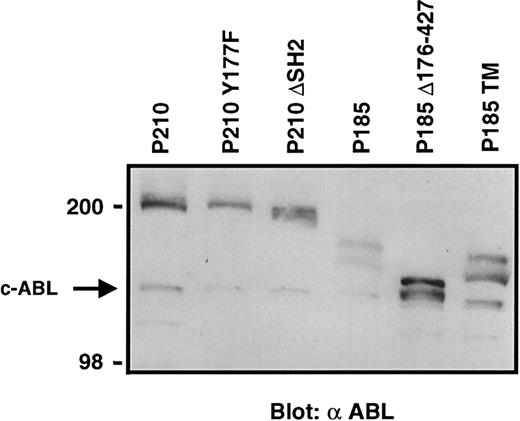
Comparable protein expression in cells transduced with BCR-ABL mutant retroviral supernatants.
Western blot analysis of expression of BCR-ABL and its mutant in 3T3 cells infected with a 1-in-10 dilution of transducing retroviral particles used in experiments summarized in Table 2. The arrow indicates the endogenous c-ABL protein.
Comparable protein expression in cells transduced with BCR-ABL mutant retroviral supernatants.
Western blot analysis of expression of BCR-ABL and its mutant in 3T3 cells infected with a 1-in-10 dilution of transducing retroviral particles used in experiments summarized in Table 2. The arrow indicates the endogenous c-ABL protein.
Discussion
Cumulative evidence in the past decade supports the notion that cell fate is determined by the cellular context in which signaling proteins are expressed.11 Tyrosine phosphorylation and activation of JAK2 is key to erythroid development. In response to Epo stimulation, JAK2 becomes tyrosine phosphorylated and activated; JAK2 then phosphorylates the EpoR on several tyrosine residues leading to the activation of multiple signaling pathways [reviewed in Constantinescu et al7]. In addition to the EpoR, JAK2 binds to other cytokine receptors and cytosolic proteins, and directly activates, in a receptor-phosphotyrosine independent fashion, signaling pathways such as STAT5 and Shc.8 9 The relative contribution of different EpoR-activated signaling pathways to erythroid development, specifically proliferation, differentiation, and survival of erythroid progenitors, is not well understood. As a first step in identifying potential EpoR signaling components sufficient to support erythropoiesis in primary erythroid cells, we activated signaling pathways downstream of the EpoR using the well-characterized BCR-ABL oncoprotein and its mutants.
The constitutively active protein tyrosine kinase BCR-ABL supports erythropoiesis in both wild-type and EpoR−/− fetal liver cells.18 In BCR-ABL–expressing erythroid cells, JAK2 is tyrosine phosphorylated (Figures 2 and 3). Thus, we asked whether JAK2 is an obligatory signaling protein for induction of erythropoiesis downstream of BCR-ABL. Using Ter119 as a cell surface marker of erythroid cells, functional progenitor assays, and morphologic analyses, we have demonstrated that JAK2 is not required for BCR-ABL induction of red cell formation. However, our data indicate that BCR-ABL does not support erythroid maturation quantitatively as well as JAK2 does and that these two protein tyrosine kinases are not identical in their ability to support terminal differentiation and maturation of erythroid cells.
Our findings also indicate that the ability of BCR-ABL to activate signaling pathways required for erythropoiesis is different in EpoR−/− and JAK2−/− fetal liver cells. As example, BCR-ABL expression in EpoR−/− fetal liver cells supports the formation of 25% of the maximum number of BFU-E–derived colonies in the absence of any added growth factors, namely IL-3, IL-6, and SF.18 In contrast, here we showed that in the absence of JAK2, BCR-ABL expression did not alleviate the requirement for SF and IL-6, in generation of BFU-E–derived erythroid colonies.
Other examples are provided by the BCR-ABL mutants lacking either the SH2 domain (P210 ΔSH2), or the Y177 residue that is essential for activation of the Ras pathway by BCR-ABL in certain cells. Despite their impaired signaling potential, P210 ΔSH2 or P210Y177F replace EpoR signaling and support erythropoiesis in EpoR−/−fetal liver cells to the same extent as does P210.18 In contrast, here we found that P210 ΔSH2 and P210Y177F do not support efficient erythroid development in the absence of JAK2 (Table 2). Taken together, these results suggest that tyrosine phosphorylation and presumably activation of JAK2 in BCR-ABL–expressing cells activates signaling pathways that overlap with those activated by the P210 SH2 domain or P210 (phospho) Y177. Activation of these pathways either via JAK2 or these BCR-ABL domains is important for normal erythropoiesis in EpoR−/− and also in wild-type fetal liver cells.
Although JAK2 is constitutively tyrosine phosphorylated in BCR-ABL–expressing erythroid (Figures 2, 3) and other myeloid cells,33 34 the mechanism of this effect is unknown. Interestingly, JAK2 immunocomplexes recovered from HCDP210 cells constantly contained P210 (data not shown), suggesting P210 and JAK2 are interacting in BCR-ABL–expressing erythroid cells and that BCR-ABL may directly phosphorylate JAK2. The tyrosine residues in JAK2 that become phosphorylated in BCR-ABL–expressing cells are unknown. Phosphorylation of certain tyrosines in JAK2 may directly activate its kinase activity. Alternatively, phosphotyrosines can provide docking sites for binding SH2 or phosphotyrosine binding domains of cytosolic proteins that subsequently become phosphorylated by JAK2.
In this study we identified at least one domain of BCR-ABL that is crucial for erythroid development in both wild-type and JAK2−/− fetal liver cells. The 176-427 deletion mutant of P185 (P185 Δ176-427) did not support erythropoiesis in the absence of Epo when expressed in fetal liver cells, regardless of whether they expressed JAK2. For several reasons the failure of P185 Δ176-427 to support wild-type fetal erythropoiesis in the absence of Epo does not appear to be due to the absence of the Grb-2–binding site Y177: first, we showed previously that Y177 is dispensable for BCR-ABL complementation of EpoR signaling in EpoR−/− fetal liver cells.18 Second, the Y177 residue is also missing in P185 TM, which we showed here supports wild-type fetal liver erythropoiesis better than does P185 Δ176-427 (Table 2). Moreover, despite the lower potency of P185 Δ176-427 as compared with P185 TM in its ability to support erythropoiesis, P185 Δ176-427 is more potent than P185 TM in its transforming ability.30 The failure of the P185 Δ176-427 mutant to support erythropoiesis in fetal liver cells is likely not due to a lack of STAT5 activation, since the BCR-ABL kinase domain and domains supporting the activation of STAT535are all conserved in this mutant.
Some clues to the deficient support of erythropoiesis by the P185 Δ176-427 mutant may come from an analysis of its missing sequence: sequences within 176-427 of BCR bind BCR-associated protein-1, a member of the 14-3-3 family of adapter proteins.36 The 14-3-3 family of proteins binds to serine/threonine phosphorylated proteins such as the MAPKKK Raf137,38; the proapoptotic protein BAD, a BCL-2 family-member; and the transcription factor Forkhead, which increases the expression of apoptotic genes,39,40all targets of the serine threonine kinase AKT.41 Upon phosphorylation by activated AKT, Forkhead and BAD bind 14-3-3 proteins and are subsequently sequestered in the cytoplasm away from their targets, thereby inhibiting their proapoptotic functions.41 Interestingly, the 176-427 sequence within BCR-ABL has been shown to regulate PI3-kinase/AKT,42Raf1,43 and the 14-3-3 family of adapter proteins,36 all implicated in erythropoiesis44-46 (and S.G. and H.L., unpublished data, August 2000). Our recent findings support an important role for AKT in erythroid development.47 In addition, the data presented here may point to a central role for the 14-3-3 family of adapter proteins in erythropoiesis and we are currently investigating this possibility.
STAT5 activation is important for erythroid survival as demonstrated by studies of STAT5a−/−b−/− mice.48 BCR-ABL expression in hematopoietic cultured cell lines clearly results in the activation of STAT533,34 and its known transcriptional target BCL-xL.49 However, the role of STAT5 in BCR-ABL function is not clear.50-52 STAT5 is moderately phosphorylated in BCR-ABL–expressing myeloid cells isolated from mice with CML-like disease.53,54 Moreover, STAT5 was not activated in Epo-independent HCDP210 cells as assessed by electrophoretic mobility shift assay (data not shown). The SH2 and SH3 domains of BCR-ABL have been reported to be required together for activation of the STAT5 pathway.35 Although it contains intact SH2 and SH3 domains, we found that P185 Δ176-427 was unable to support fetal liver erythropoiesis in the absence of Epo. Furthermore, STAT5a−/−b−/− fetal liver cells transduced with BCR-ABL generated normal numbers of erythroid colonies in the absence of Epo (S.G. and H.L., unpublished data, February 2000), and thus we exclude any significant role for STAT5 in BCR-ABL complementation of EpoR signaling. Taken together, our findings support the notion that pathways other than STAT5, potentially involving 14-3-3, PI3-kinase/AKT, and/or Raf-1 are activated by BCR-ABL in fetal liver cells and are essential for normal erythropoiesis.
Through JAK2, the EpoR activates multiple signaling pathways that function together to prevent apoptosis and to support terminal erythroid proliferation and differentiation. Here we have shown that BCR-ABL activates similar pathways independently of JAK2, and supports erythroid survival, proliferation and differentiation in JAK2−/− fetal liver cells. Since certain BCR-ABL mutants were defective in supporting erythropoiesis in JAK2−/−but not in EpoR−/− fetal liver cells, we conclude that pathways activated directly by JAK2, independently of its stimulation by the EpoR and of receptor-phosphotyrosines, overlap with BCR-ABL–activated pathways and may contribute to normal erythroid development.
We thank Luk Van Parijs (MIT) for MSCV-IRES-GFP; Norman Iscove (Ontario Cancer Institute) and Trang Hoang (Clinical Research Institute of Montreal) for the GATA-1 and L-32 probes; Gordon Keller (Mount Sinai School of Medicine) for the β-major globin probe; Ann Marie Pendergast (Duke University) for pSRαMSVtkneo-P185 TM and pSRαMSVtkneo-P185 Δ176-427; George Daley (Whitehead Institute) for pGDP185; and James Ihle (St Jude Children's Research Hospital) for pRK5-JAK2. We are grateful to Carlo Brugnara (Harvard Medical School) and Bill Schiemann (Whitehead Institute) for discussions and critical reading of the manuscript and to Glenn Paradis (MIT, Cancer Center) for cell sorting. We are indebted to Guy Sauvageau and his group (Clinical Research Institute of Montreal) for providing invaluable help with single cell polyA–RT-PCR.
Supported by a Clinician Scientist Award from The National Institutes of Health (National Cancer Institute) to S.G., and the following grants to H.F.L.: grant #HL-32262 from The National Institutes of Health, a grant from Amgen Corporation, and NSF grant #EEC-9843342/67983 from the Biotechnology Process Engineering Center (BPEC) at MIT.
Submitted June 18, 2001; accepted July 10, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Harvey F. Lodish, Whitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, MA 02142; e-mail:lodish@wi.mit.edu.

![Fig. 5. BCR-ABL rescues JAK2−/− erythroid progenitors. / The population of GFP+Ter119− cells encompasses all retrovirally transduced erythroid progenitors and, as detailed in the legend to Figure 4B, this population was FACS sorted from JAK2−/− fetal liver cells transduced with either JAK2 or P210 BCR-ABL. From these, CFU-E (A) and BFU-E (B) colonies were generated. Total numbers of diaminobenzidine-positive CFU-Es cultured in the presence or absence of 2 u/mL Epo and 100 ng/mL SF (A) and BFU-Es cultured in the presence or absence of 2 u/mL Epo, 100 ng/mL SF, and 10 ng/mL IL-6 (B) were counted after 2 (A) and 9 (B) days, respectively. Results are presented as percentage of colonies formed in the JAK2-infected populations and cultured under optimum conditions (Epo and SF for CFUE [A] and Epo, SF, and IL-6 for BFU-E [B]) shown by the hatched bars. Graphs are averages from duplicate cultures of 4 (A) and 5 (B) independent experiments. Under optimum conditions, the average absolute number of JAK2-transduced colonies derived from JAK2−/− fetal liver cells is 1248 ± 263 CFU-E and 96 ± 9 BFU-E colonies per 105GFP+Ter119− FACS-sorted cells. (C) Representative BFU-E–derived colonies from GFP+Ter119− JAK2−/− fetal liver cells transduced with either P210 or JAK2 and cultured in the presence of SF and IL-6, with (JAK2-transduced cells) or without (P210-transduced cells) Epo (× 100). (D) PolyA RT-PCR from single P210- or JAK2-generated BFU-E colonies of similar size were analyzed by southern blotting probed with β-major globin, GATA-1, and L-32. Shown are representative results from 3 independent experiments. Controls consist of HCD57 (104 cells) in lane 1 and PCR reagents with no cells in lane 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/10/10.1182_blood.v98.10.2948/5/m_h82211740005.jpeg?Expires=1769276696&Signature=FlcmtZFN2PUCdePdCASbURA-gwiaVm3Q7L6Se-BovYE-HJnMl1iAPQoy6m8AU8oCuUMiraYX45EpyFoXI90RPjmsUGB5gKOTrj7qVIla4OSa7qb4HKUH4aYHt5NQy-Be6BzQUJ9W0tZnnr7r5k~x8wXDEEkyKPIk6qJZINasp9aWFDZnvNX57FqtHeZTnIO~-dy6IXj8gFFKBM5eaHgdZiXjYSc2~juMaXPVynrnPCktiSQ36vSN4cCeWWiABiuBd0L0yQSYGmj~MRzML97z-v901Cvr3Qcn8yy8YYir-3rX9d3GBf0-fhvdEyxP8dYjpxVh7Wsgg80Gi4yroJ4uhQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal