Abstract
The antiangiogenic activity of thalidomide (Thal), coupled with an increase in bone marrow angiogenesis in multiple myeloma (MM), provided the rationale for the use of Thal in MM. Previously, the direct anti-MM activity of Thal and its analogues (immunomodulatory drugs, IMiDs) on MM cells was demonstrated, suggesting multiple mechanisms of action. In this study, the potential immunomodulatory effects of Thal/IMiDs in MM were examined. It was demonstrated that Thal/IMiDs do not induce T-cell proliferation alone but act as costimulators to trigger proliferation of anti-CD3–stimulated T cells from patients with MM, accompanied by an increase in interferon-γ and IL-2 secretion. However, an increase in autologous T-cell killing of patient MM cells could not be demonstrated. A role for natural killer (NK)- and LAK-cell–mediated killing is suggested because IL-2–primed peripheral blood mononuclear cells (PBMCs) treated with Thal/IMiDs demonstrated significantly increased lysis of MM cell lines. Cold target inhibition assays suggested NK- rather than LAK-cell–mediated killing. Furthermore, this killing was not major histocompatibility complex-class restricted, and the depletion of CD56+ cells blocked the drug-induced MM cell lysis. It was significant that increased killing of patient MM cells by autologous PBMCs treated with Thal/IMiDs was also observed. Although the in vivo relevance of NK-cell–mediated MM cell killing is unknown, phenotypic analysis performed in MM patients receiving Thal therapy demonstrated an increase in CD3−CD56+cells in patients responding to therapy. Thus in vitro and in vivo data support the hypothesis that Thal may mediate its anti-MM effect, at least in part, by modulating NK cell number and function.
Introduction
Recent reports of increased bone marrow angiogenesis in multiple myeloma (MM), coupled with the known antiangiogenic properties of thalidomide (Thal), provided the rationale for its use to treat patients with MM. Remarkably, Thal produced clinical responses in 32% of patients whose disease was refractory to conventional and high-dose therapy.1 However, there was no correlation of bone marrow angiogenesis with response to treatment, suggesting that Thal may not mediate its anti-MM activity through its antiangiogenic effects alone. We have previously demonstrated that Thal and 3 immunomodulatory drugs (IMiDs), potent Thal analogues, can act directly on MM cells.2 These drugs induce a dose-dependent inhibition of proliferation even in MM cell lines and patient MM cells resistant to conventional chemotherapy, and they add to the effect of dexamethasone (Dex). Thal and IMiDs induce growth arrest or apoptosis, and, as with Dex, apoptosis is mediated by the activation of related adhesion focal tyrosine kinase (RAFTK).2 3 For Thal, these effects were observed only at relatively high concentrations not readily achievable in plasma, suggesting that there may be alternative mechanisms for its clinical anti-MM activity.
For many years the immunomodulatory effects of Thal have provided the rationale for its use in the treatment of a broad range of diseases, including erythema nodosum leprosum, aphthous ulceration complicating human immunodeficiency virus, and graft-versus-host disease.4-6 Its mechanism of action was initially thought to be through the inhibition of cytokine production by monocytes, particularly tumor necrosis factor α (TNF-α).7However, more recent studies have suggested that Thal may also act as a costimulatory signal to T cells, inducing T-cell proliferation associated with interferon γ (IFN-γ) and interleukin 2 (IL-2) production.8,9 New analogues of Thal have been produced that are 50 000 times more potent than Thal at inhibiting TNF-α secretion from peripheral blood mononuclear cells (PBMCs).9 These analogues are also more potent inducers of T-cell proliferation with IFN-γ and IL-2 secretion and inhibitors of IL-1β and IL-6 secretion from PBMCs; hence, these drugs have been named IMiDs.9
In this study, we have investigated the immunomodulatory effects of Thal and 3 potent IMiDs in MM. Although these drugs induce the proliferation of MM patient T cells as well as IFN-γ and IL-2 secretion in vitro, these T cells are not cytotoxic and do not lyse autologous MM cells. In the in vivo setting, we were also unable to demonstrate an expansion of CD3+, CD4+, or CD8+ T cells in MM patients receiving Thal therapy. Of importance, we demonstrate that these drugs markedly enhance in vitro NK-cell–mediated lysis of both MM cell lines and autologous patient MM cells. Moreover, in vivo there is an increase in NK cell number in patients responding to Thal therapy. These data therefore suggest that Thal and the IMiDs, in addition to their direct inhibition of MM cell proliferation and survival2 and antiangiogenic effects in the bone marrow (BM),10 may also induce NK cell anti-MM immune responses.
Materials and methods
Tumor-derived cell lines and MM patient cells HS Sultan, Raji, and K562 cell lines were obtained from American Type Culture Collection (Rockville, MD). MM.1S cells were a kind gift from Dr Steven Rosen (Northwestern University, Chicago, IL). Cells were cultured in RPMI-1640 media (Sigma Chemical, St Louis, MO) containing 10% fetal bovine serum, 2 mM L-glutamine (Gibco, Grand Island, NY), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). PBMCs were obtained from normal donors and MM patients (1 newly diagnosed, 2 receiving induction chemotherapy, 1 after autologous transplantation, and 2 receiving therapy for relapsed disease) after written informed consent according to institutional guidelines. No patient had received previous thalidomide or antiangiogenic therapy. Mononuclear cells were isolated by centrifugation on Ficoll-Paque (Pharmacia Biotech, Piscataway, NJ), and cells were cultured in RPMI-1640 media supplemented with 10% human serum. Patient MM cells, greater than 90% CD38+CD45−CD138+ cells by flow cytometry, were also isolated in a similar manner from bone marrow aspirate samples.
Preparation of Thal and IMiDs
Thal and IMiDs (Celgene, Warren, NJ) were dissolved in dimethyl sulfoxide (DMSO) (Sigma) and stored at −20°C until use. Drugs were diluted in culture medium (0.1-10 μg/mL) with less than 0.1% DMSO immediately before use.
T-cell proliferation assays
T cells were obtained from PBMCs using T-cell enrichment columns (R&D Systems, Minneapolis, MN) through high-affinity negative selection (more than 95% CD3+), according to the manufacturer's instructions. T cells were incubated in 96-well U-bottomed culture plates (2 × 105 cells/well) for 5 days, with the daily addition of either DMSO or Thal/IMiDs. To demonstrate that Thal/IMiDs were acting as costimulators, these drugs were cultured in the presence of T cells activated either by T-cell receptor cross-linking using soluble murine anti–human CD3 monoclonal antibody or by culture with autologous dendritic cells (DC) derived from PBMCs by culture for 6 days with IL-4 and granulocyte macrophage–colony-stimulating factor, as previously described (T/DC ratio, 30:1).11CD4+ and CD8+ cells were obtained from CD3+ T-cell–enriched populations using magnetic bead depletion (Dynal, Lake Success, NY). Proliferation was determined by measuring the uptake of tritiated thymidine (3[H]TdR) (New England Nuclear, Boston, MA). Cells were pulsed with3[H]-TdR (1 μCi/well) during the last 12 hours of 5-day cultures, harvested onto glass filters with an automatic cell harvester (Cambridge Technology, Cambridge, MA), and counted using the LKB beta plate scintillation counter (Wallace, Gaithersburg, MD). All experiments were performed in triplicate, and results were expressed as mean counts per minute (cpm) ± SD.
Generation of CTL and lines with increased NK-cell activity
CTL lines were generated by culturing PBMCs from MM patients (1 × 107) with dendritic cells at a ratio of 40:1. After the first 48 hours of culture, IL-2 (20 U/mL; Collaborative Biomedical Products, Bedford, MA) was added. Cells were treated with Thal/IMiD (1 and 5 μg/mL) on alternate days, and standard chromium 51 Cr (51Cr) release assays were performed on days 10 to 14. Lines with increased NK-cell activity were generated by first depleting monocytes from PBMCs by adherence for 2 hours. Nonadherent cells were cultured with IL-2 (10 U/mL) for 72 hours, and this was followed by the daily addition of Thal or IMiDs for 72 hours. 51Cr release assays and flow cytometric analysis were performed at 144 hours.
Cytotoxicity assays
Effector cells generated as described above were tested for their MM-specific cytotoxicity in a standard 4-hour51Cr-release assay. Briefly, 1 × 106 target cells were labeled with 100 μCi 51Cr (New England Nuclear) for 60 minutes at 37°C and were washed 3 times to remove unincorporated isotope. Labeled targets were added to 96-well U-bottom plates (1 × 104 cells/well) and incubated with varying ratios of effector cells for 4 hours at 37°C in a 5% CO2atmosphere. Supernatants were assayed for 51Cr release in a gamma counter. Spontaneous release of 51Cr was assessed by the incubation of targets in the absence of effectors, and maximum release of 51Cr was determined by incubation of targets in 0.1% Triton X-100. Percentage of specific 51Cr release was determined using the following equation: [% specific lysis = (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100]. Assays were performed in triplicate, and results were expressed as mean ± SD.
MM.1S and HS Sultan cell lines were chosen as targets because they were relatively resistant to NK-cell–mediated lysis; in all experiments, K562 was used as a positive control. To confirm whether the CTLs were class I- or class II-restricted, anti-MHC class I and class II blocking antibodies were added to the target at a concentration of 20 μg/mL and were incubated for 60 minutes on ice before 51Cr release assay. To confirm whether cytotoxicity was NK-cell–mediated, either CD4+, CD8+, CD14+, or CD56+ cells were depleted (more than 97% depletion by flow cytometry) from PBMCs using immunomagnetic beads (Dynal, Lake Success, NY.) immediately before 51Cr release assay. Cold target inhibition assays were performed using the NK-sensitive, LAK-sensitive cell line K562 and the NK-resistant, LAK-sensitive cell line Raji. Effectors were derived from normal PBMCs as described above. Unlabeled competitor targets (Raji) were added in varying ratios to labeled targets (K562) at a standard 30:1 effector-target ratio, and percentage lysis was determined as described above. To control for possible adverse effects of the DMSO vehicle, medium alone was also used as a negative control in all experiments. In all cases, DMSO and media-alone cultures were equivalent. All cell line experiments were repeated at least 3 times.
Flow cytometry analysis
Flow cytometry analysis was performed using the Coulter Epics XL (Coulter, Miami, FL) to determine the expression of T-cell antigens (CD3, CD4, CD8), NK-cell antigen (CD56), and NK-cell–activation antigens (CD2, CD11a, CD31, CD69, HLA DR; (Beckman Coulter). Cells were washed and incubated in phosphate-buffered saline with 20% human AB serum at room temperature for 20 minutes to eliminate nonspecific Fc receptor binding. Afterward, cells were incubated with murine antibodies directly labeled with either FITC or PE for 30 minutes on ice. Cells were then washed, fixed with 2% paraformaldehyde, and evaluated by flow cytometry.
In vivo effect of Thal therapy
Patients were treated with 200 to 800 mg/day Thal according to an institutional protocol. Phenotypic analysis of T-cell subsets in the peripheral blood was performed every 4 weeks during Thal therapy.
Cytokines
Tissue culture supernatants were harvested and frozen immediately at −80°C. Assays were performed in triplicate for IFN-γ and IL-2 using enzyme-linked immunosorbent assays (R&D Systems).
Statistical analysis
Statistical significance of differences observed in drug-treated versus control cultures was determined using the Student ttest. The minimal level of significance was P < .05.
Results
Effect of Thal and IMiDs on T cells in healthy donors and patients with MM
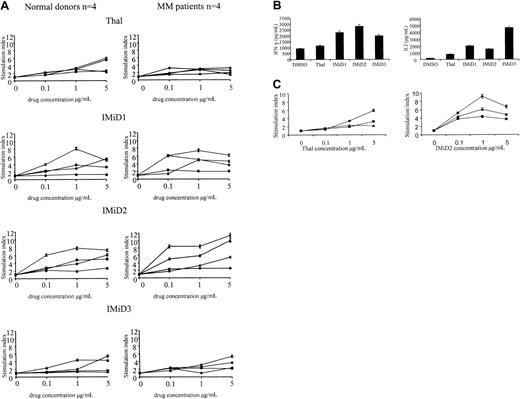
As in previous reports,8 we found no proliferation of CD3+ cells triggered by treatment with Thal or IMiDs alone (data not shown). However, Thal induced an increased proliferation (mean stimulation index, 3.2; range, 1.5-5.9) of CD3+ cells from healthy donors and patients with MM, cultured either in the presence of anti-CD3 or dendritic cells. Treatment with IMiDs 1, 2, and 3 triggered greater increments in proliferation of CD3+cells in healthy donors and patients (IMiD1 mean stimulation index, 3.9; range, 1.3-6.1; IMiD2 mean stimulation index, 6.3; range, 2.5-11.3; IMiD3 mean stimulation index, 3.5; range, 1.3-5.4) (Figure1A). Stimulation was maximal at 1 to 5 μg/mL, levels readily achievable in plasma; at doses greater than 10 μg/mL, a reduction in the fold increase in proliferation was noted (data not shown). In healthy donors and patients with MM, there were interindividual differences in the proliferative response; although the numbers in each group were small, there was no statistical difference between the response of MM patients and healthy donors and no difference between the response of newly diagnosed patients and heavily pretreated patients (Table 1). This drug-induced increase in CD3+ cell proliferation was accompanied by IFN-γ and IL-2 secretion. As can be seen in Figure 1B, the increase in cytokine secretion was significantly higher in Thal- and IMiD-treated cultures than in DMSO-treated controls (Thal,P = .03; IMiD1, IMiD2, IMiD3, P < .001).
Effect of Thal and IMiDs on T cells in healthy donors and patients with MM.
(A) CD3+ cells from 4 healthy donors (left) and 4 patients with MM (right) were stimulated with anti-CD3 and treated with Thal/IMiDs daily for 5 days. Values represent the stimulation index (± SD) in the presence of Thal/IMiDs (0.1, 1, and 5 μg/mL). (B) IFN-γ and IL-2 levels were measured using enzyme-linked immunosorbent assay in supernatants of anti-CD3–stimulated CD3+ cells cultured with Thal or IMiDs (5 μg/mL) for 5 days. (C) CD3+ (♦), CD4+ (▪), and CD8+ (▴) cells were selected using immunomagnetic beads. Cells were stimulated with anti-CD3 and treated with Thal (left panel) and IMiD2 (right panel) daily for 5 days. Proliferation was assayed as described above in the presence of Thal/IMiD2 (0.1, 1, and 5 μg/mL).
Effect of Thal and IMiDs on T cells in healthy donors and patients with MM.
(A) CD3+ cells from 4 healthy donors (left) and 4 patients with MM (right) were stimulated with anti-CD3 and treated with Thal/IMiDs daily for 5 days. Values represent the stimulation index (± SD) in the presence of Thal/IMiDs (0.1, 1, and 5 μg/mL). (B) IFN-γ and IL-2 levels were measured using enzyme-linked immunosorbent assay in supernatants of anti-CD3–stimulated CD3+ cells cultured with Thal or IMiDs (5 μg/mL) for 5 days. (C) CD3+ (♦), CD4+ (▪), and CD8+ (▴) cells were selected using immunomagnetic beads. Cells were stimulated with anti-CD3 and treated with Thal (left panel) and IMiD2 (right panel) daily for 5 days. Proliferation was assayed as described above in the presence of Thal/IMiD2 (0.1, 1, and 5 μg/mL).
Effect of thalidomide and immunomodulatory drugs on T cells in healthy donors and patients with multiple myeloma
| . | Stimulation index of CD3+ cells . | P . | |
|---|---|---|---|
| Healthy donors . | Myeloma patients . | ||
| Thal | Mean, 4.1 | Mean, 2.2 | .12 |
| Range, 2.3-5.9 | Range, 1.5-3.1 | ||
| IMiD1 | Mean, 3.7 | Mean, 4.1 | .8 |
| Range, 1.3-5.4 | Range, 1.9-6.1 | ||
| IMiD2 | Mean, 5.2 | Mean, 7.2 | .4 |
| Range, 2.6-7.3 | Range, 2.5-11.3 | ||
| IMiD3 | Mean, 3.2 | Mean, 3.3 | .92 |
| Range, 1.3-5.4 | Range, 2.1-5.3 | ||
| . | Stimulation index of CD3+ cells . | P . | |
|---|---|---|---|
| Healthy donors . | Myeloma patients . | ||
| Thal | Mean, 4.1 | Mean, 2.2 | .12 |
| Range, 2.3-5.9 | Range, 1.5-3.1 | ||
| IMiD1 | Mean, 3.7 | Mean, 4.1 | .8 |
| Range, 1.3-5.4 | Range, 1.9-6.1 | ||
| IMiD2 | Mean, 5.2 | Mean, 7.2 | .4 |
| Range, 2.6-7.3 | Range, 2.5-11.3 | ||
| IMiD3 | Mean, 3.2 | Mean, 3.3 | .92 |
| Range, 1.3-5.4 | Range, 2.1-5.3 | ||
Thal indicates thalidomide; IMiD, immunomodulatory drugs.
CD3+ cells from 4 healthy donors and 4 patients with MM were stimulated with anti-CD3 and treated with Thal/IMiDs daily (5 μg/mL) for 5 days.
To determine whether the proliferative response was in all T cells or a subset of T cells, we next examined the effect of these drugs on the proliferation of CD3+, CD4+, and CD8+ T cells from 2 patients with MM and 2 healthy donors. As can be seen in Figure 1C, the proliferation of CD4+(mean stimulation index, 2.6; range, 1.1-4.4) and CD8+(mean stimulation index, 1.5; range, 0.8-2.2) T-cell subsets was induced by Thal (P = .24). A similar response in CD4+ (mean stimulation index, 2.4; range, 0.5-3.6) and CD8+ (mean stimulation index, 6.4; range, 3.0-11.4) T-cell subsets was triggered by IMiD2 (P = .22).
To determine the functional significance of this drug-induced CD3+ cell proliferation in patients with MM, PBMCs treated with Thal/IMiDs for 10 to 14 days were used as effectors and autologous MM cell were used as targets in a standard chromium release assay. Autologous dendritic cells, rather than anti-CD3 antibody, were used to provide a physiological stimulus to trigger through the T-cell receptor. In 2 patients tested, we were unable to demonstrate increased autologous MM cell killing after drug treatment, though control K562 cells were lysed. Flow cytometry analysis of the CTL lines demonstrated a mixed population of T cells (data not shown).
Treatment of PBMCs with Thal and IMiDs increases lysis of MM cell lines
Given the lack of effect of Thal and IMiDs on CTL activity, we next examined the effect of these drugs on NK- and LAK-cell activity. MM.1S and HS Sultan MM cell lines were chosen as targets because they are relatively resistant to NK/LAK cell lysis and initial experiments demonstrated a 20% difference in MM cell lysis between normal PBMCs stimulated for 72 hours with IL-2 (10 U) and maximal lysis with normal PBMCs stimulated for 72 hours with IL-2 (100 U) (data not shown). Normal PBMCs were treated with IL-2 for 72 hours and then with IL-2 and Thal/IMiDs for 72 hours at a dose of 1 μg/mL. Thal triggered increased MM cell lysis (15.4%) compared to DMSO-treated control (8.4%; P = .07). This increase in MM cell lysis was more marked after treatment with IMiDs (DMSO control, 8.4%; IMiD1, 18.6%,P < .001; IMiD2, 18.2%, P = <.001; IMiD3, 16.5%, P = .02) (Figure2A). The increase in lysis was also dose dependent: DMSO control, 8.4%; Thal 1 μg/mL, 15.4%,P = .07; Thal 5 μg/mL, 26.2%, P = .004; IMiD1 1 μg/mL, 18.6%, P < .001; IMiD1 5μg/mL, 29.5%, P < .001 (Figure 2B). Next, we determined whether the addition of exogenous IL-2 was essential to observe the drug-induced increase in MM cell lysis. As can be seen in Figure 2C, PBMCs cultured without IL-2 showed less lysis of MM cells, though PBMCs treated with IMiD1 still triggered an increase in MM cell lysis compared to control-treated PBMCs (DMSO control, 3.8%; Thal 5 μg/mL 3.4%; IMiD1 5 μg/mL, 22.2%; P < .001).
Treatment of PBMCs with Thal and IMiDs increases MM cell line lysis.
Lysis of the MM cell line HS Sultan by normal PBMCs treated with IL-2 for 72 hours and then by Thal or IMiDs for 72 hours was measured using a standard 51Cr release assay at 30:1 to 7.5:1 effector-target ratios. Results are representative of 3 independent experiments. (A) Effect of Thal and IMiD1, IMiD2, and IMiD3 at 1 μg/mL. (B) (upper panel) Effect of Thal (1 μg/mL and 5 μg/mL). (lower panel) Effect of IMiD1 (1 μg/mL and 5 μg/mL). (C) PBMCs treated with and without the addition of exogenous IL-2 followed by the addition of Thal/IMiD1 (5 μg/mL).
Treatment of PBMCs with Thal and IMiDs increases MM cell line lysis.
Lysis of the MM cell line HS Sultan by normal PBMCs treated with IL-2 for 72 hours and then by Thal or IMiDs for 72 hours was measured using a standard 51Cr release assay at 30:1 to 7.5:1 effector-target ratios. Results are representative of 3 independent experiments. (A) Effect of Thal and IMiD1, IMiD2, and IMiD3 at 1 μg/mL. (B) (upper panel) Effect of Thal (1 μg/mL and 5 μg/mL). (lower panel) Effect of IMiD1 (1 μg/mL and 5 μg/mL). (C) PBMCs treated with and without the addition of exogenous IL-2 followed by the addition of Thal/IMiD1 (5 μg/mL).
Increased MM cell lysis induced by treatment of PBMCs with Thal and IMiDs is NK-cell mediated
To determine whether the enhanced MM cell lysis triggered by Thal and IMiDs was mediated by LAK cells or NK cells, cold-target inhibition assays were performed. K562 cells were used as hot targets because they are both NK sensitive and LAK sensitive, and Raji cells were used as the competitive cold target because they are LAK sensitive but NK resistant. There was no difference in cell lysis with the addition of the competitive cold target, suggesting that the major effect is NK-cell mediated (Figure3A).
Increased MM cell lysis induced by treatment of PBMCs with Thal and IMiDs is NK cell mediated.
(A) Normal PBMCs were treated with IL-2 for 72 hours and then by Thal 5 μg/mL (upper panel) or IMiD1 5 μg/mL (lower panel) daily for 3 days. Labeled K562 (NK sensitive/LAK sensitive) and unlabeled competitor Raji cells (NK resistant/LAK sensitive) were cultured at ratios of 0.5:1 to 2:1 in a standard 51Cr release assay at a 30:1 effector-hot target ratio. (B) Normal PBMCs were treated with IL-2 for 72 hours and then by IMiD1 5 μg/mL daily for 3 days. CD4+, CD8+, CD56+, or CD14+ cells were depleted (more than 97% depletion by flow cytometry analysis) using immunomagnetic beads immediately before performing a standard 51Cr release assay using HS Sultan, MM.1S, and K562 cells as targets at a 30:1 effector-target ratio. (C) Normal PBMCs were treated with IL-2 for 72 hours and then by IMiD1 5 μg/mL daily for 3 days. MM cell line targets were treated with antibodies to class I and class II before 51Cr release assay.
Increased MM cell lysis induced by treatment of PBMCs with Thal and IMiDs is NK cell mediated.
(A) Normal PBMCs were treated with IL-2 for 72 hours and then by Thal 5 μg/mL (upper panel) or IMiD1 5 μg/mL (lower panel) daily for 3 days. Labeled K562 (NK sensitive/LAK sensitive) and unlabeled competitor Raji cells (NK resistant/LAK sensitive) were cultured at ratios of 0.5:1 to 2:1 in a standard 51Cr release assay at a 30:1 effector-hot target ratio. (B) Normal PBMCs were treated with IL-2 for 72 hours and then by IMiD1 5 μg/mL daily for 3 days. CD4+, CD8+, CD56+, or CD14+ cells were depleted (more than 97% depletion by flow cytometry analysis) using immunomagnetic beads immediately before performing a standard 51Cr release assay using HS Sultan, MM.1S, and K562 cells as targets at a 30:1 effector-target ratio. (C) Normal PBMCs were treated with IL-2 for 72 hours and then by IMiD1 5 μg/mL daily for 3 days. MM cell line targets were treated with antibodies to class I and class II before 51Cr release assay.
To confirm NK-cell–mediated killing, CD4+, CD8+, CD56+, or CD14+ cells were depleted from PBMCs treated with IL-2 for 72 hours and then with IMiD1 (5 μg/mL) for 72 hours. As can be seen in Figure 3B, the depletion of CD4+ and CD8+ cells resulted in no reduction in MM cell lysis, whereas the depletion of CD56+ cells resulted in a significant reduction of MM cell lysis—decreases from 62.4% to 6.8% for HS Sultan cells and from 30.3% to 8.8% for MM1.S cells (P < .001). Lack of involvement of cytotoxic T cells was further suggested because there was no decrease in MM cell lysis in the presence of class I and II blocking antibodies (Figure3C).
Activation antigen profile on NK cells after treatment by Thal and IMiDs
Phenotypic analysis of PBMCs treated in this way showed no change in expression or absolute numbers of CD3+, CD4+, CD8+, or CD56+ cells. Further analysis of NK cell subsets demonstrated no change in the expression of activation markers CD2, CD31, CD38, CD69, or HLA-DR between control, Thal-, and ImiD-treated samples or with increasing drug concentrations (Figure 4).
Activation antigen profile on NK cells induced by Thal and IMIDs.
PBMCs were cultured with IL-2 for 72 hours and then by Thal or IMiD1 5 μg/mL daily for an additional 72 hours. The expression of activation molecules (CD2, CD11a, CD31, CD38, CD69, and HLA-DR) on CD56+ cells was examined by flow cytometry analysis.
Activation antigen profile on NK cells induced by Thal and IMIDs.
PBMCs were cultured with IL-2 for 72 hours and then by Thal or IMiD1 5 μg/mL daily for an additional 72 hours. The expression of activation molecules (CD2, CD11a, CD31, CD38, CD69, and HLA-DR) on CD56+ cells was examined by flow cytometry analysis.
Treatment of patient PBMCs with Thal and IMiDs induces increased lysis of autologous MM cells
Significantly, PBMCs from patients with MM treated with IL-2 and Thal also resulted in increased killing of autologous MM cells (patient 1: DMSO control 18.4% vs Thal 31.2%, P = .03; patient 2: DMSO control 1.8% vs Thal 5.5%, P = .005) (Figure5). As was observed with MM cell lines, the treatment of MM patient PBMCs with IMiDs resulted in an additional increase in autologous MM cell lysis (patient 1: DMSO control 18.4% vs IMiD1 51.6%, P < .001; IMiD2 49.7%,P < .001; IMiD3 45.9%, P < .001; patient 2: DMSO control 1.8% vs IMiD1 19.5%, P < .001; IMiD2 10.3%, P < .001; IMiD3 12.9%,P < .001).
Treatment of patient PBMCs with Thal and IMiDs triggers increased lysis of autologous MM cells.
PBMCs from 2 patients with MM were cultured with IL-2 for 72 hours, and then by 1 μg/mL Thal/IMiDs daily for 72 hours. Lysis of autologous MM cells by these cells was measured in a standard 51Cr release assay at a 30:1 effector-target ratio.
Treatment of patient PBMCs with Thal and IMiDs triggers increased lysis of autologous MM cells.
PBMCs from 2 patients with MM were cultured with IL-2 for 72 hours, and then by 1 μg/mL Thal/IMiDs daily for 72 hours. Lysis of autologous MM cells by these cells was measured in a standard 51Cr release assay at a 30:1 effector-target ratio.
Clinical relevance of NK cells in patients treated with Thal
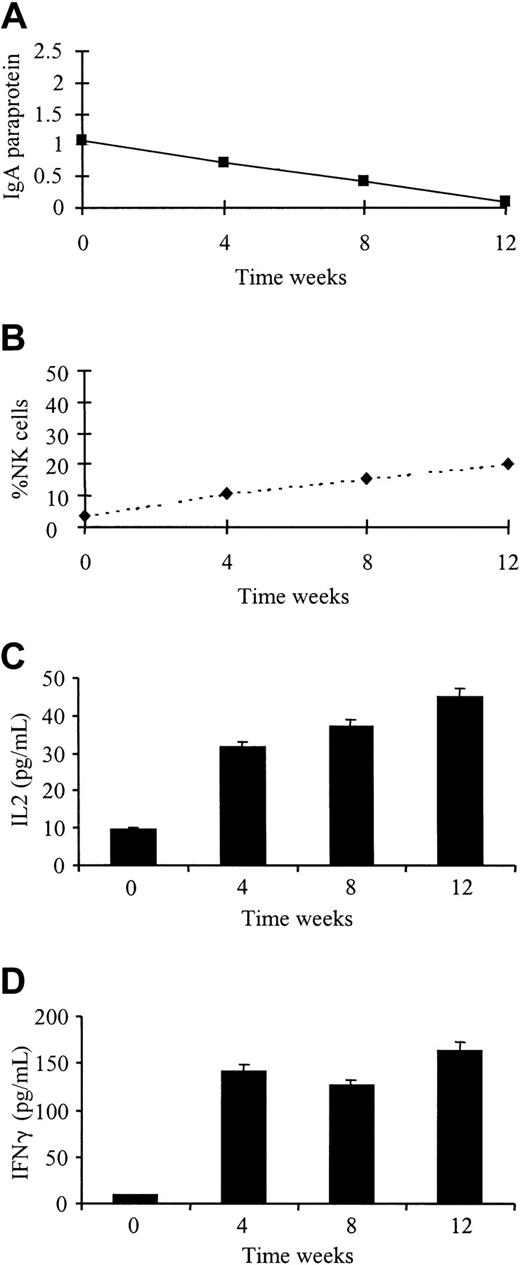
Monthly phenotypic analysis was performed on 5 patients receiving Thal treatment according to an institutional protocol. Three patients responded to treatment with Thal 200 to 800 mg/day (2 partial responses, 1 stable disease), and 2 patients failed to respond to therapy. There was no change in absolute numbers or percentages of CD3+, CD4+, or CD8+ T-cell subsets during the treatment course. All patients receiving therapy showed an increase in the absolute numbers of CD56+ NK cells (mean fold increase, 2.6; range, 1.2-3.8). The 3 patients responding to treatment showed an increase in the percentage of CD56+ NK cells (fold increases of 5.6, 2.7, and 2.1) compared to patients who failed to respond (fold increases of 0.5 and 1.2) (Table2). Cytokine levels were also measured in the plasma at monthly intervals from all 5 patients. At the beginning of treatment, only one patient had detectable levels of IL-2 or IFN-γ. As demonstrated in Figure 6, this patient achieved a good partial response, with the levels of both cytokines significantly rising with treatment (IL-2,P < .001; IFN-γ, P < .001). IL-2 and IFN-γ levels remained undetectable in the other 4 patients.
Expansion of the natural killer–cell subset in patients with multiple myeloma receiving treatment with thalidomide
| Patient . | Response to Thal . | Fold change in absolute number of NK cells . | Fold change in percentage of NK cells . |
|---|---|---|---|
| 1 | Partial response | 2.9 | 5.6 |
| 2 | Partial response | 3.9 | 2.8 |
| 3 | Stable | 2.4 | 2.1 |
| 4 | Relapse | 2.4 | 0.6 |
| 5 | Relapse | 1.3 | 1.3 |
| Patient . | Response to Thal . | Fold change in absolute number of NK cells . | Fold change in percentage of NK cells . |
|---|---|---|---|
| 1 | Partial response | 2.9 | 5.6 |
| 2 | Partial response | 3.9 | 2.8 |
| 3 | Stable | 2.4 | 2.1 |
| 4 | Relapse | 2.4 | 0.6 |
| 5 | Relapse | 1.3 | 1.3 |
NK indicates natural killer. See Table 1 for other abbreivations.
Serial phenotypic analysis of peripheral blood NK cell (CD56+) subsets was performed on 5 patients with MM receiving Thal therapy.
Response to Thal treatment is accompanied by an increase in plasma IL-2 and IFN-γ levels and an increase in percentage of NK cells.
Paraprotein measurement (A), flow cytometry (B), and cytokine levels (C, D) were performed on patients receiving Thal therapy for relapsed MM at weekly intervals for 4 weeks.
Response to Thal treatment is accompanied by an increase in plasma IL-2 and IFN-γ levels and an increase in percentage of NK cells.
Paraprotein measurement (A), flow cytometry (B), and cytokine levels (C, D) were performed on patients receiving Thal therapy for relapsed MM at weekly intervals for 4 weeks.
Discussion
Despite the use of conventional therapy, the 5-year survival of patients with MM remains poor. The introduction of high-dose therapy has resulted in an encouraging improvement in response and survival rates; however, patients eventually have relapses with refractory disease, and few, if any, are cured. Based on the observation of increased angiogenesis in the bone marrow of patients with MM, Thal was used to treat relapsed/refractory MM, and it achieved an impressive response rate of 30%.1 However, the lack of correlation of BM angiogenesis with response to Thal suggested alternative anti-MM mechanisms of action of the drug. To define other potential mechanisms of anti-MM activity, we first demonstrated in vitro a direct effect of Thal and IMiDs against human MM cell lines and patient cells, including those resistant to conventional therapy.2 These drugs either induced growth arrest or apoptosis, associated with the activation of RAFTK, in MM cell lines and patient cells. However, the effects of Thal in vitro are noted at higher concentrations than are achievable in patient plasma, suggesting that other mechanisms of action may be important in mediating its anti-MM effect.
Our previous studies have delineated mechanisms whereby MM cells home, grow, and survive in the BM microenvironment,12-14 and recent studies suggest that Thal and IMiDs may impact MM through its effects on cytokines in the BM milieu (manuscript submitted). Specifically, we have demonstrated that MM cells in the BM trigger interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF) secretion. IL-6 is the major growth and survival factor in MM. Our recent studies suggest that VEGF not only mediates angiogenesis, but also directly triggers low-level proliferation and migration of human MM cells.15 Thal and the IMiDs abrogate the up-regulation of IL-6 and VEGF secretion triggered by MM cells in the BM. The inhibition of cytokine secretion therefore has an impact not only on the microenvironment, ie, angiogenesis, but also directly inhibits MM cell growth, survival, and migration.
The above demonstration that Thal acts through cytokines in the BM of patients with MM—coupled with the traditional use of Thal to treat diseases because of its marked immunomodulatory effects—provided the impetus to delineate potential cytokine-related immunomodulatory effects of these drugs against MM cells. Although its initial mechanism of action was thought to be through the inhibition of TNF-α secretion,7 more recent reports have also suggested that Thal may have a direct effect on T cells.8 Specifically, studies in normal donors demonstrate that Thal and IMiDs trigger the proliferation of CD3+ cells only in the presence of anti-CD3 or dendritic cell priming, suggesting that these drugs provide a secondary costimulatory signal and are not mitogenic. In this study, we demonstrate that Thal and IMiDs act as costimulatory molecules that trigger increased proliferation (1.5- to 5.9-fold) of cells of patients with MM. The proliferative response in MM patients is similar to that in normal donors and is associated with an increase in IL-2 and IFN-γ secretion. However, we have been unable to demonstrate any increase in T-cell cytolytic activity of patient PBMCs treated with Thal or IMiDs against autologous MM targets in standard CTL assays. Moreover, we have not observed any expansion in CD4+ or CD8+cells in patients receiving Thal therapy. Therefore, despite the proliferation in CD3+ cells induced by Thal, these cells do not mediate its anti-MM activity.
In this study, we describe a novel immunomodulatory mechanism of action of Thal and IMiDs. These drugs induced NK-cell–mediated lysis of MM cell lines and patient cells. Cold-target inhibition assays showed that this killing is NK cell rather than LAK cell mediated. Moreover, the depletion of CD56+ cells, but not of CD4+ or CD8+ cells, blocked drug-induced MM cell lysis, confirming that NK cells are the effectors mediating this response. Thal- and IMiD-induced MM cell lysis was not altered by class I or class II blocking, further implicating NK cells.
To confirm the in vivo significance of the in vitro NK cell-mediated MM cell lysis triggered by Thal, we determined whether NK cell number and function were similarly altered in patients treated with Thal for relapsed MM after transplantation. We demonstrate that all patients treated with Thal show an increase in absolute number of NK cells; however, only those patients who responded to treatment showed an increase in the percentage of NK cells. Measurement of cytokines in the plasma from patients also showed that the decrease in paraprotein and the increase in NK cells was accompanied by an increase in IL-2 and IFN-γ secretion. Our data, therefore, suggest that the immunomodulatory effects of Thal mediate, at least in part, its anti-MM activity. Specifically, the direct effect of Thal on T cells results in an increase in IL-2 and IFN-γ secretion, which augments NK cell number and function. The increased levels of these circulating cytokines leads to the enhancement of NK cell activity and an increase in MM cell lysis. Other drugs also appear to be active, at least in part, by altering host anti-MM immunity. For example, bisphosphonates were originally demonstrated to reduce bone complications in MM; however, recent studies show that they directly inhibit MM cell proliferation and survival.16,17 Moreover, Kunzmann et al18 reported a significant expansion of γδ T cells mediating specific lysis against MM cell lines in vitro and in pamidronate-treated BM cultures compared to untreated control cultures. That report, coupled with the present study, highlights the importance of assessing the action of potential new drugs in MM not only for their direct effect on tumor cells but also for their impact on the BM microenvironment and host immune response. These new insights not only lead to a better understanding of MM pathogenesis, they may also help to define at what stage of disease a therapy should be most effective. Specifically, our results suggest that Thal and new analogues may not only be useful in the treatment of refractory/relapsed disease, but also be effective in the maintenance of minimal residual disease after transplantation by enhancing NK-cell–mediated anti–MM cell immunity.
Supported by the British Society of Haematology Fellowship (F.E.D.), the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.), and National Institutes of Health grant PO-1 78378.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Department of Adult Oncology, Dana-Farber Cancer Institute, Harvard Medical School, 44 Binney St, Rm M557, Boston, MA 02115; e-mail: kenneth_anderson@dfci.harvard.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal