Abstract
It was recently reported that autoreactive CD4+ T cells to glycoprotein IIb-IIIa (GPIIb-IIIa) mediate antiplatelet autoantibody production in patients with immune thrombocytopenic purpura (ITP). To further examine the antigenic specificity of the GPIIb-IIIa–reactive T cells, 6 recombinant fragments encoding different portions of GPIIbα or GPIIIa were generated and tested for their ability to stimulate antigen-specific T-cell proliferation and anti–GPIIb-IIIa antibody production in vitro. T cells from the peripheral blood of 25 patients with ITP and 10 healthy donors proliferated in response to recombinant GPIIb-IIIa fragments in various combinations. The amino-terminal portions of both GPIIbα and GPIIIa (IIbα18-259 and IIIa22-262) were frequently recognized (60% and 64%, respectively) compared with other fragments (4%-28%) in patients with ITP, but this tendency was not detected in healthy donors. In subsequent analyses in patients with ITP, T-cell reactivities to IIbα18-259 and IIIa22-262 were consistently detected, whereas those to other fragments were sometimes lost. In vitro antigenic stimulation of peripheral blood mononuclear cells with IIbα18-259 or IIIa22-262 promoted the synthesis of anti–GPIIb-IIIa antibodies in patients with ITP, but not in healthy donors. Of 15 CD4+ T-cell lines specific for platelet-derived GPIIb-IIIa generated from 5 patients with ITP, 13 lines recognized IIbα18-259, IIIa22-262, or both. T-cell lines reactive to IIbα18-259 or IIIa22-262 promoted the production of anti–GPIIb-IIIa antibodies that were capable of binding to normal platelet surfaces. These results indicate that the immunodominant epitopes recognized by pathogenic CD4+ T cells in patients with ITP are located within the amino-terminal portions of both GPIIbα and GPIIIa.
Introduction
Chronic immune thrombocytopenic purpura (ITP) is an autoimmune disease characterized by increased platelet clearance caused by antiplatelet autoantibodies.1,2 These antibodies bind to circulating platelets, resulting in platelet destruction by the reticuloendothelial system. The major target of the antiplatelet autoantibodies is platelet membrane glycoprotein IIb-IIIa (GPIIb-IIIa),3-5 also designated αIIbβ3 integrin or CD41/CD61, which is a calcium-dependent heterodimeric membrane receptor for fibrinogen and other ligands.6 Although earlier studies reported the presence of platelet-reactive T cells in patients with ITP,7-9 we have recently found that GPIIb-IIIa is one of the major target antigens recognized by platelet-reactive CD4+ T cells.10 GPIIb-IIIa–reactive CD4+ T cells in patients with ITP have a helper activity that promotes the production of anti–GPIIb-IIIa antibodies capable of binding to normal platelets, indicating that these autoreactive T cells are involved in the production of pathogenic antiplatelet autoantibodies in patients with ITP.10
Our previous study demonstrated that GPIIb-IIIa–reactive T cells respond to chemically modified GPIIb-IIIa and recombinant GPIIb-IIIa fragments expressed in bacteria, but not to GPIIb-IIIa in its native form.10 One possible explanation for this finding is that autoreactive T cells recognize “cryptic” epitopes on GPIIb-IIIa that are not produced from native GPIIb-IIIa by the normal processing pathway. However, the precise locations of these cryptic epitopes have not been reported. T-cell epitope mapping on GPIIb-IIIa is potentially useful for detecting foreign cross-reactive proteins that elicit autoantibody responses to GPIIb-IIIa11 and for developing therapeutic strategies that suppress harmful T-cell responses in patients with ITP.12 In this study, a series of recombinant fragments encompassing different portions of GPIIbα and GPIIIa were constructed, and the responses of T cells from patients with ITP to these fragments were analyzed.
Patients, materials, and methods
Patients and controls
T cells from the peripheral blood of 25 patients with chronic ITP (3 men, 22 women) were analyzed. Chronic ITP was defined as thrombocytopenia (platelet count less than 150 × 109/L) persisting longer than 6 months, normal or increased levels of bone marrow megakaryocytes without morphologic evidence for dysplasia, and no secondary immune or nonimmune disease that could account for the thrombocytopenic state.1 2 The mean age at examination was 50.4 years (range, 21-71 years), and the mean platelet count was 36 × 109/L (range, 17 × 109-68 × 109). Anti–GPIIb-IIIa antibodies in plasma and in platelet eluates were positive in 12 and 22 patients, respectively, but 3 patients had negative findings for anti–GPIIb-IIIa antibodies. Seven patients had newly diagnosed disease, and the remaining 18 patients underwent various treatment regimens. At the time of blood examination, 12 patients had been on low-dose corticosteroids for 1.2 to 22.0 years. Previous medical treatment was with corticosteroids in 6 patients, splenectomy in 7, danazol in 2, slow infusion of vincristine in 1, and azathioprine in 1 (Table1). Peripheral blood samples from 14 patients with ITP were examined on 2 or 3 occasions at intervals ranging from 3 to 24 months.
T-cell proliferation and in vitro anti–GPIIb-IIIa antibody production in response to recombinant GPIIb-IIIa fragments in 25 patients with immune thrombocytopenic purpura
| Patients with ITP . | Age/sex . | Treatment . | Anti–GPIIb-IIIa . | Fragment-induced T-cell proliferation (SI)* . | Anti–GPIIb-IIIa antibody (OD450) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma . | Platelet eluates . | IIbα18-259 . | IIbα244-575 . | IIbα566-841 . | IIIa22-262 . | IIIa254-462 . | IIIa708-762 . | GST . | IIbα18-259 . | IIbα244-575 . | IIbα566-841 . | IIIa22-262 . | IIIa254-462 . | IIIa708-762 . | |||
| ITP1 | 53/F | cs, (sp) | − | + | 3.50 | — | — | — | — | — | NT | ||||||
| ITP2 | 51/M | None | − | + | 6.80 | — | 3.98 | — | — | 3.90 | NT | ||||||
| ITP3 | 42/F | None | + | + | — | — | — | 4.23 | — | — | NT | ||||||
| ITP4 | 60/F | cs | + | + | 5.08 | — | 4.55 | 8.52 | — | 7.54 | 0.064 | 0.547† | 0.097 | 0.399† | 0.694† | 0.102 | 0.071 |
| ITP5 | 29/F | None | − | + | 17.98 | — | 3.73 | 11.36 | — | 3.85 | NT | ||||||
| ITP6 | 72/F | None | − | + | — | — | 17.20 | — | — | — | NT | ||||||
| ITP7 | 51/F | (cs, sp) | − | + | 5.34 | — | — | — | 4.00 | — | NT | ||||||
| ITP8 | 56/M | cs, (sp) | + | + | 3.29 | — | — | — | — | — | 0.037 | 0.317† | 0.030 | 0.034 | 0.043 | 0.037 | 0.022 |
| ITP9 | 23/F | (cs) | − | + | 3.42 | 7.57 | 3.39 | 5.68 | — | — | 0.039 | 0.243† | 0.099 | 0.219† | 0.549† | 0.044 | 0.036 |
| ITP10 | 43/F | cs | + | + | — | — | — | 8.58 | — | — | 0.089 | 0.079 | 0.086 | 0.094 | 0.428† | 0.088 | 0.079 |
| ITP11 | 62/F | cs | + | + | 6.58 | — | — | — | — | — | 0.057 | 0.671† | 0.056 | 0.049 | 0.087 | 0.054 | 0.029 |
| ITP12 | 59/F | cs | − | − | 3.90 | — | — | — | — | — | NT | ||||||
| ITP13 | 56/F | None | + | + | 4.75 | — | — | 4.07 | — | — | 0.034 | 0.279† | 0.024 | 0.051 | 0.605† | 0.028 | 0.026 |
| ITP14 | 57/F | (cs, sp) | − | + | 3.54 | — | — | 3.15 | — | — | 0.039 | 0.197† | 0.046 | 0.041 | 0.372† | 0.061 | 0.056 |
| ITP15 | 49/F | None | + | + | — | 5.32 | — | 5.08 | — | — | 0.069 | 0.049 | 0.072 | 0.054 | 0.397† | 0.069 | 0.039 |
| ITP16 | 33/F | None | − | + | 4.62 | — | — | 3.19 | — | — | NT | ||||||
| ITP17 | 42/F | (cs) | + | + | — | — | — | 3.68 | — | — | NT | ||||||
| ITP18 | 60/M | cs | − | − | — | — | — | 4.03 | — | — | 0.008 | 0.001 | 0.002 | 0.009 | 0.112† | 0.000 | 0.004 |
| ITP19 | 46/F | cs | + | + | — | — | — | 11.08 | — | — | 0.026 | 0.028 | 0.036 | 0.024 | 0.239† | 0.026 | 0.021 |
| ITP20 | 71/F | cs, (sp, dz, vcr, az) | + | + | 5.14 | — | — | — | — | — | 0.038 | 0.551† | 0.052 | 0.050 | 0.072 | 0.047 | 0.048 |
| ITP21 | 62/F | cs | + | + | 6.40 | — | — | — | — | — | 0.039 | 0.603† | 0.061 | 0.038 | 0.071 | 0.036 | 0.045 |
| ITP22 | 54/F | cs, (sp) | − | − | — | 5.70 | — | 5.06 | — | — | NT | ||||||
| ITP23 | 53/F | cs | + | + | — | — | — | 5.19 | — | — | 0.046 | 0.049 | 0.051 | 0.084 | 0.695† | 0.042 | 0.041 |
| ITP24 | 21/F | (cs) | − | + | 8.20 | — | 3.73 | 9.62 | — | — | 0.022 | 0.146† | 0.012 | 0.028 | 0.320† | 0.012 | 0.019 |
| ITP25 | 54/F | (cs, sp, dz) | − | + | — | — | 7.48 | 8.25 | — | — | 0.019 | 0.017 | 0.021 | 0.061 | 0.409† | 0.049 | 0.021 |
| Patients with ITP . | Age/sex . | Treatment . | Anti–GPIIb-IIIa . | Fragment-induced T-cell proliferation (SI)* . | Anti–GPIIb-IIIa antibody (OD450) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma . | Platelet eluates . | IIbα18-259 . | IIbα244-575 . | IIbα566-841 . | IIIa22-262 . | IIIa254-462 . | IIIa708-762 . | GST . | IIbα18-259 . | IIbα244-575 . | IIbα566-841 . | IIIa22-262 . | IIIa254-462 . | IIIa708-762 . | |||
| ITP1 | 53/F | cs, (sp) | − | + | 3.50 | — | — | — | — | — | NT | ||||||
| ITP2 | 51/M | None | − | + | 6.80 | — | 3.98 | — | — | 3.90 | NT | ||||||
| ITP3 | 42/F | None | + | + | — | — | — | 4.23 | — | — | NT | ||||||
| ITP4 | 60/F | cs | + | + | 5.08 | — | 4.55 | 8.52 | — | 7.54 | 0.064 | 0.547† | 0.097 | 0.399† | 0.694† | 0.102 | 0.071 |
| ITP5 | 29/F | None | − | + | 17.98 | — | 3.73 | 11.36 | — | 3.85 | NT | ||||||
| ITP6 | 72/F | None | − | + | — | — | 17.20 | — | — | — | NT | ||||||
| ITP7 | 51/F | (cs, sp) | − | + | 5.34 | — | — | — | 4.00 | — | NT | ||||||
| ITP8 | 56/M | cs, (sp) | + | + | 3.29 | — | — | — | — | — | 0.037 | 0.317† | 0.030 | 0.034 | 0.043 | 0.037 | 0.022 |
| ITP9 | 23/F | (cs) | − | + | 3.42 | 7.57 | 3.39 | 5.68 | — | — | 0.039 | 0.243† | 0.099 | 0.219† | 0.549† | 0.044 | 0.036 |
| ITP10 | 43/F | cs | + | + | — | — | — | 8.58 | — | — | 0.089 | 0.079 | 0.086 | 0.094 | 0.428† | 0.088 | 0.079 |
| ITP11 | 62/F | cs | + | + | 6.58 | — | — | — | — | — | 0.057 | 0.671† | 0.056 | 0.049 | 0.087 | 0.054 | 0.029 |
| ITP12 | 59/F | cs | − | − | 3.90 | — | — | — | — | — | NT | ||||||
| ITP13 | 56/F | None | + | + | 4.75 | — | — | 4.07 | — | — | 0.034 | 0.279† | 0.024 | 0.051 | 0.605† | 0.028 | 0.026 |
| ITP14 | 57/F | (cs, sp) | − | + | 3.54 | — | — | 3.15 | — | — | 0.039 | 0.197† | 0.046 | 0.041 | 0.372† | 0.061 | 0.056 |
| ITP15 | 49/F | None | + | + | — | 5.32 | — | 5.08 | — | — | 0.069 | 0.049 | 0.072 | 0.054 | 0.397† | 0.069 | 0.039 |
| ITP16 | 33/F | None | − | + | 4.62 | — | — | 3.19 | — | — | NT | ||||||
| ITP17 | 42/F | (cs) | + | + | — | — | — | 3.68 | — | — | NT | ||||||
| ITP18 | 60/M | cs | − | − | — | — | — | 4.03 | — | — | 0.008 | 0.001 | 0.002 | 0.009 | 0.112† | 0.000 | 0.004 |
| ITP19 | 46/F | cs | + | + | — | — | — | 11.08 | — | — | 0.026 | 0.028 | 0.036 | 0.024 | 0.239† | 0.026 | 0.021 |
| ITP20 | 71/F | cs, (sp, dz, vcr, az) | + | + | 5.14 | — | — | — | — | — | 0.038 | 0.551† | 0.052 | 0.050 | 0.072 | 0.047 | 0.048 |
| ITP21 | 62/F | cs | + | + | 6.40 | — | — | — | — | — | 0.039 | 0.603† | 0.061 | 0.038 | 0.071 | 0.036 | 0.045 |
| ITP22 | 54/F | cs, (sp) | − | − | — | 5.70 | — | 5.06 | — | — | NT | ||||||
| ITP23 | 53/F | cs | + | + | — | — | — | 5.19 | — | — | 0.046 | 0.049 | 0.051 | 0.084 | 0.695† | 0.042 | 0.041 |
| ITP24 | 21/F | (cs) | − | + | 8.20 | — | 3.73 | 9.62 | — | — | 0.022 | 0.146† | 0.012 | 0.028 | 0.320† | 0.012 | 0.019 |
| ITP25 | 54/F | (cs, sp, dz) | − | + | — | — | 7.48 | 8.25 | — | — | 0.019 | 0.017 | 0.021 | 0.061 | 0.409† | 0.049 | 0.021 |
SI indicates stimulation index; OD450, optical density at 450 nm; NT, not tested; GST, glutathione S-transferase; cs, corticosteroids; sp, splenectomy; dz, danazol; vcr, vincristine; az, azathioprine. Previous treatment is shown in parentheses.
Negative results (SI < 3) are indicated by dashes.
Significant anti–GPIIb-IIIa antibody levels in culture supernatants.
Control T cells were obtained from 10 healthy donors (4 men, 6 women) who had no history of ITP and showed a T-cell proliferative response to trypsin-digested GPIIb-IIIa. The mean age at examination was 36.7 years (range, 23-56 years). All healthy controls had normal platelet counts and were negative for anti–GPIIb-IIIa antibodies in plasma and platelet eluates.
Human leukocyte antigen (HLA)-DRB1, -DQB1, and -DPB1 alleles were determined for all patients and controls, using polymerase chain reaction (PCR) followed by analysis of restriction fragment length polymorphisms.13 14 Written informed consent approved by the Institutional Review Board guidelines was granted by all study participants.
GPIIb-IIIa preparations for T-cell stimulation
Human GPIIb-IIIa was purified from outdated platelet concentrates using affinity chromatography and chemically modified by treatment with porcine trypsin (0.1 μg/mL) as described previously.10 Phosphate-buffered saline (PBS) containing porcine trypsin in the absence of GPIIb-IIIa was also prepared for use as a control antigen.
Seven different portions of GPIIbα and GPIIIa were expressed as recombinant glutathione S-transferase (GST) fusion proteins.15 These included IIbα18-259, IIbα244-575, and IIbα566-841, which encompass amino acid residues 18-259, 244-575, and 566-841, respectively, of the 871 amino acids of GPIIbα. The GPIIIa fragments used were IIIa22-262, IIIa254-462, IIIa455-723, and IIIa708-762, which encompass amino acid residues 22-262, 254-462, 455-723, and 708-762, respectively, of the 762 amino acids of GPIIIa. A series of GPIIbα and GPIIIa complementary DNA (cDNA) constructs prepared by reverse transcription and PCR from the bone marrow cells of a patient with ITP were subcloned into the 3′ end of theSchistosoma japonicum GST gene in the bacterial expression plasmid vector, pGEX 6P-1 (Amersham Pharmacia Biotech, Piscataway, NJ). To eliminate possible PCR errors and to verify the translational frames, both strands of each DNA construct were sequenced on an ABI Prism 310 genetic analyzer (Applied Biosystems, Foster City, CA). Expression of the recombinant GST-fusion proteins was induced with 1 mM isopropyl-β-D-thiogalactopyranoside, and bacterial lysates containing recombinant proteins were prepared by sonication in the presence of 8 M urea. After stepwise dialysis to remove excess denaturant, soluble bacterial proteins in the presence of 2 M urea were fractionated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis, and the recombinant GPIIb-IIIa fragments were directly eluted from the gels.16 Briefly, both the right and the left edges of the gel were stained with 0.05% Coomassie blue, and the portion of the gel corresponding to the recombinant protein was excised and crushed by mechanical compression in PBS. After the gel components were removed by quick centrifugation, the eluted proteins were dialyzed against PBS and filter-sterilized. Specificity of the GST-fusion proteins was confirmed on immunoblots probed with goat anti-GST polyclonal antibodies (Amersham Pharmacia Biotech). To evaluate the amounts of contaminating bacterial proteins in the purified preparations, a sample from each preparation was incubated with glutathione–Sepharose 4B (Amersham Pharmacia Biotech) for affinity depletion of GST fusion proteins.15 Purified preparations before and after affinity depletion were fractionated on SDS–10% polyacrylamide gels, stained with 0.05% Coomassie blue or Silver Staining Plus kit (Bio-Rad Laboratories, Hercules, CA), and subsequently analyzed using a Molecular Imager FX (Bio-Rad Laboratories). Protein bands absorbed by incubation with glutathione–Sepharose beads were regarded as recombinant GST–GPIIb-IIIa fusion proteins.
Detection of anti–GPIIb-IIIa antibodies
The level of immunoglobulin G (IgG) anti–GPIIb-IIIa antibodies in plasma, platelet eluates, and culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA) using affinity-purified GPIIb-IIIa as an antigen, as described elsewhere.5 10 All samples were tested in duplicate, and the results were calculated as the duplicate mean. Cutoff values for plasma and platelet eluates were considered the mean plus 3 × SD of 20 samples from healthy donors.
GPIIb-IIIa–reactive T-cell lines
GPIIb-IIIa–reactive T-cell lines were generated from patients with ITP using a previously described method17 with some modifications. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood using Lymphoprep (Nycomed Pharma AS, Oslo, Norway) density gradient centrifugation and were cultured in RPMI 1640 containing 8% pooled human AB serum, 2 mM L-glutamine, 50 U/mL penicillin, and 50 mg/mL streptomycin in the presence of trypsin-digested, platelet-derived GPIIb-IIIa (5 μg/mL) in a humidified atmosphere of 5% CO2 at 37°C. On day 3, 20 U/mL interleukin-2 (IL-2) (Life Technologies, Grand Island, NY) was added to the cultures. Cells were restimulated with trypsin-digested GPIIb-IIIa and IL-2 (50 U/mL) and irradiated (30 Gy) autologous PBMCs in fresh medium on day 10. Seven days after the second stimulation, T-cell blasts were cloned by limiting dilution at 1 to 100 cells/well using round-bottomed, 96-well plates in the presence of trypsin-digested GPIIb-IIIa, IL-2, and irradiated PBMCs. Wells that contained growing cells were selected, and these cells were expanded by repeated stimulation with trypsin-digested GPIIb-IIIa, IL-2, and autologous Epstein-Barr virus–transformed lymphoblastoid B cells irradiated at 100 Gy. The specificity of each T-cell line was assessed by antigen-induced T-cell proliferation assays, and T-cell lines that proliferated in response to trypsin-digested GPIIb-IIIa, but not to mock-treated PBS containing trypsin alone, were selected as GPIIb-IIIa–specific T-cell lines. Cell surface expression of CD4 and CD8 was examined by flow cytometry using fluorescein isothiocyanate–conjugated monoclonal antibodies (mAbs) against CD4 and CD8 (Becton Dickinson, San Jose, CA).
T-cell proliferation assay
The antigenic specificity of T cells was determined by antigen-induced T-cell proliferation.17,18 PBMCs were cultured in the presence or absence of antigen for 7 days unless otherwise indicated. GPIIb-IIIa–reactive T-cell lines were cultured with irradiated autologous lymphoblastoid B cells in the presence or absence of antigen for 3 days. After a final 16-hour incubation with 0.5 μCi/well of 3H-thymidine, the cells were harvested, and 3H-thymidine incorporation was determined in a TopCount microplate scintillation counter (Packard, Meriden, CT). Antigens were used at a concentration of 5 μg/mL and included native GPIIb-IIIa, trypsin-digested GPIIb-IIIa, mock-treated PBS, GST, tetanus toxoid (List Biological Laboratories, Campbell, CA), and individual recombinant GPIIb-IIIa fragments. All cultures were prepared in triplicate, and all values represent the mean of triplicate determinations. For bulk PBMC cultures, Δcpm was calculated by subtracting the cpm value obtained in cultures incubated without antigen from those obtained in cultures with antigen. Antigen-specific T-cell proliferation in response to each recombinant GPIIb-IIIa fragment was expressed as a stimulation index, which was calculated as the Δcpm incorporated into cultures with recombinant GPIIb-IIIa fragment divided by the Δcpm incorporated into cultures with GST. A positive response was defined as having a stimulation index greater than 3. Standard deviations were less than 20% of the mean or less than 100 cpm, unless indicated otherwise. To examine the inhibitory effects of anti-HLA class II mAb on antigen-specific T-cell proliferation, mAbs were added at the start of the cultures.10 Anti–HLA-DR (L243; IgG2a), anti–HLA-DQ (1a3; IgG2a), anti–HLA-DP (B7/21; IgG3), and isotype-matched control mAbs (Leinco Technologies, Ballwin, MO) were dialyzed against PBS and used at a final concentration of 1 μg/mL.
In vitro assay for anti–GPIIb-IIIa antibody production
An in vitro assay to analyze the antigen-induced anti–GPIIb-IIIa antibody synthesis in PBMC cultures or in cultures of GPIIb-IIIa–reactive T-cell lines and autologous peripheral blood B cells was carried out as described.10,19 Briefly, PBMCs (5 × 105/well) or GPIIb-IIIa–reactive T cells (2 × 105) plus autologous peripheral blood B cells (105) were cultured in complete medium with or without antigen in the presence of pokeweed mitogen (1 μg/mL) for 10 days. Individual recombinant GPIIb-IIIa fragments and GST were used at 5 μg/mL as antigens. In some experiments, anti–interferon γ (IFN-γ) (25718.11; IgG2a), anti–IL-4 (34019.111; IgG2b), or anti–IL-6 (6708.111; IgG1) mAb (Genzyme Techne, Cambridge, MA) were added at the initiation of the culture. IgG anti–GPIIb-IIIa antibody levels in undiluted culture supernatants were measured by ELISA using purified GPIIb-IIIa as an antigen source, as described above. All cultures were prepared in duplicate, and anti–GPIIb-IIIa antibody results represent the mean of duplicate values. Significant anti–GPIIb-IIIa antibody production in response to antigenic stimulation with recombinant GPIIb-IIIa fragments was defined as having both a value greater than 2 for the anti–GPIIb-IIIa antibody levels in cultures with a recombinant GPIIb-IIIa fragment divided by the anti–GPIIb-IIIa antibody levels in cultures with GST and an increase in OD450 (optical density) greater than 0.1 associated with antigenic stimulation. In some experiments, anti–GPIIb-IIIa antibodies produced in culture supernatants were absorbed by incubation with platelets, erythrocytes, or PBMCs obtained from 2 independent healthy donors, as described elsewhere.10 Standard deviations were less than 20% of the mean or less than 0.01 (OD450), unless indicated otherwise.
Cytokine production assay
For the determination of cytokine profiles in individual GPIIb-IIIa–reactive T-cell lines, T cells were cultured with phytohemagglutinin (1 μg/mL) and an anti-CD3 mAb (OKT3; 30 ng/mL) for 48 hours, and the supernatants were collected and stored at −80°C until analysis. The levels of human IFN-γ, IL-4, and IL-6 in the culture supernatants were measured in duplicate using ELISA kits (BioSource International, Camarillo, CA) according to the manufacturer's instructions.
Statistical analysis
All comparisons between the 2 groups were tested for statistical significance using the Fisher 2-tailed exact test or the Studentt test. Correlation coefficient (r) was determined using a single regression model.
Results
Expression and purification of recombinant GPIIbα and GPIIIa fragments
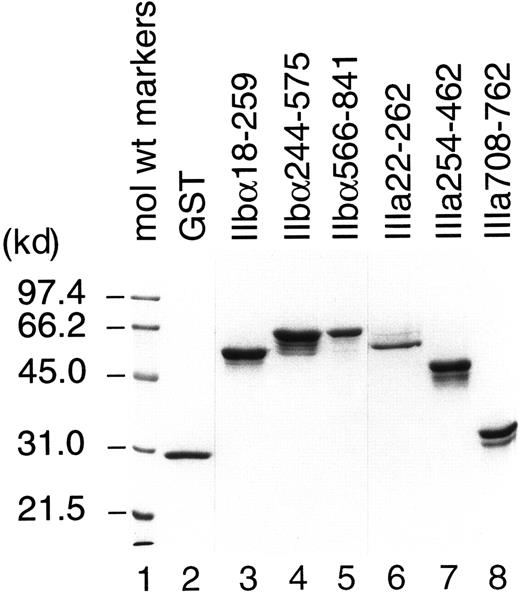
All recombinant fusion proteins except IIIa455-723 were successfully purified and used as antigens for T-cell stimulation. We failed to purify IIIa455-723, which contains cysteine-rich, tandemly repeated domains of GPIIIa, because only a trace amount of recombinant protein was produced by bacteria transfected with cDNA encoding this region. IIbα18-259, IIbα244-575, IIbα566-841, IIIa22-262, IIIa254-462, and IIIa708-762 were expressed in large quantities, but the yields by affinity purification were low because of the formation of insoluble aggregates. Therefore, these recombinant fragments and GST were purified directly from bacterial lysates fractionated on SDS-polyacrylamide gels. As shown in Figure1, each purified preparation represented major protein band consistent with the predicted molecular weight mass, with or without additional bands. These extra protein bands were completely absorbed by incubation with glutathione–Sepharose beads and, therefore, were degradation products of recombinant GPIIb-IIIa fragments. Densitometric analysis on silver-stained gels revealed that more than 92% of the proteins in our preparations were recombinant GPIIb-IIIa fragments.
Analysis of recombinant human GPIIb-IIIa fragments by SDS–polyacrylamide gel electrophoresis.
Purified GST and recombinant human GPIIb-IIIa fusion proteins were fractionated on a 10% polyacrylamide-SDS gel and stained with Coomassie blue.
Analysis of recombinant human GPIIb-IIIa fragments by SDS–polyacrylamide gel electrophoresis.
Purified GST and recombinant human GPIIb-IIIa fusion proteins were fractionated on a 10% polyacrylamide-SDS gel and stained with Coomassie blue.
T-cell proliferative responses to GPIIb-IIIa fragments
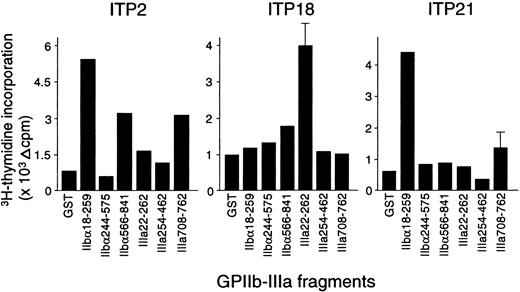
The proliferative responses of peripheral blood T cells with the 6 recombinant GPIIb-IIIa fragments were examined, and representative results from 3 patients with ITP are shown in Figure2. ITP2 responded to IIbα18-259, IIbα566-841, and IIIa708-762; ITP18 responded to IIIa22-262; and ITP21 responded to IIbα18-259. Table 1 summarizes the T-cell proliferative responses to recombinant GPIIb-IIIa fragments in 25 patients with ITP. Each patient showed a significant response to at least one fragment, though 3 patients (ITP12, ITP18, and ITP22) were negative for anti–GPIIb-IIIa antibodies at the time of examination. T cells from the patients with ITP recognized the GPIIb-IIIa fragments in various combinations, and 12 patients responded to 2 or more fragments. Amino-terminal regions of both IIbα and IIIa (IIbα18-259 and IIIa22-262) were frequently recognized (60% and 64%, respectively), compared with the other fragments (IIbα566-841, IIbα244-575, IIIa254-462, and IIIa708-762), which were recognized by 28%, 12%, 4%, and 12% of the patients, respectively. T-cell proliferative responses to IIbα18-259 or IIIa22-262 were detected in all but one patient. These findings indicate that several distinct T-cell epitopes are present on GPIIb-IIIa, but the immunodominant T-cell epitopes are located within IIbα18-259 and IIIa22-262.
Peripheral blood T-cell proliferative responses to recombinant GPIIb-IIIa fragments.
PBMCs from patients with ITP were stimulated with GST or individual GPIIb-IIIa fragments for 7 days, and 3H-thymidine incorporation was measured by liquid-scintillation counting.
Peripheral blood T-cell proliferative responses to recombinant GPIIb-IIIa fragments.
PBMCs from patients with ITP were stimulated with GST or individual GPIIb-IIIa fragments for 7 days, and 3H-thymidine incorporation was measured by liquid-scintillation counting.
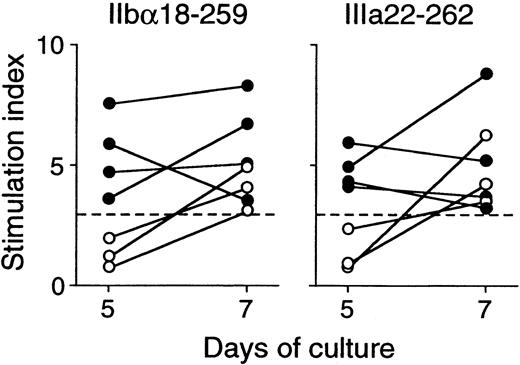
All healthy donors who responded to trypsin-digested GPIIb-IIIa also showed T-cell proliferative responses to 2 or more recombinant GPIIb-IIIa fragments in various combinations (Table2). In contrast to findings in patients with ITP, all 6 recombinant fragments were recognized in similar frequencies. It was of note that T-cell responses to IIbα18-259 and IIIa22-262 were detected in 3 healthy donors each, whereas IIbα566-841 was recognized in 6 healthy controls. To examine the kinetics of T-cell proliferative responses to IIbα18-259 and IIIa22-262 in healthy donors versus patients with ITP, T-cell responses to these fragments were measured on day 5 and day 7 in responders, including 4 patients with ITP and 3 healthy donors (Figure3). T-cell responses to IIbα18-259 and IIIa22-262 were detected on day 5 and day 7 in patients with ITP, whereas T cells from healthy donors did not show a T-cell response on day 5 but responded on day 7.
T-cell proliferation and in vitro anti–GPIIb-IIIa antibody production in response to recombinant GPIIb-IIIa fragments
| Healthy donors . | Age/sex . | Fragment-induced T-cell proliferation (SI)* . | Anti–GPIIb-IIIa antibody (OD450) . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IIbα18-259 . | IIbα244-575 . | IIbα566-841 . | IIIa22-262 . | IIIa254-462 . | IIIa708-762 . | GST . | IIbα18-259 . | IIbα244-575 . | IIbα566-841 . | IIIa22-262 . | IIIa254-462 . | IIIa708-762 . | ||
| HD1 | 37/M | 3.03 | 4.17 | — | — | 3.71 | — | 0.002 | 0.003 | 0.000 | 0.001 | 0.003 | 0.002 | 0.005 |
| HD2 | 38/F | — | — | 3.17 | 4.19 | — | — | NT | ||||||
| HD3 | 23/F | — | — | 3.21 | — | 3.93 | — | 0.005 | 0.000 | 0.006 | 0.002 | 0.002 | 0.000 | 0.004 |
| HD4 | 22/M | — | 4.11 | — | 6.31 | — | — | 0.001 | 0.005 | 0.007 | 0.000 | 0.003 | 0.012 | 0.001 |
| HD5 | 44/M | — | 3.33 | — | — | — | 5.61 | 0.011 | 0.013 | 0.001 | 0.004 | 0.004 | 0.007 | 0.005 |
| HD6 | 47/F | — | — | 4.07 | — | 5.00 | — | NT | ||||||
| HD7 | 32/M | 3.96 | — | 4.01 | — | — | 5.94 | 0.000 | 0.000 | 0.003 | 0.002 | 0.000 | 0.005 | 0.001 |
| HD8 | 28/F | — | — | 3.78 | 3.44 | — | — | 0.003 | 0.005 | 0.000 | 0.003 | 0.000 | 0.007 | 0.002 |
| HD9 | 56/F | — | 3.94 | 6.50 | — | — | — | 0.003 | 0.008 | 0.009 | 0.001 | 0.003 | 0.000 | 0.003 |
| HD10 | 40/F | 4.88 | — | — | — | 4.36 | — | NT | ||||||
| Healthy donors . | Age/sex . | Fragment-induced T-cell proliferation (SI)* . | Anti–GPIIb-IIIa antibody (OD450) . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IIbα18-259 . | IIbα244-575 . | IIbα566-841 . | IIIa22-262 . | IIIa254-462 . | IIIa708-762 . | GST . | IIbα18-259 . | IIbα244-575 . | IIbα566-841 . | IIIa22-262 . | IIIa254-462 . | IIIa708-762 . | ||
| HD1 | 37/M | 3.03 | 4.17 | — | — | 3.71 | — | 0.002 | 0.003 | 0.000 | 0.001 | 0.003 | 0.002 | 0.005 |
| HD2 | 38/F | — | — | 3.17 | 4.19 | — | — | NT | ||||||
| HD3 | 23/F | — | — | 3.21 | — | 3.93 | — | 0.005 | 0.000 | 0.006 | 0.002 | 0.002 | 0.000 | 0.004 |
| HD4 | 22/M | — | 4.11 | — | 6.31 | — | — | 0.001 | 0.005 | 0.007 | 0.000 | 0.003 | 0.012 | 0.001 |
| HD5 | 44/M | — | 3.33 | — | — | — | 5.61 | 0.011 | 0.013 | 0.001 | 0.004 | 0.004 | 0.007 | 0.005 |
| HD6 | 47/F | — | — | 4.07 | — | 5.00 | — | NT | ||||||
| HD7 | 32/M | 3.96 | — | 4.01 | — | — | 5.94 | 0.000 | 0.000 | 0.003 | 0.002 | 0.000 | 0.005 | 0.001 |
| HD8 | 28/F | — | — | 3.78 | 3.44 | — | — | 0.003 | 0.005 | 0.000 | 0.003 | 0.000 | 0.007 | 0.002 |
| HD9 | 56/F | — | 3.94 | 6.50 | — | — | — | 0.003 | 0.008 | 0.009 | 0.001 | 0.003 | 0.000 | 0.003 |
| HD10 | 40/F | 4.88 | — | — | — | 4.36 | — | NT | ||||||
See Table 1 for abbreviations.
Negative results (SI < 3) are indicated by dashes.
Peripheral blood T-cell proliferative responses to IIbα18-259 and IIIa22-262 on day 5 and day 7.
PBMCs from 4 patients with ITP (closed circles) and 3 healthy donors (open circles) were stimulated with GST or GPIIb-IIIa fragments, and3H-thymidine incorporation was measured on day 5 and day 7 by liquid-scintillation counting. T-cell proliferative responses induced by IIbα18-259 and IIIa22-262 are expressed by stimulation index.
Peripheral blood T-cell proliferative responses to IIbα18-259 and IIIa22-262 on day 5 and day 7.
PBMCs from 4 patients with ITP (closed circles) and 3 healthy donors (open circles) were stimulated with GST or GPIIb-IIIa fragments, and3H-thymidine incorporation was measured on day 5 and day 7 by liquid-scintillation counting. T-cell proliferative responses induced by IIbα18-259 and IIIa22-262 are expressed by stimulation index.
HLA and clinical associations with T-cell reactivities to GPIIb-IIIa fragments
An association between the T-cell reactivities to individual GPIIb-IIIa fragments and the HLA-DRB1, -DQB1, and -DPB1 alleles was examined. No statistically significant associations were found in patients with ITP. However, when patients with ITP and healthy donors were combined, T-cell reactivity to IIbα18-259 was detected in 9 of 10 of them with DRB1*0901 and in 9 of 25 without DRB1*0901 (P = .007).
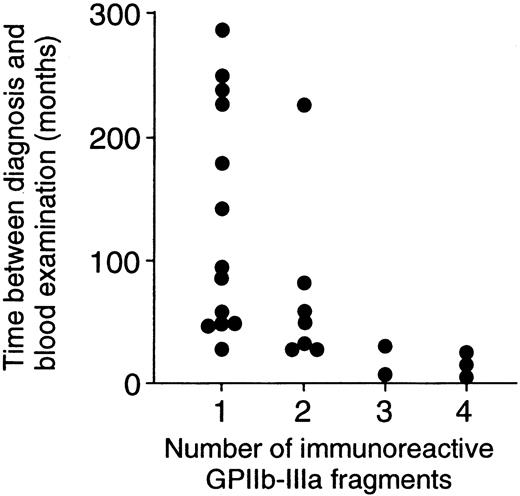
Thirteen patients with ITP had T-cell responses to one fragment, and the remaining 12 patients had responses to 2 or more fragments. When demographic and laboratory findings were compared between these 2 patient groups, no differences were found in sex, age at the time of diagnosis, platelet count at the time of blood examination, levels of anti–GPIIb-IIIa antibodies in plasma and platelet eluates, or current and previous treatment regimens (data not shown). However, the time between diagnosis and blood examination for patients who responded to one fragment was significantly longer than that for patients who responded to 2 or more fragments (135.1 ± 91.7 vs 50.3 ± 60.2 months; P = .01). Figure 4illustrates that a significant negative correlation between the number of immunoreactive fragments and the time between diagnosis and blood examination was detected (r = 0.52;P = .008).
Negative correlation between the number of immunoreactive GPIIb-IIIa fragments and the time between diagnosis and blood examination.
The number of immunoreactive GPIIb-IIIa fragments was negatively correlated with the time between diagnosis and blood examination in 25 patients with ITP (r = 0.52; P = .008).
Negative correlation between the number of immunoreactive GPIIb-IIIa fragments and the time between diagnosis and blood examination.
The number of immunoreactive GPIIb-IIIa fragments was negatively correlated with the time between diagnosis and blood examination in 25 patients with ITP (r = 0.52; P = .008).
Serial analysis of T-cell reactivities to GPIIb-IIIa fragments in patients with ITP
T-cell proliferative responses to GPIIb-IIIa fragments were serially examined in 14 patients with ITP. Immunoreactive fragments detected at the initial and later examinations were concordant in 11 patients. None of the patients developed T-cell responses to additional fragments during the follow-up, but 3 patients demonstrated a loss of fragment reactivity. ITP4 responded to 4 fragments—IIbα18-259, IIbα566-841, IIIa22-262, and IIIa708-762—at the first examination but responded to IIbα18-259 and IIIa22-262 at follow-up examinations 10 and 18 months later. ITP2 lost T-cell reactivity to IIIa708-762, and ITP7 lost reactivity to IIIa254-462 at the follow-up examinations. It was noted that T-cell reactivities to immunodominant fragments were consistently detected in all 14 patients examined. In contrast, T-cell reactivities lost during follow-up were to nondominant fragments.
In vitro anti–GPIIb-IIIa antibody production in response to GPIIb-IIIa fragments
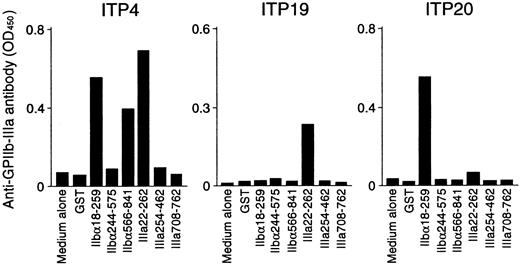
Anti–GPIIb-IIIa antibody production was measured in PBMC cultures with recombinant GPIIb-IIIa fragments, and representative results are shown in Figure 5. PBMCs from ITP4 produced anti–GPIIb-IIIa antibodies in response to recombinant fragments IIbα18-259, IIbα566-841, and IIIa22-262. Samples from this patient showed T-cell proliferative responses to these 3 fragments in addition to IIIa708-762, which did not promote in vitro anti–GPIIb-IIIa antibody production. In vitro anti–GPIIb-IIIa antibody production was detected in ITP19 and ITP20 in response to IIIa22-262 and IIbα18-259, respectively. For these patients, the fragments inducing T-cell proliferation were consistent with those promoting in vitro anti–GPIIb-IIIa antibody production. Levels of anti–GPIIb-IIIa antibodies in PBMC culture supernatants stimulated with GST or individual GPIIb-IIIa fragments for 15 patients with ITP and 7 healthy donors are summarized in Tables 1 and 2, respectively. In patients with ITP, in vitro anti–GPIIb-IIIa antibody production was induced by antigenic stimulation with at least one of the GPIIb-IIIa fragments. In contrast, none of the PBMCs from healthy donors produced anti–GPIIb-IIIa antibodies, though they showed T-cell proliferation to 2 or more GPIIb-IIIa fragments. To examine whether anti–GPIIb-IIIa antibodies produced in in vitro cultures react with platelets, 10 randomly selected culture supernatants containing anti–GPIIb-IIIa antibodies were incubated with platelets, erythrocytes, or PBMCs obtained from healthy donors. Anti–GPIIb-IIIa antibody reactivity was suppressed by incubation with platelets, but not by incubation with erythrocytes or PBMCs (data not shown).
In vitro anti–GPIIb-IIIa antibody production in PBMC cultures in response to recombinant GPIIb-IIIa fragments.
PBMCs from patients with ITP were cultured with GST or individual recombinant GPIIb-IIIa fragments for 10 days, and anti–GPIIb-IIIa antibody levels were measured by ELISA.
In vitro anti–GPIIb-IIIa antibody production in PBMC cultures in response to recombinant GPIIb-IIIa fragments.
PBMCs from patients with ITP were cultured with GST or individual recombinant GPIIb-IIIa fragments for 10 days, and anti–GPIIb-IIIa antibody levels were measured by ELISA.
When the results of in vitro anti–GPIIb-IIIa antibody production were compared with those of T-cell proliferation assays, it was found that the immunodominant fragments IIbα18-259 and IIIa22-262 promoted anti–GPIIb-IIIa antibody production exclusively in responders to these fragments in the proliferation assay. In contrast, anti–GPIIb-IIIa antibody production was detected in none of the PBMC cultures with IIbα244-575, whereas samples from 2 patients showed a T-cell proliferative response to IIbα244-575 (ITP9 and ITP15). Similarly, IIbα566-841 failed to induce anti–GPIIb-IIIa antibody production in 2 of 4 responders to IIbα566-841 in the proliferation assay (ITP24 and ITP25). Samples from none of the patients with ITP, including ITP4, induced anti–GPIIb-IIIa antibody production in response to IIIa708-762, though ITP4 showed a T-cell proliferative response to this fragment.
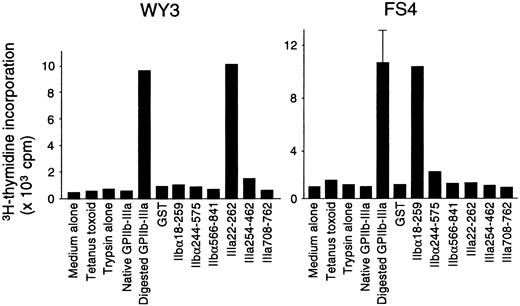
Characterization of GPIIb-IIIa–reactive T-cell lines
Fifteen T-cell lines specific for platelet-derived GPIIb-IIIa were established from 5 patients with ITP. All these lines had a CD4+CD8− phenotype and responded to trypsin-digested GPIIb-IIIa, but not to the mock-treated PBS control. Antigen-induced proliferative responses in representative GPIIb-IIIa–reactive T-cell lines are shown in Figure6. Lines WY3 and FS4 responded to IIIa22-262 and IIbα18-259, respectively, in addition to trypsin-digested GPIIb-IIIa. Eight T-cell lines reacted with one GPIIb-IIIa fragment (2 with IIbα18-259, 1 with IIbα566-841, and 5 with IIIa22-262), and 6 lines reacted with 2 or 3 fragments (5 with IIbα18-259 and IIIa22-262, and 1 with IIbα18-259, IIbα566-841, and IIIa22-262). One GPIIb-IIIa–reactive T-cell line did not respond to any of the 6 fragments. In total, 13 of 15 T-cell lines that were reactive with platelet-derived GPIIb-IIIa recognized IIbα18-259, IIIa22-262, or both, indicating again that T cells reactive with these 2 amino-terminal fragments are the predominant GPIIb-IIIa–reactive T cells in the peripheral blood of patients with ITP.
Proliferative responses to a series of GPIIb-IIIa preparations in GPIIb-IIIa–reactive CD4+ T-cell lines.
GPIIb-IIIa–reactive T-cell lines were cultured with autologous antigen-presenting cells in the presence or absence of various antigens, including tetanus toxoid, mock-treated PBS containing trypsin alone, native GPIIb-IIIa, trypsin-digested GPIIb-IIIa, GST, or individual recombinant GPIIb-IIIa fragments for 7 days, and3H-thymidine incorporation was measured by liquid-scintillation counting. Only standard deviation > 20% of the mean and 100 cpm is shown as an extended line above a bar.
Proliferative responses to a series of GPIIb-IIIa preparations in GPIIb-IIIa–reactive CD4+ T-cell lines.
GPIIb-IIIa–reactive T-cell lines were cultured with autologous antigen-presenting cells in the presence or absence of various antigens, including tetanus toxoid, mock-treated PBS containing trypsin alone, native GPIIb-IIIa, trypsin-digested GPIIb-IIIa, GST, or individual recombinant GPIIb-IIIa fragments for 7 days, and3H-thymidine incorporation was measured by liquid-scintillation counting. Only standard deviation > 20% of the mean and 100 cpm is shown as an extended line above a bar.
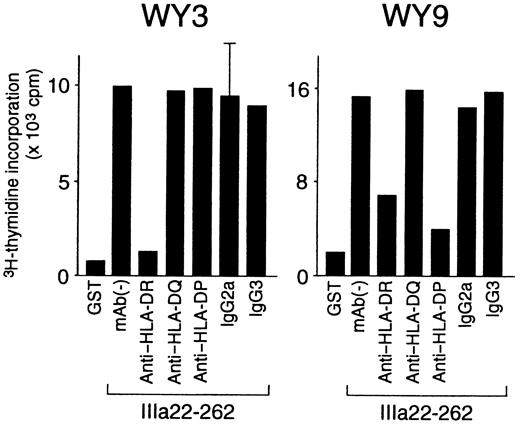
HLA class II restriction, T-cell helper activity, and cytokine profiles were further analyzed in the 8 GPIIb-IIIa–reactive T-cell lines that responded to a single GPIIb-IIIa fragment (Table3). HLA class II restriction was determined according to the inhibitory effect of an anti–HLA class II mAb on antigen-induced T-cell proliferation. As shown in Figure7, IIIa22-262–induced proliferation was inhibited by an anti–HLA-DR mAb in line WY3 and by both anti–HLA-DR and anti–HLA-DP mAb in line WY9. All GPIIb-IIIa–reactive T-cell lines were restricted by HLA-DR, but line WY9 was restricted by both HLA-DR and HLA-DP.
Immunoreactive GPIIb-IIIa fragment, human leukocyte antigen class II restriction, T-cell helper activity inducing anti–GPIIb-IIIa antibody production, and cytokine profiles in GPIIb-IIIa–reactive CD4+ T-cell lines generated from patients with immune thrombocytopenic purpura
| Line . | Donor . | Immunoreactive fragment . | HLA class II restriction . | Anti-GPIIb-IIIa antibody in culture (OD450)3-150 . | Cytokine level in culture (pg/mL)3-151 . | ||||
|---|---|---|---|---|---|---|---|---|---|
| With immunoreactive fragment . | With GST . | ||||||||
| No treatment . | +Platelets3-152 . | IFN-γ . | IL-4 . | IL-6 . | |||||
| FS4 | ITP8 | IIbα18-259 | HLA-DR | 0.267 | 0.055 | 0.029 | 256 | < 1 | 360 |
| SiM4 | ITP14 | IIbα18-259 | HLA-DR | 0.351 | 0.101 | 0.048 | 456 | 9 | 296 |
| SuM7 | ITP25 | IIbα566-841 | HLA-DR | 0.042 | 0.039 | 0.011 | 780 | 76 | 18 |
| WY3 | ITP10 | IIIa22-262 | HLA-DR | 0.422 | 0.084 | 0.096 | NT | NT | NT |
| WY5 | ITP10 | IIIa22-262 | HLA-DR | NT | NT | NT | NT | NT | NT |
| WY9 | ITP10 | IIIa22-262 | HLA-DR, -DP | 0.567 | 0.109 | 0.124 | 280 | 86 | 567 |
| NE4 | ITP24 | IIIa22-262 | HLA-DR | 0.196 | NT | 0.014 | 892 | 16 | 117 |
| SuM8 | ITP25 | IIIa22-262 | HLA-DR | 0.397 | 0.051 | 0.017 | 667 | 67 | 396 |
| Line . | Donor . | Immunoreactive fragment . | HLA class II restriction . | Anti-GPIIb-IIIa antibody in culture (OD450)3-150 . | Cytokine level in culture (pg/mL)3-151 . | ||||
|---|---|---|---|---|---|---|---|---|---|
| With immunoreactive fragment . | With GST . | ||||||||
| No treatment . | +Platelets3-152 . | IFN-γ . | IL-4 . | IL-6 . | |||||
| FS4 | ITP8 | IIbα18-259 | HLA-DR | 0.267 | 0.055 | 0.029 | 256 | < 1 | 360 |
| SiM4 | ITP14 | IIbα18-259 | HLA-DR | 0.351 | 0.101 | 0.048 | 456 | 9 | 296 |
| SuM7 | ITP25 | IIbα566-841 | HLA-DR | 0.042 | 0.039 | 0.011 | 780 | 76 | 18 |
| WY3 | ITP10 | IIIa22-262 | HLA-DR | 0.422 | 0.084 | 0.096 | NT | NT | NT |
| WY5 | ITP10 | IIIa22-262 | HLA-DR | NT | NT | NT | NT | NT | NT |
| WY9 | ITP10 | IIIa22-262 | HLA-DR, -DP | 0.567 | 0.109 | 0.124 | 280 | 86 | 567 |
| NE4 | ITP24 | IIIa22-262 | HLA-DR | 0.196 | NT | 0.014 | 892 | 16 | 117 |
| SuM8 | ITP25 | IIIa22-262 | HLA-DR | 0.397 | 0.051 | 0.017 | 667 | 67 | 396 |
See Table 1 for abbreviations.
T-cell lines were cultured with autologous peripheral blood B cells in the presence of the immunoreactive GPIIb-IIIa fragment or GST for 10 days, and anti–GPIIb-IIIa antibody levels in culture supernatants were measured by ELISA.
T-cell lines were stimulated with phytohemagglutinin and anti-CD3 mAb for 3 days, and cytokine levels in culture supernatants were measured by ELISA.
Culture supernatants were incubated with normal platelets before being applied to anti–GPIIb-IIIa antibody ELISA.
Effects of anti–HLA class II mAb on antigen-induced proliferation of GPIIb-IIIa–reactive CD4+ T-cell lines.
GPIIb-IIIa–reactive T-cell lines were cultured with autologous antigen-presenting cells and IIIa22-262 for 3 days in the presence or absence of anti–HLA-DR, anti–HLA-DQ, anti–HLA-DP, or isotype-control mAbs, and 3H-thymidine incorporation was measured by liquid-scintillation counting. Anti–HLA class II mAb and isotype-control mAbs were added at the initiation of cultures. Only standard deviation > 20% of the mean and 100 cpm is shown as an extended line above a bar.
Effects of anti–HLA class II mAb on antigen-induced proliferation of GPIIb-IIIa–reactive CD4+ T-cell lines.
GPIIb-IIIa–reactive T-cell lines were cultured with autologous antigen-presenting cells and IIIa22-262 for 3 days in the presence or absence of anti–HLA-DR, anti–HLA-DQ, anti–HLA-DP, or isotype-control mAbs, and 3H-thymidine incorporation was measured by liquid-scintillation counting. Anti–HLA class II mAb and isotype-control mAbs were added at the initiation of cultures. Only standard deviation > 20% of the mean and 100 cpm is shown as an extended line above a bar.
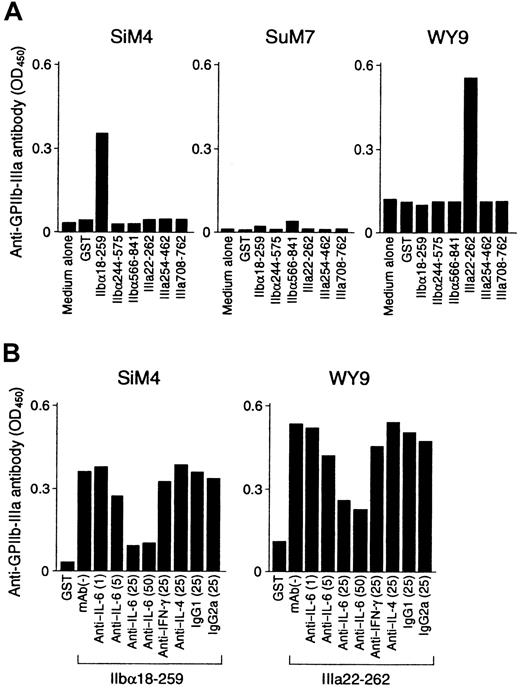
Representative results of the helper activity promoting anti–GPIIb-IIIa antibody production in GPIIb-IIIa–reactive T-cell lines are shown in Figure 8A. The IIbα18-259–specific T-cell line SiM4 and the IIIa22-262–specific T-cell line WY9 promoted anti–GPIIb-IIIa antibody production from autologous B cells in the presence of IIbα18-259 and IIIa22-262, respectively. Anti–GPIIb-IIIa antibodies synthesized in these cultures were specifically absorbed by preincubation with normal platelets (Table 3), indicating that the anti–GPIIb-IIIa antibodies produced in vitro had the pathogenic activity of binding to platelet surfaces. In contrast, the IIbα566-841–specific T-cell line SuM7 had only a minimal effect on anti–GPIIb-IIIa antibody production on antigenic stimulation with IIbα566-841. When cytokine expression profiles for IFN-γ, IL-4, and IL-6 were examined in 6 GPIIb-IIIa–reactive CD4+ T-cell lines, all lines produced IFN-γ with no or minimal IL-4 expression, indicating a Th1- or a Th0-like phenotype (Table 3). The capacity of these cell lines to produce individual cytokines was then compared with their T-cell helper activity to promote anti–GPIIb-IIIa antibody production. SuM7, which produced only a trace amount of IL-6, lacked significant helper activity, whereas the remaining 5 lines, which were capable of producing a larger amount of IL-6 (more than 100 pg/mL), promoted anti–GPIIb-IIIa antibody production. These findings suggest that T-cell–derived IL-6 plays an important role in activating B cells. To confirm this, we examined whether neutralization of IL-6 by an anti–IL-6 mAb inhibited the T-cell helper activity that promotes anti–GPIIb-IIIa antibody production. As shown in Figure 8B, the anti–IL-6 mAb inhibited anti–GPIIb-IIIa antibody production in a dose-dependent manner, but anti–IFN-γ and anti–IL-4 mAb had no effect.
T-cell helper activity promoting anti–GPIIb-IIIa antibody production from B cells in GPIIb-IIIa–reactive CD4+ T-cell lines.
(A) GPIIb-IIIa–reactive T-cell lines were cultured with autologous peripheral blood B cells in the presence or absence of GST or individual recombinant GPIIb-IIIa fragments for 10 days. (B) GPIIb-IIIa–reactive T-cell lines were cultured with autologous peripheral blood B cells and the antigenic GPIIb-IIIa fragment in the presence or absence of anti–IL-6 (1, 5, 25, and 50 μg/mL), anti–IFN-γ (25 μg/mL), anti–IL-4 (25 μg/mL), or isotype-control (25 μg/mL) mAbs for 10 days. The level of anti–GPIIb-IIIa antibodies in culture supernatants was measured by ELISA.
T-cell helper activity promoting anti–GPIIb-IIIa antibody production from B cells in GPIIb-IIIa–reactive CD4+ T-cell lines.
(A) GPIIb-IIIa–reactive T-cell lines were cultured with autologous peripheral blood B cells in the presence or absence of GST or individual recombinant GPIIb-IIIa fragments for 10 days. (B) GPIIb-IIIa–reactive T-cell lines were cultured with autologous peripheral blood B cells and the antigenic GPIIb-IIIa fragment in the presence or absence of anti–IL-6 (1, 5, 25, and 50 μg/mL), anti–IFN-γ (25 μg/mL), anti–IL-4 (25 μg/mL), or isotype-control (25 μg/mL) mAbs for 10 days. The level of anti–GPIIb-IIIa antibodies in culture supernatants was measured by ELISA.
Discussion
In this study, we examined T-cell responses to a series of recombinant GPIIb-IIIa fragments in 25 patients with ITP. Our results indicate that the immunodominant epitopes recognized by GPIIb-IIIa–reactive T cells in patients with ITP are located within the amino-terminal regions of both GPIIbα and GPIIIa, though several T-cell epitopes are located in other portions of the molecule as well. These findings were drawn from 2 independent assay systems, one measuring antigen-induced T-cell proliferation and the other determining the T-cell helper activity promoting autoantibody production in response to antigenic stimulation. Furthermore, the results obtained from bulk peripheral blood T cells were principally concordant with those obtained from CD4+ T-cell lines specific for platelet-derived GPIIb-IIIa. Our results further suggest that autoreactive T cells to the immunodominant epitopes within the amino-terminal portions of GPIIbα and GPIIIa are present in the peripheral blood of patients with ITP for a long period and are involved in the production of anti–GPIIb-IIIa antibodies. Anti–GPIIb-IIIa antibodies synthesized in our in vitro cultures appeared to be pathogenic because they could bind to normal platelet surfaces. In this regard, it was interesting to examine whether anti–GPIIb-IIIa antibodies produced in vitro–induced thrombocytopenia in mice, as has been shown in HIV-1–associated ITP.20 21It was less likely that an additional dominant T-cell epitope was present within the cysteine-rich domain of GPIIIa because 14 of 15 T-cell lines specific for platelet-derived GPIIb-IIIa recognized at least 1 of the 6 recombinant fragments used in this study.
The current study further confirmed the presence of autoreactive T cells to GPIIb-IIIa in healthy donors without having detectable anti–GPIIb-IIIa antibodies.10,22 The kinetics of T-cell responses to immunodominant GPIIb-IIIa fragments in patients with ITP were accelerated compared with that of healthy donors, as observed in a T-cell response to trypsin-digested GPIIb-IIIa.10Anti–GPIIb-IIIa antibody synthesis was observed in PBMC cultures of patients with ITP, but not in cultures of healthy donors, in response to recombinant GPIIb-IIIa fragments. This is analogous to autoantibody responses to topoisomerase I in scleroderma23 and to β2-glycoprotein I in antiphospholid syndrome.24 Using an in vitro culture system consisting of topoisomerase I–specific T-cell clones and peripheral blood B cells from patients with scleroderma and healthy donors in different combinations, we have recently shown that topoisomerase I–specific T-cell clones established from healthy donors are capable of driving patients' B cells to produce anti–topoisomerase I antibodies.19 Thus, the failure of PBMCs from healthy donors to produce anti–GPIIb-IIIa antibodies in in vitro cultures is probably owing to the absence of circulating B cells capable of producing anti–GPIIb-IIIa antibodies.
B-cell epitopes on GPIIb-IIIa recognized by autoantibodies from patients with ITP have been extensively analyzed by examining competitive binding between human antibodies and murine mAbs as well as B-cell reactivities to enzyme-cleaved GPIIb-IIIa fragments or synthetic peptides.25-32 Previously reported antigenic epitopes include a 33-kd chymotryptic core fragment of GPIIIa, encoding cysteine-rich domains,25 a carboxyl-terminal cytoplasmic portion of GPIIIa,26 a 65-kd chymotryptic carboxyl-terminal fragment of IIbα,27 and amino acid residues 231-238 of IIbα,28 which are included in IIbα18-259. However, several recent studies showed that anti–GPIIb-IIIa antibodies, especially platelet-associated antibodies, recognize cation-dependent conformational epitopes expressed exclusively on the GPIIb-IIIa complex.29-32 McMillan et al33 just recently reported that anti–GPIIb-IIIa antibodies bind primarily to the Ca++-binding site on GPIIbα. These findings demonstrate that several distinct B-cell epitopes, including continuous and conformational determinants, are present on GPIIb-IIIa. Taken together, the recognition of multiple epitopes on GPIIb-IIIa by autoreactive T and B cells indicates that the entire GPIIb-IIIa molecule is a target for the autoimmune response in patients with ITP.
Here, we found that anti–GPIIb-IIIa antibody production was induced in vitro by antigenic stimulation with IIbα18-259 or IIIa22-262 exclusively in patients who showed T-cell proliferative responses to these fragments. In contrast, nondominant GPIIb-IIIa fragments failed to induce the production of anti–GPIIb-IIIa antibodies in some samples that had responded to these fragments in the proliferation assay. This discrepancy might be explained by a low frequency of autoreactive T cells to nondominant fragments in peripheral blood, which would have insufficient power to activate B cells in in vitro cultures. However, analysis using GPIIb-IIIa–reactive T-cell lines showed that the helper activity that induces anti–GPIIb-IIIa antibody production was variable among T-cell lines and was correlated with the amount of IL-6 produced on stimulation. This finding is consistent with our recent report that the T-cell helper activity that promotes autoantibody production in topoisomerase I–specific CD4+ T-cell clones generated by scleroderma patients is largely dependent on their cytokine profiles, and especially on their ability to produce IL-6.19 In this study, the T-cell line SuM7, which was reactive with the nondominant fragment IIbα566-841, expressed a trace amount of IL-6 and failed to induce significant anti–GPIIb-IIIa antibody production, whereas T-cell lines that responded to the immunodominant fragments expressed a higher level of IL-6 and had greater helper activity. Therefore, it is also possible that autoreactive T cells to immunodominant epitopes have stronger helper activity than do those to nondominant epitopes, though the number of T-cell lines analyzed in this study was too small to draw a conclusion.
Findings from the current study and our previous report10suggest that the epitopes recognized by GPIIb-IIIa–reactive T cells are cryptic but the factors that induce the expression of cryptic determinants and activate GPIIb-IIIa–reactive CD4+ T cells in patients with ITP are unknown. Lehmann et al34 proposed that, in autoimmunity, de novo presentation of a previously cryptic self-determinant is induced by up-regulated antigen presentation capacity and shifts in peptide hierarchy. In this process, antigen-presenting cells in a local milieu play a central role through an activated antigen-processing pathway and increased expression of adhesion and costimulatory molecules. In addition, shifts in peptide hierarchy resulting in the expression of cryptic self-peptides are shown to be induced by events that affect the normal processing of self-proteins, such as an unusual cleavage or a complex formation with other proteins.35-37 These modifications are presumed to subsequently mask or unmask cleavage sites for proteinases and reductases in endosomes, resulting in the expression of previously cryptic self-peptides. In this regard, it is plausible that the changes of structural conformation of GPIIbα and GPIIIa in recombinant GPIIb-IIIa fusion proteins by fusion with GST or lack of glycosylation contribute to the expression of the cryptic self-peptides in the current study. Our results here indicate that immunodominant T-cell epitopes are located within the amino-terminal extracellular domains of both GPIIbα and GPIIIa, which are known to be exposed on the surface of the molecule. In particular, IIbα18-259 and IIIa22-262 contain the Ca++-binding site and Arg-Gly-Asp binding site, respectively.38 Therefore, it is possible that the amino-terminal regions of GPIIbα and GPIIIa are preferentially attacked by chemicals or foreign proteins that can change the molecular context and lead to the efficient presentation of previously cryptic peptides in these regions.
It is interesting to note that the number of immunoreactive GPIIb-IIIa fragments was negatively correlated with the time between diagnosis and blood examination. Serial analysis of GPIIb-IIIa fragment-induced T-cell proliferation revealed that 3 of 14 patients lost T-cell reactivity to the previously recognized fragments during follow-up. These findings suggest that patients with ITP have T cells that are responsive to a variety of epitopes on GPIIb-IIIa early in the course of the disease and that their T-cell repertoires to the immunodominant epitopes are selectively expanded, whereas those to nonimmunodominant epitopes fade during the course of the disease. It has been shown in animal models that an autoimmune response is initiated to one epitope and subsequently spreads to other sites on the same molecules, a concept called epitope spreading.39,40 However, epitope spreading is rarely observed in patients, probably because it has already occurred before the clinical onset of the disease. On the other hand, a reduction in isotype expression and epitope reactivity of autoantibody responses during follow-up was observed in patients with mixed connective tissue disease41 and scleroderma,42 independent of treatment. It was also noted that peripheral blood T cells from healthy donors reacted with 2 or more GPIIb-IIIa fragments, but IIbα18-259 and IIIa22-262 were not dominant. Taken together, it is possible that T-cell responses to GPIIb-IIIa are skewed to immunodominant epitopes in the amino-terminal portions of both GPIIbα and GPIIIa during the pathogenic process of ITP.
In summary, our results indicate that GPIIb-IIIa–reactive CD4+ T cells in patients with ITP recognize several distinct epitopes on GPIIb-IIIa, but the amino-terminal extracellular domains of both GPIIbα and GPIIIa are the major targets for autoreactive T cells that mediate antiplatelet autoantibody production. Further investigation to identify the T-cell epitope peptides within the immunodominant regions may provide a clue to the pathogenesis of chronic ITP.
We thank Yuka Okazaki and Kyoko Kimura for their expert technical assistance and Dr Kenichi Furihata for helpful suggestions.
Supported by the Keio University Medical Science Fund (M.K.), by a grant from the Japanese Ministry of Health and Welfare (Y.I.), and by grants from the Ministry of Education, Science, Sports and Culture of Japan (S.K. and Y.I.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masataka Kuwana, Institute for Advanced Medical Research, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail: kuwanam@sc.itc.keio.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal