Abstract
Experimental and clinical data suggest the presence of multiple types of adenosine diphosphate (ADP) receptors, one coupled to ligand-gated cation channels (P2X) and others coupled to G-protein–coupled (P2Y) receptors. This report identifies cDNA for a structurally altered P2X1-like receptor in megakaryocytic cell lines (Dami and CMK 11-5) and platelets that, when transfected into nonresponsive 1321 cells, confers a specific sensitivity to ADP with the pharmacologic rank order of ADP > > ATP > > > α,β-methylene-ATP as measured by Ca++ influx. This receptor (P2X1del) contains a deletion of 17 amino acids (PALLREAENFTLFIKNS) that includes an NFT consensus sequence for N-linked glycosylation. Glycosylated forms of the P2X1deland P2X1wt receptors were indistinguishable electrophoretically by Western blot or by immunoprecipitation using available antihuman and antirat antibodies. These results indicate that the expression of the P2X1del receptor results in an influx of Ca++ induced by ADP. Expression of P2X1delreceptor homomeric subunits is sufficient to express a receptor preferentially activated by ADP and suggests that this altered form, alone or in combination with P2X1wt receptors, is a component of an ADP-activated ion channel.
Introduction
Adenosine diphosphate (ADP) is known to play a key role in the development and extension of arterial thrombosis, the deposition of platelets onto collagen under flow conditions, collagen-induced aggregation, and the stabilization of thrombin-induced human platelet aggregates independent of fibrinogen binding to GPIIb/IIIa, and it plays an important role in irreversible aggregation induced by PAR-1.1-5 Reduced thrombus formation is observed when platelets from patients with storage pool deficiency are exposed to vascular subendothelium and when removal of ADP from blood-perfusing damaged arteries results in increased bleeding times.4,6 Platelet aggregation is impaired in 2 patients with a congenital defect of platelet response to ADP,1,7further demonstrating that released ADP plays an important role in platelet activation though the mechanism of activation has not been elucidated. Significantly, inhibitors of ADP-induced aggregation are effective antithrombotic drugs.8 9
Two subclasses of purinergic receptors have been described: P2Y metabotropic receptors coupled to heterotrimeric G-proteins and P2X ion channel receptors. Evidence has been provided for 2 distinct G-protein–coupled receptors in human platelets, one coupled to the inhibition of stimulated adenylate cyclase (the P2YAC receptor) and the other a P2Y1 receptor (the P2TPLC receptor) coupled to the activation of phospholipase C.10-12 The P2TAC (or P2YAC) receptor, now termed the P2Y12 receptor, has been recently identified.13 AR-C69096, an adenosine triphosphate (ATP) analog, is a selective inhibitor of ADP-induced platelet aggregation that antagonizes ADP-induced activation through the P2Y12receptor.14 ATP is a competitive inhibitor of ADP-induced aggregation and adenylyl cyclase inhibition.15 A model for the activation of platelets by ADP involving a 3-receptor model has been proposed10-12 and is a refinement of the model16 involving rapid calcium influx mediated by a receptor-operated Ca++ channel, whereas adenylyl cyclase inhibition and intracellular Ca++ mobilization are mediated by a single P2Y receptor involving multiple G proteins. The crucial role of heterotrimeric G proteins has been demonstrated by using platelets from P2Y1-null mice17,18 and in Gαq-deficient mice, the latter of which are deficient, in part, in their activation by ADP19 although partial aggregation and potentiation with other agonists are retained.20
In platelets, the presence of P2X1 receptors and mRNA has been reported using polymerase chain reaction (PCR), Fura-2–measured Ca++ influx, protein blotting,21-26 and activation by α,β-methylene ATP, a selective activator of P2X1 receptors.22,25,26 P2X1receptors demonstrate rapid and transient activation by ATP.27,28 ADP-induced Ca++ mobilization is a biphasic process29 implying multiple receptors and pathways. It is unclear whether the P2X1 ion channel receptor in platelets is required or sufficient for cellular activation or whether multiple subunits of P2X-like receptors are responsible for Ca++ influx. Recent data suggest that although P2X1 receptors play functional roles in human platelets,30 it has been suggested that ADP is not an agonist at P2X1wt receptors,31 in contrast to its activation by ATP.
We have previously demonstrated that ADP and ADP–α-S mobilize Ca++ in Xenopus laevis oocytes injected with mRNA derived from megakaryocytic CMK 11-5 cells, and this mobilization is specifically blocked by ATP and ATP–α-S, as expected for a platelet-type receptor.32 We have now used a homology PCR approach based on cDNA sequences of previously identified ion-gated P2X receptors to identify purinergic receptors on platelets, megakaryocytic CMK 11-5 cells, and megakaryoblastic Dami and CMK cells.
Materials and methods
Reagents and solutions
Carbachol, ADP, and ATP were purchased from Sigma Chemical (St Louis, MO); nucleotides were purified to homogeneity using ion exchange chromatography with triethyl ammonium bicarbonate buffer. Pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), suramin, α,β-methylene-ATP, 2-methylthio-ADP, and β,γ-methylene-ATP were from Research Biochemicals (Natick, MA). Fura-2 acetoxymethylester and SK&F 96365 were from Calbiochem (San Diego, CA). Geneticin and all tissue culture reagents were from Gibco-BRL (Gaithersburg, MD).
RNA isolation and polymerase chain reaction amplification
Three bands with P2X1 sequence homology were identified using PCR amplification (expected product size, 450 bp) based on an internal primer pair (5′ sense603ATCCGCACGGGCAAGTGTGT and 3′ antisense,1059GCCTGGCAAACCTGAAGTTG) corresponding to the human bladder wild-type P2X1 receptor (accession number, X83688). Total RNA was isolated (TRIzol; Gibco-BRL) from CMK 11-5 and CMK cells, kindly provided by Dr A. Sato (Mochida Pharmaceutical, Mochida, Japan), and from platelets (American Red Cross) and Dami cells (ATCC, Rockville, MD). Preparations of platelet RNA33 were found to be negative for TAPA-1, a widely expressed white blood cell antigen using PCR primers (5′-ACAAGGACCAGATCGCCAAGG-3′ (sense) and 5′-GTACAGCTTCCCGGAGAAGAG-3′ (antisense) as described.26Ficoll-purified neutrophils from whole human blood and Jurkat cells (ATCC) revealed a positive PCR band at 266 bp (data not shown). In brief, platelet-rich plasma was centrifuged 3 times at 800g, and the upper two-thirds volume was removed for further processing. Multiple centrifugation steps and the removal of the upper plasma fraction reduced the presence of white blood cells to undetectable levels by PCR analysis. The Dami cells express epitopes for CD42a, CD42b, and CD42d (data not shown) measured by FACS analysis, indicating identity with the original cell line.34 35 Cell lines were maintained in RPMI 1640 media containing 10% fetal bovine serum without penicillin–streptomycin. Reverse transcription reactions (20 μL) consisted of RNA (9 μg), dNTP, RNAase inhibitor, random primer, oligo-dT, and MuLV reverse transcriptase. RNA was heated to 65°C for 10 minutes and allowed to cool to 4°C. Reagents were added, and the reaction (45 minutes at 42°C) was followed by a 10-minute room temperature hold. Samples were heated to 100°C for 5 minutes, cooled to 4°C, then used for PCR reactions or stored at −20°C. First-strand cDNA synthesis used 500 ng total RNA and Superscript II (Gibco-BRL). PCR reactions, performed in 40 μL, consisted of cDNA, 0.5 μM PCR primers, 2.0 mM Mg2+, 200 μM dNTP, and 5 U Taq DNA polymerase. The PCR thermal cycle consisted of 2 minutes at 94°C and 40 cycles of 30 seconds at 94°C, 60 seconds at 55 to 60°C, and 60 seconds at 72°C. To amplify the entire open reading frame of both cDNAs, PCR was carried out with these identical conditions except that 35 cycles were used for the amplification and a 5-minute elongation step was included to allow for the completion of full-length cDNA. PCR reactions used a 5′ sense primer, TAA [GGATCC]CCACCATGGACGGCGGTTCCAG that included a Kozak sequence and aBamHI site (brackets), and a 3′ antisense primer CA [TCTAGA] TCAGGATGTCCTCATGTT that included an XbaI site (brackets). Two BamHI–XbaI fragments, corresponding to nucleotides 1 to 1199 of the human bladder wild-type P2X1 clone and one reduced by 51 bp (full-length P2X1del clone), were directionally cloned into pcDNA3 (Invitrogen, Carlsbad, CA) and transformed into TOP10F′ or INVF′ cells grown in LB agar.
Specific amplification of the P2X1delcDNA from platelets
To selectively amplify P2X1del cDNA, 2 primers were used—sense primer 711ACATCCCGCGC–ATCAGCT (the dashes indicate where the 51-bp–deleted cDNA sequence would be observed in the P2X1wt receptor) and the antisense primer1082GCCTGGCAAACCTGAAGTTG. The first primer contains a 5′ 11-base sequence and a 3′ 7-base sequence that span the deleted 51-base sequence and are found only in the P2X1del cDNA. An expected PCR amplicon is calculated to measure 371 bp (Figure1C). This PCR primer pair was used to amplify a 371 bp band from cDNA isolated from platelets and from a 400-bp partial P2X1del cDNA sequence (amplified using the primers described in the preceding paragraph) inserted into the pCRII plasmid. For high-stringency conditions, 35 PCR cycles using the annealing temperature of 60°C were performed.
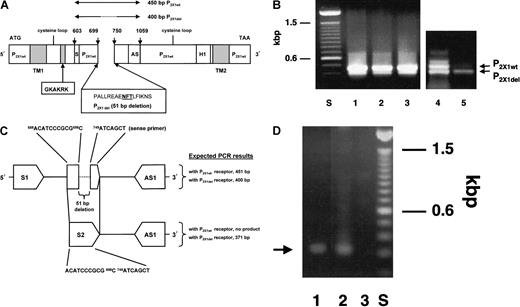
Identification of the P2X1del receptor.
(A) Schematic cDNA representation of 2 P2X1 receptor homologs (P2X1wt, the wild-type clone, and P2X1del, the deletion clone). PCR amplification using P2X1-derived ORF primers (S or 5′ sense603ATCCGCACGGGCAAGTGTGT and AS or 3′ antisense,1059GCCTGGCAAACCTGAAGTTG) to amplify a partial P2X sequence revealed the homologous wild-type P2X1 receptor and an in-frame 51-bp deletion clone (P2X1del). Lines above the figure represent the expected 450-bp P2X1wt sequence and the identified 400-bp P2X1del sequence. Sequence numbering is relative to a P2X1 receptor (accession number, X83688). ORF primers include 5′ BamHI and 3′ XbaI sites facilitating subcloning into pcDNA3.1 and subsequent transfection into 1321 cells. Within the 51-bp deletion variant is a glycosylation site indicated in bold, underlined type. The 51-bp deleted DNA sequence is shown with its deduced amino acid sequence relative to its location with the 2 transmembrane regions (TM1 and TM2), the location of 2 proposed extracellular cysteine loops, and a pore site (H1). GKAKRK is an amino acid antibody determinant deduced by the PCgene program and used for antibody production. (B) Comparison of PCR amplification products using CMK 11-5 cells and platelets. Primers (S and AS) were used to amplify a partial sequence in the extracellular portion of the P2X1wt receptor. Besides the expected amplicon of 450 bp, a band of 400 bp was observed. PCR results with CMK 11-5–derived mRNA showed approximately equal or greater amplification of the 450-bp band (wild-type P2X1 cDNA) than the 400-bp band (deletion P2X1 cDNA) (compare lanes 1-4), whereas platelets (lane 5) demonstrated a greater proportion of the 400-bp amplicon. Varying the annealing temperature for the PCR reaction at 50°C, 55°C, or 60°C (lanes 1-3, respectively) did not vary the intensity of the bands. PCR products were subcloned into pCRII and were sequenced for confirmation. (C). Selective PCR amplification of the P2X1del receptor in platelets. Two primers (sense primer (S2)688ACATCCCGCGCATCAGCT and antisense primer (AS1)1082GCCTGGCAAACCTGAAGTTG) were used to selectively amplify platelet P2X1del cDNA (note that AS and AS1 primers are identical). An 11-base sequence (ACATCCCGCGC) and a 7-base sequence (ATCAGCT) span the 51-bp deletion sequence and are found only in the P2X1del cDNA. (D) Agarose gel of PCR amplicon. Using the PCR primer pair (S2 and AS1), an approximately 371-bp PCR band from both cDNA isolated from platelets (lane 1) and from a 400-bp partial P2X1del cDNA sequence inserted into the pcDNA3.1 plasmid (lane 2) are amplified. PCR reactions used an annealing temperature of 60°C. Although an expected PCR reaction would result in 2 PCR products using the primers designated S1 and AS1—one of 451 bp for the P2X1wt receptor and one of 400 bp for the P2X1del receptor (see Figure 1B)—a single PCR product of approximately 371 bp using primers S2 and AS1 directly indicates the presence of the P2X1del receptor. Lane 3 is a PCR reaction with the S2 and AS1 primers with a pcDNA3.1 plasmid containing irrelevant DNA.
Identification of the P2X1del receptor.
(A) Schematic cDNA representation of 2 P2X1 receptor homologs (P2X1wt, the wild-type clone, and P2X1del, the deletion clone). PCR amplification using P2X1-derived ORF primers (S or 5′ sense603ATCCGCACGGGCAAGTGTGT and AS or 3′ antisense,1059GCCTGGCAAACCTGAAGTTG) to amplify a partial P2X sequence revealed the homologous wild-type P2X1 receptor and an in-frame 51-bp deletion clone (P2X1del). Lines above the figure represent the expected 450-bp P2X1wt sequence and the identified 400-bp P2X1del sequence. Sequence numbering is relative to a P2X1 receptor (accession number, X83688). ORF primers include 5′ BamHI and 3′ XbaI sites facilitating subcloning into pcDNA3.1 and subsequent transfection into 1321 cells. Within the 51-bp deletion variant is a glycosylation site indicated in bold, underlined type. The 51-bp deleted DNA sequence is shown with its deduced amino acid sequence relative to its location with the 2 transmembrane regions (TM1 and TM2), the location of 2 proposed extracellular cysteine loops, and a pore site (H1). GKAKRK is an amino acid antibody determinant deduced by the PCgene program and used for antibody production. (B) Comparison of PCR amplification products using CMK 11-5 cells and platelets. Primers (S and AS) were used to amplify a partial sequence in the extracellular portion of the P2X1wt receptor. Besides the expected amplicon of 450 bp, a band of 400 bp was observed. PCR results with CMK 11-5–derived mRNA showed approximately equal or greater amplification of the 450-bp band (wild-type P2X1 cDNA) than the 400-bp band (deletion P2X1 cDNA) (compare lanes 1-4), whereas platelets (lane 5) demonstrated a greater proportion of the 400-bp amplicon. Varying the annealing temperature for the PCR reaction at 50°C, 55°C, or 60°C (lanes 1-3, respectively) did not vary the intensity of the bands. PCR products were subcloned into pCRII and were sequenced for confirmation. (C). Selective PCR amplification of the P2X1del receptor in platelets. Two primers (sense primer (S2)688ACATCCCGCGCATCAGCT and antisense primer (AS1)1082GCCTGGCAAACCTGAAGTTG) were used to selectively amplify platelet P2X1del cDNA (note that AS and AS1 primers are identical). An 11-base sequence (ACATCCCGCGC) and a 7-base sequence (ATCAGCT) span the 51-bp deletion sequence and are found only in the P2X1del cDNA. (D) Agarose gel of PCR amplicon. Using the PCR primer pair (S2 and AS1), an approximately 371-bp PCR band from both cDNA isolated from platelets (lane 1) and from a 400-bp partial P2X1del cDNA sequence inserted into the pcDNA3.1 plasmid (lane 2) are amplified. PCR reactions used an annealing temperature of 60°C. Although an expected PCR reaction would result in 2 PCR products using the primers designated S1 and AS1—one of 451 bp for the P2X1wt receptor and one of 400 bp for the P2X1del receptor (see Figure 1B)—a single PCR product of approximately 371 bp using primers S2 and AS1 directly indicates the presence of the P2X1del receptor. Lane 3 is a PCR reaction with the S2 and AS1 primers with a pcDNA3.1 plasmid containing irrelevant DNA.
In vitro transcription–translation
35S-methionine–labeled receptors were prepared from the P2X1wt and P2X1del pcDNA3.1 plasmids using a coupled TnT (Promega, Madison, WI) transcription–translation (reaction time, 75 minutes) and were immunoprecipitated with the MAP anti-P2X1 antibody (described below) or subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. Both glycosylated and nonglycosylated receptor forms generated from both plasmids using the TnT (Promega) system were immunoprecipitated with the anti-hP2X1 antibody, which is directed against a common GKAKRK peptide sequence (data not shown).
Preparation of anti–human P2X1 receptor antibody
An octavalent core matrix of a multiple antigenic peptide (human, [h]) was used to generate an anti-GKAKRKAQGIRTGF polyclonal antibody (anti-hP2X1 pAb) and was used directly for injection as the immunogen. Analysis of the purity of the peptide by reversed phase high-performance liquid chromatography confirmed the stoichiometry of the composite amino acids. Rabbits (male New Zealand White) were bled (preinjection sample) before intradermal injections of 200 μg emulsified MAP antigen in 600 μL PBS mixed with 600 μL Freund complete adjuvant. After booster injections at 14, 28, 56, and 84 days, total IgG was isolated using protein G–Sepharose columns, and IgG was eluted with 0.1 M glycine, pH 2.5, and 0.15 M NaCl and immediately neutralized with 1.0 M Tris, pH 8.0. Before use, antibodies were dialyzed against PBS.
Immunoprecipitation and Western blot analysis of P2X receptors
For immunoprecipitation experiments, surface-biotinylated (20 μM sulfo-NHS-biotin-SO3 (Pierce, Rockford, IL) 1321 cells transfected with either the P2X1wt or P2X1delreceptors were solubilized in RIPA buffer (0.05 M Tris-HCl, pH 7.4, 150 mM NaCl, 1.0% Nonidet P-40, 0.25% deoxycholate, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 200 mM Na3VO4, and 1 μg/mL each of aprotonin, leupeptin, and pepstatin). Native Dami, CMK 11-5, or CMK cells were treated similarly. Surface-labeled receptors were immunoprecipitated with the anti-P2X1 pAb (5 μg total IgG) and complexed with protein A/G beads, and supernatants were analyzed under reducing conditions on 7% SDS-PAGE gels. After overnight electrophoretic transfer to nitrocellulose (30 mA, 18 hours; 300 mA, 2-3 hours), surface-labeled and precipitated antigens were detected by a streptavidin–horseradish peroxidase (1:20 000 dilution) enhanced chemiluminescence method. For Western blotting experiments, proteins on nitrocellulose membranes were recognized with 2.5 μg/mL anti–C-terminus anti-P2X1 receptor antibody.21 Bound antibody was visualized by a goat-antirabbit–HRP-conjugated secondary antibody and the enhanced chemiluminescence method. Protein molecular weights were compared to Rainbow (Amersham, Arlington Heights, IL) prestained markers.
Flow cytometry
Flow cytometry36 confirmed the surface expression of a P2X1 receptor(s) using both the anti-hP2X1 antibody (described above) and an anti–rat P2X1 antibody (kindly provided by Dr Julian Barden, University of Sydney, Australia) directed against an identical amino acid sequence in the human receptor. Both Dami cells and CMK 11-5 cells demonstrated binding of both antibodies (data not shown).
Cell transfection
Astrocytoma cells (1321N1; kindly provided by Dr Myron Toews, University of Nebraska) were maintained in a low-glucose (1 g/L) Dulbecco modified Eagle medium (DMEM) buffer (Gibco-BRL) supplemented with 3.7 g NaHCO3/L at 37°C and 5% CO2. Transfection reactions in the presence of Opti-MEM (Gibco-BRL) used 2 μg circular plasmid DNA and 10 μL Lipofectamine (Gibco-BRL) for 12 hours at 37°C. Complete medium was added for 24 hours. Cells were then removed with trypsin–EDTA for replating onto 100-mm Petri dishes (Falcon 3803), and 600 μg/mL Geneticin (G418) was added for selection. After selection for 12 to 30 days, individual colonies of cells were isolated using cloning cylinders. Stably transfected 1321 cells, maintained in 240 μg/mL G418, were grown to 100% confluency for 2 to 5 days on coverslips coated with 0.2% gelatin (Bio-Rad, Hercules, CA) dissolved in PBS. Four P2X1wt clones (W1, W2, W3, W4) and 5 P2X1delclones (DL1, DL2, DL3, DL4, DL5) were further characterized in this report.
Intracellular Ca++ influx
For Ca++ influx studies, adherent cells on coverslips were incubated for 30 to 60 minutes at 37°C in 1 mL DMEM media with 2.5 μM Fura-2 acetoxymethyl ester dissolved in dimethyl sulfoxide before a single exchange of DMEM-Tyrode-HEPES buffer as previously described.21,22,24,25,37,38 Ca++influx was measured with single monolayers (0.6 × 1.1 cm) of adherent nontransfected or transfected 1321 cells in a Time Drive program with a PerkinElmer LS50B fluorometer (λex 340 nm and λem 510 nm, slit widths 10 nm in matched quartz cuvettes). This program is approximately 10 times more sensitive than the Intracellular Biochemistry program (Perkin Elmer, Buckinghamshire, England) and has been validated in work with the P2X1receptor.21,22,24-26 Ca++ influx is shown in arbitrary linear units of Fura-2 emission measured at 510 nm. Fura-2–labeled cells in 1.2 mL Tyrode-HEPES, pH 7.4 buffer were incubated with 2 mM Ca++ before the addition of reagents under high-stirring conditions (approximately 1000 rpm). Of importance, there was no Ca++ influx to ADP or ATP in the absence of exogenous Ca++ or using nontransfected 1321 cells, demonstrating that Ca++ influx with transfected cells results only from the activation of expressed receptors. Purinergic receptor inhibitors were incubated with cells for 1 minute before the addition of nucleotides. Results show qualitative comparisons between P2X1wt and P2X1del receptors and the concentrations of nucleotides used to activate the receptors. In all experiments (200-240 seconds), nucleotide agonists were sequentially added, and then 50 μM carbachol, a muscarinic agonist, was added to elicit a positive standard response from its endogenous receptor. The addition of digitonin, as used in previous experiments32 36-38 to permeabilize cells to obtain maximal Ca++ concentrations for calibration curves, removed adherent cells from the coverslips and therefore maximum and minimum Ca++ concentrations were not determined.
This Ca++ influx method measures the total cumulative Ca++ signal for the entire population of adherent cells, not for an individual cell as in the electrophysiological method. Although the method is incapable of resolving the kinetics of influx of Ca++ through the opening and closing of individual receptor gates, the method is capable of measuring the cumulative signals from many adherent activated cells. Our expectations are that the action potentials generated by the Fura-2 method and the electrophysiological method would be different because Fura-2 measures the mass action of Ca++ influx by averaging the values from many intact cells. Fura-2 is distributed in the cytoplasm and is not localized as the electrophysiological measurements. Therefore, it is expected that the Fura-2 method will be slower in the time-course of activation, and this reflects the slower diffusion of the agonist over the cell surfaces. Because we are using Fura-2 to relate qualitative differences (and are not measuring quantitative kinetic results), this method is valid to measure Ca++ influx from many cells. In addition, the Fura-2 method has been successfully used and reported for comparative studies of P2X1 receptors.21,22,25,26,30,31Traces in Figures 4C and 4D show only the initial activation time of 30 seconds, whereas in other studies,21,22,25 26 the figures are displayed over 200 to 300 seconds; these different presentations result in significant differences in the appearance of the activation profiles.
Results
PCR amplification of P2X1homologs
PCR amplification was carried out using mRNA from Dami cells, CMK, CMK 11-5 cells, and platelets in reactions containing nondegenerate primers based on a human urinary bladder P2X1 receptor (accession number, X83688).27 Primers (5′ sense603ATCCGCACGGGCAAGTGTGT and 3′ antisense1059GCCTGGCAAACCTGAAGTTG) were selected to amplify approximately one third of the 1200-bp open-reading frame (ORF) in the extracellular portion of the P2X1 wild-type (P2X1wt) receptor (450 bp) and, in addition, yielded a band of 400 bp. Subcloning and sequencing (Figure 1A) revealed these 2 related clones27 28 and, most important, directly demonstrated the presence of multiple mRNAs for these receptors in these cells (Figure 1B). The 400-bp band (designated the P2X1 deletion clone or P2X1del) has a 51-bp in-frame deletion and would correspond to the deletion of 17 amino acids PALLREAENFTLFIKNS (calculated MWt, 1961) in the extracellular domain. This region is flanked by 5′ and 3′ ORF sequences identical to the P2X1wt receptor (Figure 1A). PCR amplification of CMK 11-5–derived mRNA (and mRNA from Dami cells; data not shown) showed equal or slightly greater amplification of the 450-bp (P2X1wt clone) than the 400-bp P2X1del band. In contrast, platelets demonstrated a greater proportion of the P2X1del 400-bp amplicon (Figure 1B) with apparent, but reduced, P2X1wt 450-bp amplicon levels.
Using the primer pair identified in “Materials and methods” (Figure1C), a single band of approximately 350 to 375 bp (expected size, 371 bp) was amplified using platelet cDNA (Figure 1D, lane 1) or with a pcDNA3.1 plasmid containing a partial 400-bp P2X1del cDNA (Figure 1D, lane 2). In the P2X1wt cDNA, the 11- and 7-base sequences comprising the sense primer (Figure 1C) are separated by 51 bases that have been deleted in the P2X1del cDNA (Figure1C); therefore, the 11- and 7-base sequences form a linear 18-base sequence for primer annealing only in P2X1del cDNA.
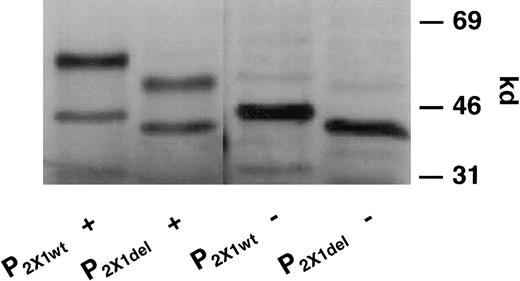
When pcDNA3.1 plasmids containing the entire ORF of the P2X1wt and P2X1del clones were transcribed and translated in vitro, differences were observed in the molecular size of translated protein, whether in the presence of microsomes to effect glycosylation (Figure 2; P2X1wt +, lane 1; P2X1del +, lane 2) or in their absence (P2X1wt −, lane 3; P2X1del −, lane 4). Only one type of P2X1wt or P2X1delplasmid DNA was observed in each plasmid preparation because only a single translated and glycosylated protein product was observed. This indicated that only homomeric receptor complexes were formed and expressed in the transfected 1321 cells. Both glycosylated and nonglycosylated forms of each receptor were detectable in each preparation, and both forms were immunoprecipitated by the polyclonal antipeptide anti-hP2X1 antibody (data not shown).
SDS-PAGE analysis of 35S-methionine–labeled P2X1wt and P2X1del receptors after transcription and translation reactions.
Proteins translated from P2X1wt and P2X1delplasmid (pcDNA3.1) DNAs were radiolabeled with35S-methionine using a coupled transcription–translation (TnT) rabbit reticulocyte system in the presence or absence of microsomes. Note the differences in the apparent molecular sizes for translated proteins between different plasmid constructs, separated by SDS-PAGE, in the presence of microsomes (lanes 1 and 2) or in their absence (lanes 3 and 4). Note that each plasmid preparation contains only P2X1wt or P2X1del DNA and that, therefore, only homomeric receptor complexes can be formed.
SDS-PAGE analysis of 35S-methionine–labeled P2X1wt and P2X1del receptors after transcription and translation reactions.
Proteins translated from P2X1wt and P2X1delplasmid (pcDNA3.1) DNAs were radiolabeled with35S-methionine using a coupled transcription–translation (TnT) rabbit reticulocyte system in the presence or absence of microsomes. Note the differences in the apparent molecular sizes for translated proteins between different plasmid constructs, separated by SDS-PAGE, in the presence of microsomes (lanes 1 and 2) or in their absence (lanes 3 and 4). Note that each plasmid preparation contains only P2X1wt or P2X1del DNA and that, therefore, only homomeric receptor complexes can be formed.
P2X1wt and P2X1del receptors are surface expressed
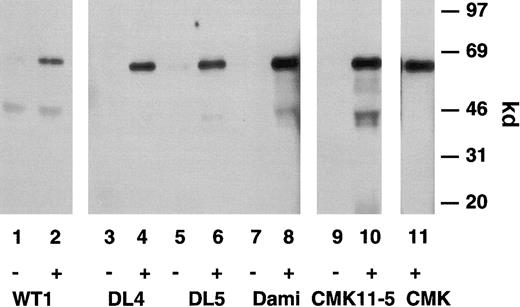
A single band of 67 kd was immunoprecipitated from stably transfected 1321 cells expressing either P2X1wt (Figure3, lane 2) or P2X1del (Figure3, lanes 4 and 6) receptors using a polyclonal anti-hP2X1antibody, and this apparent molecular size was identical to that found in identically prepared surface-biotinylated Dami cells (Figure 3, lane 8), CMK 11-5 cells (lane 10), or CMK cells (lane 11). Most important, no apparent size difference was observed between the single protein that was immunoprecipitated from these preparations. These experiments demonstrated the specificity of the antibody receptor interaction (lanes 2, 4, 6, 8, 10, 11). Proteins were not immunoprecipitated using preimmune antisera (lanes 1, 3, 5, 7, 9); these immunoprecipitation results were corroborated using an anti–rat P2X1antibody39 (data not shown). These results are from different SDS-PAGE gels, but chromatography of biotin-labeled P2X1wt and P2X1del receptors in adjacent lanes showed no significant differences in size.
Immunoreactive characterization of the P2X1receptor.
Immunoprecipitation of surface-expressed P2X1wt and P2X1del receptors stably transfected into 1321 astrocytoma cells or on native megakaryoblastic or megakaryocytic cells. Surface-biotinylated 1321 cells transfected with P2X1wt(lanes 1 and 2) or 2 individual P2X1del receptors (DL 4 and DL 5, lanes 3-6), Dami cells (lanes 7 and 8), CMK 11-5 cells (lanes 9 and 10), or CMK cells (lane 11) were solubilized in RIPA buffer and antigens immunoprecipitated with the anti-hP2X1 pAb (+) but not with the preimmune antibody (−). Note that the apparent size of the single 67-kd protein is identical in direct comparisons between the P2X1wt and P2X1del clones and native Dami, CMK 11-5, and CMK cells.
Immunoreactive characterization of the P2X1receptor.
Immunoprecipitation of surface-expressed P2X1wt and P2X1del receptors stably transfected into 1321 astrocytoma cells or on native megakaryoblastic or megakaryocytic cells. Surface-biotinylated 1321 cells transfected with P2X1wt(lanes 1 and 2) or 2 individual P2X1del receptors (DL 4 and DL 5, lanes 3-6), Dami cells (lanes 7 and 8), CMK 11-5 cells (lanes 9 and 10), or CMK cells (lane 11) were solubilized in RIPA buffer and antigens immunoprecipitated with the anti-hP2X1 pAb (+) but not with the preimmune antibody (−). Note that the apparent size of the single 67-kd protein is identical in direct comparisons between the P2X1wt and P2X1del clones and native Dami, CMK 11-5, and CMK cells.
P2X1wt and P2X1del receptors are equivalent in molecular size
In addition, Western blot analysis with an anti–human P2X1 polyclonal antibody directed against the C terminus of the human P2X1 receptor21 (kindly provided by Drs George Dubyak and Karen Parker, Case Western Reserve University, Cleveland, OH) showed an identically sized 60-kd band comparing Dami cells with P2X1wt- and P2X1del-transfected 1321 cells, whereas nontransfected 1321 cells were negative (data not shown). As with the immunoprecipitation experiments (Figure 3), no apparent differences were noted in the molecular size of the single blotted protein band.
Selective activation of Ca++ influx by ADP in P2X1del receptors
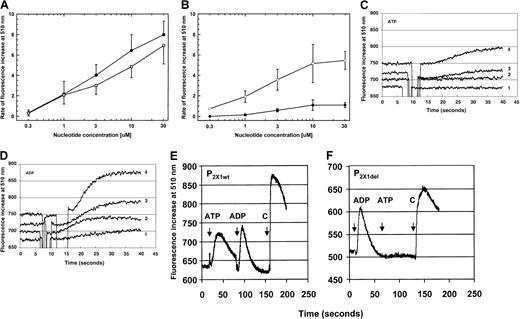
Both ATP and ADP activated Ca++ influx to comparable levels, as shown in dose-response curves of P2X1wt-receptor transfectants (Figure4A). In contrast, a direct comparison of dose-response curves using 1321 cells expressing the P2X1del receptor demonstrated a 10- to 30-fold increased sensitivity of the P2X1del receptor to ADP when compared to activation by ATP (Figure 4B); 2-methylthio-ADP (30 μM) caused Ca++ influx to a similar extent as for ADP (data not shown). The rightward shift in the dose-response curve for the activation by ATP resulted from both the reduced amount of Ca++ influx and the increased time required to reach peak influx. Differences in the rate of Ca++ influx between ATP and ADP demonstrated the increased sensitivity of the P2X1del receptor to activation by ADP and was particularly evident at concentrations as low as 3 μM (Figure 4B).
Ca++ mobilization of transfected P2X1wt and P2X1del receptors.
(A) Dose-response Ca++ influx (rate of fluorescence increase at 510 nm) for the P2X1wt receptor (summarized for P2X1wt clones designated W1, W2, W3, and W4) exposed to ATP (●, 2-6 individual experiments per concentration) or ADP (■, 2-7 individual experiments per concentration). (B) Dose-response Ca++ influx for the P2X1del receptor (summarized for P2X1del clones designated DL1, DL3, DL4, and DL5) exposed to ATP (●, 2-14 individual experiments per concentration) or ADP (■, 2-16 individual experiments per concentration). Values shown in panels A and B are mean ± SD (C) Ca++ influx profiles for the P2X1del receptor exposed to differing concentrations of ATP. A delayed activation by ATP is observable only at concentrations of 3 μM (trace 3) or greater. (D) Ca++ influx profiles for the P2X1delreceptor exposed to differing concentrations of ADP. Ca++influx is evident at concentrations of 0.3 μM (trace 1) or greater. Transfected cells expressing P2X1del receptors were exposed to increasing concentrations (0.3, 1, 3, or 10 μM, traces 1-4, respectively) of ATP (C) or ADP (D). Note that although the P2X1wt receptor is activated at similar concentrations by both ATP and ADP, the P2X1del receptor is activated to a greater extent by ADP at all concentrations. No activation was observed by either ATP or ADP in the absence of exogenous Ca++ or using nontransfected 1321 cells, confirming both the absence of endogenously activated nucleotide receptors and the requirement for influx of Ca++ as expected for an ion channel functioning as a receptor. (E) Ca++ influx profile for the P2X1wt receptor with the sequential addition of agonists. ATP (30 μM), ADP (30 μM), and carbachol (C, 50 μM) were added as indicated. (F) Ca++ influx profile for the P2X1del receptor with the sequential addition of agonists. ADP (30 μM), ATP (30 μM), and carbachol (C, 50 μM) were added as indicated. Note the different x-axis values between panels E or F (200 seconds) and C or D (40 seconds).
Ca++ mobilization of transfected P2X1wt and P2X1del receptors.
(A) Dose-response Ca++ influx (rate of fluorescence increase at 510 nm) for the P2X1wt receptor (summarized for P2X1wt clones designated W1, W2, W3, and W4) exposed to ATP (●, 2-6 individual experiments per concentration) or ADP (■, 2-7 individual experiments per concentration). (B) Dose-response Ca++ influx for the P2X1del receptor (summarized for P2X1del clones designated DL1, DL3, DL4, and DL5) exposed to ATP (●, 2-14 individual experiments per concentration) or ADP (■, 2-16 individual experiments per concentration). Values shown in panels A and B are mean ± SD (C) Ca++ influx profiles for the P2X1del receptor exposed to differing concentrations of ATP. A delayed activation by ATP is observable only at concentrations of 3 μM (trace 3) or greater. (D) Ca++ influx profiles for the P2X1delreceptor exposed to differing concentrations of ADP. Ca++influx is evident at concentrations of 0.3 μM (trace 1) or greater. Transfected cells expressing P2X1del receptors were exposed to increasing concentrations (0.3, 1, 3, or 10 μM, traces 1-4, respectively) of ATP (C) or ADP (D). Note that although the P2X1wt receptor is activated at similar concentrations by both ATP and ADP, the P2X1del receptor is activated to a greater extent by ADP at all concentrations. No activation was observed by either ATP or ADP in the absence of exogenous Ca++ or using nontransfected 1321 cells, confirming both the absence of endogenously activated nucleotide receptors and the requirement for influx of Ca++ as expected for an ion channel functioning as a receptor. (E) Ca++ influx profile for the P2X1wt receptor with the sequential addition of agonists. ATP (30 μM), ADP (30 μM), and carbachol (C, 50 μM) were added as indicated. (F) Ca++ influx profile for the P2X1del receptor with the sequential addition of agonists. ADP (30 μM), ATP (30 μM), and carbachol (C, 50 μM) were added as indicated. Note the different x-axis values between panels E or F (200 seconds) and C or D (40 seconds).
No activation by ATP is apparent until 3 μM or greater (Figure 4C), although activation of P2X1del-transfected cells is apparent at 0.3 and 1 μM ADP (Figure 4D, curves 1 and 2). A marked delay (relative to ADP) is observed during ATP activation that is only partially reduced by increasing the nucleotide concentration to 10 μM (Figure 4C) or even 30 μM (not shown). These results were observed for 4 P2X1del clones. Note that these profiles show the first 30 seconds of Ca++ influx.
Sequential exposure of P2X1wt receptors (shown for the W1 clone) to nucleotides (Figure 4E) demonstrated that the addition of both ATP (30 μM) and ADP (30 μM) activated Ca++ influx. In contrast, activation of P2X1del receptors (shown for the DL4 clone) by ADP (30 μM) was not followed by any Ca++influx elicited by ATP (30 μM) (Figure 4F). Carbachol (C) was added to confirm Fura-2 labeling of the adherent monolayers.
P2X1del receptors are not activated by α,β- methylene-ATP
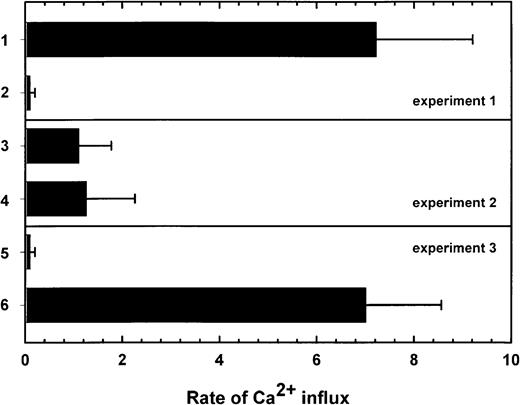
To compare the ability of ADP and ATP to sequentially activate P2X1del receptors, stably transfected 1321 cells expressing P2X1del receptors were labeled with Fura-2 and grown on coverslips designated experiments 1, 2, and 3 (Figure5). In data expressed as the rate of Ca++ influx, adherent cells on different coverslips were sequentially exposed (experiment 1) to ADP (30 μM, column 1) before ATP (30 μM, column 2) or, conversely, in experiment 2, to ATP (30 μM, column 3) before ADP (30 μM, column 4) (Figure 5). Experiment 3 shows the results of the addition of α,β-methylene-ATP (100 μM, column 5) before ADP (30 μM, column 6). Data in Figure 5, expressed as the rate of influx, is summarized for 3 individual P2X1del receptor clones (designated DL1, DL2, and DL5) expressed in 1321 cells. ADP (30 μM) induced a rapid peak of Ca++ influx (column 1), yet a secondary addition of ATP (30 μM) or ADP (30 μM) was completely ineffective at causing further Ca++ influx. Primary exposure to ATP (column 3) acted as a weak agonist, but this maximal activation required an increased time compared to the more rapid peak influx of Ca++ by ADP (Figure 4C). In contrast to the absent secondary activation by ATP after the addition of ADP (column 2), a secondary addition of ADP after the primary addition of ATP (column 4) continued to be effective at causing the influx of a secondary but significantly reduced peak of Ca++. In experiment 3 and in contrast to the results observed with the primary addition of ATP (column 3), the primary addition of α,β-methylene-ATP (100 μM) was completely ineffective at causing Ca++ influx (column 5). In addition, this prior exposure of the cells to α,β-methylene-ATP did not affect the Ca++ influx elicited by 30 μM ADP (column 6). 2-Methylthio-ADP at 30 μM caused Ca++ influx with a potency equivalent to that of ADP using the P2X1deltransfected cells (data not shown).
Rate of Ca++ influx in P2X1delreceptors stably transfected in 1321 astrocytoma cells.
In experiment 1, 3 P2X1del cell lines (DL 1, DL 2, DL 5) labeled with Fura-2 responded rapidly to 30 μM ADP (column 1, mean ± SD) but were completely unresponsive to a second exposure to 30 μM ATP (column 2). Initial exposure to 30 μM ATP in experiment 2 resulted in a significantly reduced rate of Ca++ influx (column 3) compared to column 1, but a secondary exposure to 30 μM ADP continued to activate, albeit at a significantly reduced level, the P2X1del transfected cells (column 4). The initial activation by ATP decreased a secondary activation by ADP but influenced only the maximal extent of influx without significantly affecting the time required for maximal activation. In experiment 3, the inability of α,β-methylene-ATP (100 μM) to activate the P2X1del-tranfected cells is shown in column 5. In addition, both α,β-methylene-ATP (100 μM, column 5) and β,γ-methylene-ATP (100 μM, data not shown) were ineffective at blocking influx induced by a secondary addition of 30 μM ADP (column 6). The rate of Ca++ influx is expressed as the maximal peak of Ca++ influx divided by the time required for maximum activation.
Rate of Ca++ influx in P2X1delreceptors stably transfected in 1321 astrocytoma cells.
In experiment 1, 3 P2X1del cell lines (DL 1, DL 2, DL 5) labeled with Fura-2 responded rapidly to 30 μM ADP (column 1, mean ± SD) but were completely unresponsive to a second exposure to 30 μM ATP (column 2). Initial exposure to 30 μM ATP in experiment 2 resulted in a significantly reduced rate of Ca++ influx (column 3) compared to column 1, but a secondary exposure to 30 μM ADP continued to activate, albeit at a significantly reduced level, the P2X1del transfected cells (column 4). The initial activation by ATP decreased a secondary activation by ADP but influenced only the maximal extent of influx without significantly affecting the time required for maximal activation. In experiment 3, the inability of α,β-methylene-ATP (100 μM) to activate the P2X1del-tranfected cells is shown in column 5. In addition, both α,β-methylene-ATP (100 μM, column 5) and β,γ-methylene-ATP (100 μM, data not shown) were ineffective at blocking influx induced by a secondary addition of 30 μM ADP (column 6). The rate of Ca++ influx is expressed as the maximal peak of Ca++ influx divided by the time required for maximum activation.
It must be noted that despite the weak activation of the P2X1del receptor by higher concentrations of ATP, as shown in Figure 4C, the rate of ATP-induced activation (expressed as the peak Ca++ mobilization measured at 510 nm [arbitrary units] divided by the time required for maximum activation or expressed as U/s) was significantly prolonged compared with activation by ADP (Figure 4D). ADP-induced Ca++ influx is more rapid than ATP-induced Ca++ influx; the latter requires 2- to 3-fold more time, and this is reflected in rate values only approximately one third of the ADP-induced maximum rate. For example, with the P2X1del receptor, the activation rate for 30 μM ADP (7.2 ± 2.0 U/s) is significantly greater than that observed with 30 μM ATP (1.1 ± 0.67 U/s), reflecting both the increased time required for ATP activation and the reduced overall peak of Ca++ influx (Figure 5). Prior exposure to 30 μM ATP decreased the secondary activation of Ca++ influx by 30 μM ADP to 1.3 ± 1.0 U/s, a reduction of 85%. The nonhydrolyzable ATP analog α,β-methylene-ATP, at concentrations to 100 μM, was completely ineffective at causing measurable Ca++ influx (Figure 5, column 5), as was β,γ-methylene-ATP (data not shown). In addition, both α,β-methylene-ATP (100 μM) and β,γ-methylene-ATP (100 μM) were ineffective at blocking Ca++ influx induced by 1 μM ADP (data not shown) indicating that neither interacts with the expressed receptors.
These quantitative differences are not the result of differences in the surface expression of the transfected receptors, in contrast to results with a P2X2 receptor, in which densensitization40 or surface expression41 is dependent on the extent of glycosylation. In the current study, using equivalent cell numbers, the intensity of the immunoprecipitated band at 67 kd and the Western blotted band at 60 kd were similar, demonstrating that similar levels of P2X1wt and P2X1del receptors were surface expressed.
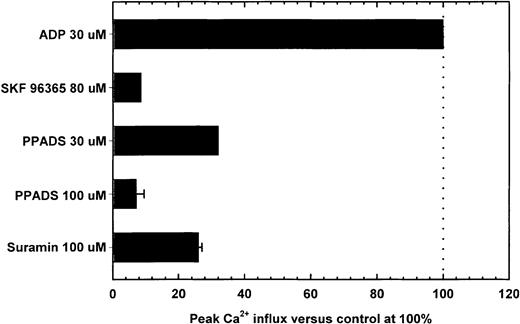
Purinergic receptor antagonists block Ca++ influx induced by ADP
To compare the pharmacology of Ca++ mobilization by the P2X1del receptors, experiments were conducted in the presence of suramin (a P2 antagonist), PPADS (a P2X antagonist), or SK&F 96365 (which antagonizes fast, responsive ion channels)42,43 (Figure6). Prior exposure of P2X1delor P2X1wt-transfected cells to PPADS, suramin, or SK&F 96365—neither of which caused Ca++ influx—resulted in reduced responses to activation by ADP for P2X1del (Figure6) and ATP for P2X1wt (data not shown) receptors. For the P2X1del receptor, SK&F 96365 at 80 μM inhibited Ca++ influx by 30 μM ADP (column 1) by 95% or more (Figure 6, column 2), whereas PPADS (at 30 μM and 100 μM) inhibited 30 μM ADP-induced peak Ca++ influx by 75% and 96% (columns 3 and 4), respectively. Suramin at 100 μM inhibited Ca++ influx by 75% (Figure 6, column 5). As with intact platelets,37 Ca++ mobilization was inhibited by 30 μM ATP-α-S which, by itself, induced a slow increase in rate of activation, comparable to that for ATP (data not shown).
Inhibition of Ca++ influx by the P2X1del receptor by purinergic receptor antagonists.
Peak Ca++ influx by 30 μM ADP (column 1) was inhibited by purinergic receptor antagonists. Adherent P2X1del-transfected cells were incubated with the indicated concentrations of SK&F 96365 (column 2, identified from the top), PPADS (columns 3 and 4), or suramin (column 5) before exposure to 30 μM ADP. Essentially identical patterns of inhibition were observed using 1 μM ADP (data not shown). Despite the alterations in the pharmacology of the expressed P2X1del receptor, inhibition by these antagonists was similar to that for the ATP-activated P2X1wt receptor (data not shown).
Inhibition of Ca++ influx by the P2X1del receptor by purinergic receptor antagonists.
Peak Ca++ influx by 30 μM ADP (column 1) was inhibited by purinergic receptor antagonists. Adherent P2X1del-transfected cells were incubated with the indicated concentrations of SK&F 96365 (column 2, identified from the top), PPADS (columns 3 and 4), or suramin (column 5) before exposure to 30 μM ADP. Essentially identical patterns of inhibition were observed using 1 μM ADP (data not shown). Despite the alterations in the pharmacology of the expressed P2X1del receptor, inhibition by these antagonists was similar to that for the ATP-activated P2X1wt receptor (data not shown).
Discussion
The current study shows that several megakaryocytic cell lines contain a P2X1-like receptor in which there is a deletion of a 17-amino acid extracellular sequence. We have termed this the P2X1del receptor. Expression of the P2X1delreceptor in 1321 cells confers a selective sensitivity to ADP and 2-methylthioADP, in contrast to expression of the P2X1wtreceptor in which both ATP and ADP induce Ca++ influx. These pharmacologic changes may reflect conformational changes in the P2X1del receptor resulting from the loss of the 17-amino acid extracellular sequence normally observed in the P2X1wtreceptor. The loss of the N708FT glycosylation site and the amino acid sequence that separates 2 species-conserved extracellular cysteine-folding domains in the deleted peptide, based on amino acid sequence alignment of 7 known members of the rat P2Xreceptor family,39 may result in these changes. Antagonism of Ca++ influx (Figure 6) demonstrates that the structure of the P2X1del receptor retains the ability to interact with purinergic receptor inhibitors (PPADS,44suramin,45,46 or SK&F 9636542), despite structural changes that result in altered pharmacologic responses.
Peptide-sequence alignment of known P2X2 and P2X7 receptors also revealed that the deleted PALLREAENFTLFIKNS sequence corresponds to exon 6 and may be a naturally occurring variant of P2X1 receptors possibly generated by alternative splicing, as observed in the pituitary and cochlea.47 These conformational changes in the P2X1del receptor may affect the oligomerization of the receptor subunits, but they do not affect its quantitative expression because P2X1wt and P2X1del receptors are expressed in equal amounts in transfected 1321 cells.
Using PCR primers restricted to the 5′ region of the P2X1wtreceptor, we have identified P2X1del and P2X1wtreceptor RNA in megakaryocytic cell lines and platelets and showed by DNA sequencing that the latter is identical to the previously described P2X1wt receptor.27,28 These results differ from those previously obtained using PCR primers designed to amplify cDNA corresponding to the entire extracellular domain of the P2X1wt receptor that indicate the preferential amplification of a P2X1wt receptor.26 ATP and α,β-methylene-ATP cause weak, transient activation of Ca++ mobilization in human platelets, but the relevance of this functional activation is unknown because ATP and α,β-methylene-ATP neither cause platelet aggregation nor alter ADP-induced activation,21,22,25,31,48 but there is some evidence that P2X1 receptors are functionally active.30 Activation of the P2X1wt receptor by ATP is in contrast to the P2X1del receptor, which is activated preferentially by ADP and not by ATP or α,β-methylene-ATP. In addition, we have used a PCR primer with a sequence found only in the P2X1del receptor to demonstrate directly the presence of P2X1del receptor cDNA in platelets.
Although it is possible that the P2X1 ADP receptors expressed on megakaryocytes consist of both P2X1wt and P2X1del subunits, it is clear from the current study that expression of the P2X1del receptor alone in 1321 cells is sufficient to effect sensitive and preferred activation by ADP. The inability of α,β-methylene-ATP to activate P2X1delreceptors at concentrations (100 μM) that exceed by 330 times a concentration of ADP (0.3 μM), which causes measurable Ca++ influx, clearly indicates the specificity of the P2X1del receptor for activation by ADP. The time to effect maximal Ca++ influx on the order of several seconds (peak Ca++ influx, approximately 10-20 seconds), with monolayers of adherent cells expressing either P2X1wt or P2X1del receptors using Fura-2 (this study), parallels those studies (after adjusting for x-axis differences) using platelet suspensions using the Fura-2 indicator.21,22,25,26 As discussed in “Materials and methods,” Ca++ influx measured using Fura-2 results in fluorescence signals summarized from the activation of a population of adherent cells, not an individual cell. In contrast, the electrophysiological technique is capable of extremely rapid millisecond measurements29 of extremely rapid (20 msec) activation.22,43 Another alternative method—for example, the nystatin-permeabilized patch technique, records subsecond Ca++ fluxes of individual platelets.22 43
Pharmacologic evidence indicates that platelets express several types of nucleotide receptors. The P2Y1 (or the P2TPLC) receptor is coupled to Gq proteins, activates Ca++ mobilization, and mediates platelet shape change and aggregation.10-12 The involvement of the P2Y1 receptor and the pathway involving the Gqprotein in ADP-induced platelet activation was investigated using knockout mice for the P2Y1 receptor and the Gαq subunit.17-20 The recently identified P2Y12 Gi-linked receptor is coupled to the inhibition of adenylyl cyclase and is a target for the antithrombotic reagents ticlopidine, clopidogrel, and AR-C69096.13 It should be noted that the determination of cytosolic Ca++levels in experiments17,19,20 are all conducted in the presence of EDTA, which would completely block any contribution of Ca++ influx to the Fura-2 or Indo-1 signals. Under these conditions, only Ca++ mobilization, not ADP-activated Ca++ influx, would be observed. The involvement of the ADP-activated calcium influx channel to the activation of platelets remains under investigation, but several reports (this study and21,25) have shown that ADP is an agonist at P2X1wt receptors in contrast to other findings.31
We propose that previous reports of P2X1 receptors on platelets21,22,24-26 have actually been recognizing both the P2X1wt receptor and the P2X1del receptor identified in this report: (1) the P2X1wt (399 amino acids) and P2X1del (382 amino acids) receptors in glycosylated form have identical electrophoretic mobilities and are not distinguishable by Western blotting or immunoprecipitation techniques using surface-biotinylated native megakaryocytic cell lines or transfected cells containing P2X1wt or P2X1delreceptors; and (2) the available anti-P2X1 receptor antibodies target amino acid sequences that are common to both receptor forms and, therefore, fail to differentiate between the P2X1wt and P2X1del receptors. In the literature, slight size differences between P2X1wtreceptors and cross-reactive proteins have been previously noted.21,24 Specifically, an antibody recognizing a 60-kd P2X1 receptor with transiently transfected 293T cells cross-reacts with a more abundant smaller protein of 55 to 57 kd in purified human platelets.24 Similarly, the rat vas deferens P2X1wt receptor is larger than the cross-reactive human platelet protein, even though the rat and the human P2X1 receptors have identical (399-amino acid) ORFs. We hypothesize that in both of these reports, the smaller protein recognized by the anti-P2X1 antibody is the P2X1del receptor identified in the current study.
In summary, the current study shows that stable expression of the P2X1del receptor in 1321 cells confers a preferential activation by ADP resulting in Ca++ influx and that this receptor is expressed in megakaryocytic cell lines and platelets and may be involved in ADP-induced platelet activation.
The authors express their appreciation for the generous contributions of their colleagues, including Drs H. Tran, M. Rinaudo, E. Gubina, R. Friesel, and W. Burgess; Sharon Brown; Donna Sobieski; E. Szylobryt (for MAP peptide synthesis); and Ni Yasong.
Supported by United States Public Health Service Merit Award HL 39438 (G.A.J.). Supported in part by the A. Bianchi Bonomi Foundation, Milan, Italy (G.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicholas J. Greco, Jerome H. Holland Laboratories, American Red Cross, 15601 Crabbs Branch Way, Rockville, MD 20855; e-mail: greco@usa.redcross.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal