Abstract
To investigate B-cell receptor evolution in follicular lymphomas (FLs), immunoglobulin variable heavy chain (VH) gene regions of 3 FLs were analyzed at different time points. One FL with a high somatic mutation load and intraclonal VH gene diversity was investigated in situ. VH gene transcripts were amplified and sequenced from samples of approximately 50 tumor cells isolated from frozen tissue sections by laser microdissection. Interestingly, the mutation pattern of the prevalent subclone in the relapse biopsy was virtually identical to that of a subclone isolated by microdissection from the presentation biopsy 9 years earlier. In a second FL, proof was obtained that the subclone that dominated the relapse sample had already been present in the initial biopsy. The finding that subclones found in the relapses of these FLs had not evolved over time but were preexistent, challenges the concept of antigen-driven B-cell receptor evolution during disease course.
Introduction
Follicular lymphomas (FLs) are thought to originate from germinal-center B cells.1 It is assumed, based on observed intraclonal immunoglobulin variable (IgV) gene diversity,2-5 that FLs have retained the capacity of ongoing somatic hypermutation. This assumption implies that mutations may occur that give rise to frameshifts or stop codons or that affect the overall structure of the immunoglobulin.6 The fact that most FLs express intact B-cell receptors (BCRs) during many years of disease7 therefore suggests that counterselection for such alterations takes place. Indeed, the patterns of somatic mutation in IgV genes of FLs show the characteristics of such a selection process.8-10 Moreover, clonal analyses of individual FLs revealed genealogic relations between the tumor cells, compatible with clonal evolution.11-13 This finding led to the hypothesis that FLs, similar to normal B cells, do not only depend on the expression of a BCR but also on signals elicited by potential ligands of this receptor.3,11 12
Recently, we analyzed the IgV genes of a large panel of FLs.10 Interestingly, in 3 of 4 FLs for which tissue of different time points was available, we found no obvious accumulation of the number of somatic mutations over time, whereas the mutation patterns were not compatible with clonal evolution. On the basis of these data, we questioned whether BCR ligands had played a role in the evolution of some of these FLs. Here, the issue of clonal evolution is investigated in more detail in 3 of these FLs.
Study design
Patient material
Fresh tissue of FLs 3, 6, and 8 and their respective relapse samples were obtained from surgically removed lymph nodes from the departments of pathology of the Academic Medical Center, Amsterdam, the Westeinde Hospital, The Hague, and of the Leiden University Medical Center, the Netherlands. FL 3 was first diagnosed in 1993 (FL 3-'93) and relapsed in 1995 (FL 3-'95). From patient 6, FL tissue was available from 1994 (FL 6-'94) and from 1998 (FL 6-'98). FL 8 was established in 1983 (FL 8-'83) and relapsed in 1992 (FL 8-'92). Clinical data have been described previously.10
Microdissection of samples
Microdissection was performed with a laser-microbeam system (PALM GmbH, Bernried, Germany). Samples of approximately 50 cells were dissected from a 10-μm unstained frozen tissue section from FL 8-'83, “catapulted” into 3 μL complementary DNA (cDNA) reaction mixture, and stored on ice until cDNA synthesis.
cDNA synthesis
RNA of bulk material was isolated from frozen tissue sections and cDNA was synthesized as described.14 From microdissected samples, cDNA was synthesized without prior RNA isolation. Samples were incubated with the cDNA reaction mixture as described14 in a total volume of 20 μL. After incubating for 15 minutes at 37°C, the enzyme was inactivated during 10 minutes at 95°C. Next, 20 μL water was added.
Amplification of the VH gene
cDNA reaction mixture (1 μL) was used in a 25-μL polymerase chain reaction (PCR) volume by using a forward primer specific for the leader of the VH3 gene family in combination with a reverse primer specific for Cμ (Cμ1-: 5′-CGTATCCGACGGGGAATTCTC-3′) or Cγ.14 Next, a nested PCR was performed using 2.5 μL of the first PCR product in a 25-μL reaction. A VH3 primer that anneals in the FR1 region (VH3FR, 5′-TCCCTGAGACTCTCCTGTG-3′) was combined with the appropriate reverse primer, either Cμ, or Cγ2.14 PCR conditions were the same as those described for the CDR3-specific PCR.14
Amplification of time point–specific clones of FL 3 and 6
Time point-specific primers used were 5218+: (5′-GGTGTCCAGTGTGAGGTG-3′) as forward primer and 5218-: (5′-ACGTCCATACCGTAGTCTG-3′) as downstream primer for FL 3 (Figure1B), and 5′-GTGTCCAGTGTGGGAGCAA-3′ as forward primer and either 5′-TCTCAGACTGTTCATTTGTAA-3′ or 5′-CCCTTGGTGGAAGCTGAG-3′ as downstream primer for FL 6. cDNA (1 μL) was used in 25-μL reaction mixture. The PCR protocol was the same as for the VH family-specific PCR.14 The obtained PCR products were subsequently sequenced.
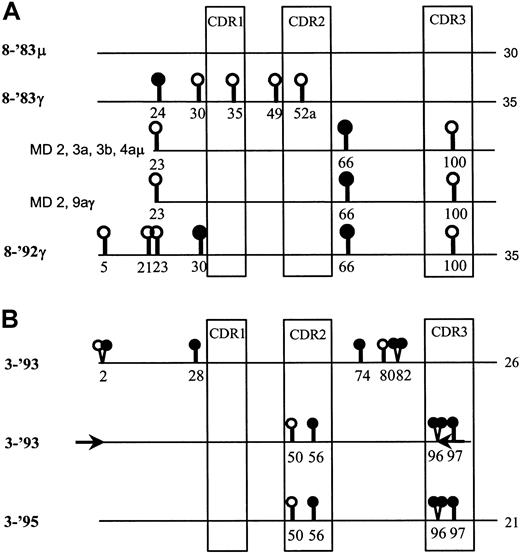
Evidence for subclone selection over time in FL 8 and FL 3.
(A) The IgM- and IgG-derived VH sequences from FL 8-'83 (8-'83μ and 8-'83γ, respectively) and from the relapse, 8-'92, are represented as lines. The consensus sequences had been determined by crude tissue analyses.10 The total number of somatic mutations compared with the germline gene, V3-23, is indicated at the end of each line. In addition, the IgM-derived sequences from microdissected samples (MD) 2, 3a, 3b, and 4a, as well as the IgG-derived sequences from samples 2 and 9a from FL 8-'83 are shown. The sample numbers represent the different follicles from which they were taken. Different samples from one follicle are designated with a different letter. Replacement and silent mutations are represented as closed and open circles, respectively. Codon numbers are indicated underneath the symbols. Only the mutations that differ between the sequences are indicated. The sequences depicted of the microdissected samples of FL 8-'83 had an identical mutation pattern and were remarkably similar to that of the consensus sequence of FL 8-'92. (B) Comparison of the consensus sequences of FL 3-'93 and 3-'95 (top and bottom line).10 Only the nucleotide differences between them are shown. The total number of mutations compared with the germline gene, V3-7, is indicated at the end of each line. A PCR was performed on cDNA of FL 3-'93 using primers (depicted as arrows) designed on the 3-'95–specific sequence in the indicated areas. The 3′ termini of these primers matched the critical 3-'95 positions in codons 2 and 96, respectively. The middle line depicts the sequence of the product obtained by this time point–specific PCR.
Evidence for subclone selection over time in FL 8 and FL 3.
(A) The IgM- and IgG-derived VH sequences from FL 8-'83 (8-'83μ and 8-'83γ, respectively) and from the relapse, 8-'92, are represented as lines. The consensus sequences had been determined by crude tissue analyses.10 The total number of somatic mutations compared with the germline gene, V3-23, is indicated at the end of each line. In addition, the IgM-derived sequences from microdissected samples (MD) 2, 3a, 3b, and 4a, as well as the IgG-derived sequences from samples 2 and 9a from FL 8-'83 are shown. The sample numbers represent the different follicles from which they were taken. Different samples from one follicle are designated with a different letter. Replacement and silent mutations are represented as closed and open circles, respectively. Codon numbers are indicated underneath the symbols. Only the mutations that differ between the sequences are indicated. The sequences depicted of the microdissected samples of FL 8-'83 had an identical mutation pattern and were remarkably similar to that of the consensus sequence of FL 8-'92. (B) Comparison of the consensus sequences of FL 3-'93 and 3-'95 (top and bottom line).10 Only the nucleotide differences between them are shown. The total number of mutations compared with the germline gene, V3-7, is indicated at the end of each line. A PCR was performed on cDNA of FL 3-'93 using primers (depicted as arrows) designed on the 3-'95–specific sequence in the indicated areas. The 3′ termini of these primers matched the critical 3-'95 positions in codons 2 and 96, respectively. The middle line depicts the sequence of the product obtained by this time point–specific PCR.
Sequencing of PCR products
Both strands of the PCR products were directly sequenced with an ABI sequencer by using the dye-terminator cycle-sequencing kit (PerkinElmer, Norwalk, CT).
Results and discussion
BCR configuration was studied over time in 3 FLs. In the presentation biopsy of FL 8 (FL 8-'83) IgM- and IgG-switch variants were found that contained 30 and 35 somatic mutations compared with the germline gene V3-23, respectively (Figure 1A). The second time point (FL 8-'92) 9 years later consisted of IgG-expressing tumor cells only that contained 35 mutations compared with V3-23, 30 of which were shared with the IgM- and IgG-sequences of the 1983 sample (Figure 1A). At both time points high intraclonal variation was observed,10 generally believed indicative of ongoing somatic hypermutation.
The subclones present in FL 8-'83 were studied in more detail by dissecting samples of approximately 50 cells from the 20 neoplastic follicles. Of each sample, the VH-Cμ and VH-Cγ gene transcripts were amplified and sequenced. All PCR and sequence reactions were carried out in duplicate, and “consensus” sequences thus obtained of each sample were compared. In general, we found a random distribution of subclones over the follicles without obvious subclone dominance within individual tumor follicles (data not shown). Significant intraclonal sequence variation was found (ie, 2.7 and 3.1 somatic mutations per immunoglobulin sequence) compared with the IgM- or IgG-derived consensus sequences as derived from crude tissue analyses,10 respectively (Table1). Interestingly, we noticed that the IgM-derived sequences from samples 2, 3a, 3b, and 4a, as well as the IgG-derived sequences from samples 2 and 9a of FL 8-'83 clearly differed from the consensus 8-'83 sequences but instead were almost identical to the mutation pattern of the IgG+ subclone that dominated the 8-'92 sample (Figure 1A). The only difference was a replacement mutation at amino acid position 30 present in FL 8-'92 but not found in any of the sequences of FL 8-'83 (Figure 1A). This close resemblance and the fact that we found significant intraclonal variation makes it very likely that the dominant subclone of 8-'92 was already present in the presentation biopsy. The findings suggest that over time subclone selection occurred instead of evolution of a subclone by continued somatic hypermutation in combination with BCR selection.
Diversity of subclones found in follicular lymphoma 8-'83
| Isotype . | No. of clone sequences . | Total no. of intraclonal nucleotide differences* . | Intraclonal variation (no. of mutations/clone) . |
|---|---|---|---|
| 8-'83 IgM | 22 | 59 | 2.7 |
| 8-'83 IgG | 20 | 61 | 3.1 |
| 8-'92 IgG | 61-153 | 8 | 1.3 |
| Isotype . | No. of clone sequences . | Total no. of intraclonal nucleotide differences* . | Intraclonal variation (no. of mutations/clone) . |
|---|---|---|---|
| 8-'83 IgM | 22 | 59 | 2.7 |
| 8-'83 IgG | 20 | 61 | 3.1 |
| 8-'92 IgG | 61-153 | 8 | 1.3 |
IgM, immunoglobulin M; IgG, immunoglobulin G.
The majority of the nucleotide differences found were mutations that were shared by more than one clone, which argues strongly for somatic hypermutation rather than Taq errors.
Clones were not obtained by microdissection of samples, but bacterial clones were made from the VH3–polymerase chain reaction product derived from 8-'92 complementary DNA.10
On the basis of this finding, we assayed the initial biopsies of FLs 3 and 6 for the presence of the subclones that dominated their respective relapses. For this purpose, we designed time point–specific PCR primers of which critical 3′-position(s) matched solely with the sequences of the relapse populations. In FL 3, we had previously found a decrease in the number of somatic mutations over time and successive mutation patterns that were not in favor of clonal evolution.10 Now, we obtained a PCR product from cDNA of FL 3-'93 with 3-'95–specific primers. Sequencing of this product indeed proved that the clone that dominated the relapse 3-'95 had already been present in the initial biopsy 3-'93, most likely at a very low frequency (Figure 1B). The fact that we found this subclone with an identical mutation pattern at both time points is not in support of ongoing somatic hypermutation. In FL 6, this PCR approach was not successful (data not shown). Interestingly, in this case, the number of somatic mutations had increased over time, from 44 to 50 mutations compared with the germline gene, whereas the successive mutation patterns were also potentially compatible with clonal evolution.
Thus, we provided evidence for selective outgrowth of minor subclones in the course of FL disease. Previously, the gradual overgrowth by an already major subclone, without alteration of the consensus mutation pattern, has been documented.4 Matolcsy et al13 described a diffuse large B-cell lymphoma (DLBL) that evolved from a FL.13 The mutation patterns of the FL and its DLBL relapse were different and more suggestive of subclone selection than of clonal evolution.13 However, the investigators were not able to demonstrate, by PCR with subclone-specific primers, the presence of the DLBL clonotype in the FL.
On the basis of observed mutation patterns, intraclonal variation and genealogic relationships between tumor subclones in FL, a role for antigen-receptor ligands in lymphomagenesis has been proposed.11,12 However, the evidence we obtained for subclone selection rather than clonal evolution questions a role for BCR ligands in the growth of at least some FLs. Interestingly, Ottensmeier et al5 recently described a FL subclone with a stop codon in the functionally rearranged VH gene. Among other clones, this subclone was also found in the relapse sample 10 months later, suggesting that BCR expression was not essential for the propagation of this FL.5 In conclusion, we think it is worth considering that the expansion of FLs is independent of the quality of the BCR but is determined by various other genetic alterations that give selective growth advantage during disease course.
Supported by grant AMC 95-957 from the Dutch Cancer Society. C.J.M.v.N. is a fellow of The Netherlands Royal Academy of Arts and Sciences.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carel J. M. van Noesel, Dept of Pathology, Academic Medical Center, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands; e-mail: c.j.vannoesel@amc.uva.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal