Abstract
This work aimed at investigating the function of the [C674R] mutation in GPIIb that disrupts the intramolecular 674 to 687 disulfide bridge. Individuals heterozygous for this mutation show a platelet GPIIb-IIIa content approximately 30% of normal controls, which is less than expected from one normal functioning allele. Coexpression of normal [674C]GPIIb and mutant [674R]GPIIb with normal GPIIIa produced a [674R]GPIIb concentration-dependent inhibition of surface exposure of GPIIb-IIIa complexes in Chinese hamster ovary (CHO) cells, suggesting that [674R]GPIIb interferes with the association and/or intracellular trafficking of normal subunits. Mutation of either 674C or 687C had similar effects in reducing the surface exposure of GPIIb-IIIa. However, substitution of 674C for A produced a much lesser inhibition than R, suggesting that a positive-charged residue at that position renders a less efficient subunit conformation. The mutant [674R]GPIIb but not normal GPIIb was found associated with the endoplasmic reticulum chaperone BiP in transiently transfected CHO cells. BiP was also found associated with [674R]GPIIb-IIIa heterodimers, but not with normal GPIIIa or normal heterodimers. Overexpression of BiP did not increase the surface exposure of [674R]GPIIb-IIIa complexes, indicating that its availability was not a limiting step. Platelets from the thrombasthenic patient expressing [674R]GPIIb-IIIa were found to bind soluble fibrinogen in response to physiologic agonists or dithiothreitol treatment. Thus, the [674R]GPIIb mutation leads to a retardation of the secretory pathway, most likely related to its binding to the molecular chaperone BiP, with the result of a defective number of functional GPIIb-IIIa receptors in the cell surface.
Introduction
Glanzmann thrombasthenia (GT)1 is a recessively inherited bleeding disorder characterized by appearance of symptoms immediately after birth, normal platelet count, prolonged bleeding time, and low to absent clot retraction. A distinct feature of GT is the lack of platelet aggregation both spontaneously or in response to physiologic agonists and a normal ristocetin-induced aggregation. GT is a rather heterogeneous entity,2 but, in all cases studied, the thrombasthenic phenotype is associated with either a deficient or defective platelet fibrinogen (Fg) receptor.3,4 GT is currently classified as type I or type II.5 In type I GT there is absence of platelet GPIIb-IIIa and Fg and lack of clot retraction; in type II GT the platelet content may be around 10%, Fg may be detectable, and weak clot retraction may be observed. The platelet from the so-called GT variants may contain normal or near normal dysfunctional GPIIb-IIIa complexes.4,6 The calcium-dependent heterodimer formed by the glycoproteins GPIIb and GPIIIa, also known as integrin αIIbβ3, is a receptor for Fg and other adhesive proteins and is the major platelet plasma membrane protein component. Since the primary nucleotide structure of the human GPIIb and GPIIIa has become available,7-11 a significant number of mutations in both GPIIb and GPIIIa have been detected that are associated with thrombasthenic phenotypes.12,13 We have reported a compound heterozygote for GPIIb associated with type II GT.14 One of the mutations found in this patient was a splice site mutation, resulting in insertion of intron 5, appearance of a premature stop codon, and instability of messenger RNA (mRNA). The second mutation was a T2113C transition that changes C674R, disrupting the 674-687 intramolecular disulfide bridge. The platelets from the proband contained only subnormal amounts of [674R]GPIIb transcript, as expected from the expression of only one allele, and the platelet content of GPIIb-IIIa was about 10% of the normal platelets. Even though both parents of the proband carried one normal functional allele, the platelet GPIIb-IIIa content was approximately 30% and 50% of the normal platelet control in the mother and father, respectively. The difference between both parents is that the father has only transcripts from a normal allele, whereas the mother transcribes at similar rates normal and mutant [674R]GPIIb alleles. On the basis of these observations, we hypothesized that the decreased surface expression of GPIIb-IIIa in the platelets of the mother could be the result of interference between the translational products of both normal and mutant transcripts. This possibility has been investigated by coexpressing variable proportions of normal and mutant [674R]GPIIb with normal GPIIIa in Chinese hamster ovary (CHO) cells. The present work also aimed at elucidating the molecular mechanism responsible for the reduced surface expression of [674R]GPIIb-IIIa complexes in the proband's platelets as well as determining the functionality of the platelet receptor. It has been observed that intracellular retention of pro[674R]GPIIb associated with the chaperone BiP may play a role in determining the reduced surface exposure of [674R]GPIIb-IIIa. However, platelets from the proband showed normal responsiveness to physiologic agonists, manifested by enhanced binding of Fg and the activation-dependent monoclonal antibody (moab) PAC1. According to these observations, preservation of the 674-687 intramolecular disulfide bridge is essential to maintain a normal rate of GPIIb and GPIIIa dimerization and intracellular trafficking but does not compromise the physiologic responsiveness of the exposed receptor.
Materials and methods
Construction of expression vectors with normal or mutant GPIIb complementary DNAs
The complementary DNA (cDNA) for the [674R]GPIIb mutant was prepared by the splicing overlap extension polymerase chain reaction (PCR) procedure as previously described,15 using 2 sets of oligonucleotide primers: GPIIb-sense-(955-979) 5′-TATTTTGGGCATTCAGTGGCTGTCA-3′, GPIIb-antisense-(2123-2103) 5′-TTCTGATTACGGATGAGTCTC-3′, GPIIb-sense-(2103-2123) 5′-GAGACTCATCCGTAATCAGAA-3′, and GPIIb-antisense-(3154-3133) 5′-CACCCTCCTGCTAGAATAGT-3′. Bases substituted to generate the mutation are underlined. The final PCR carrying the mutation was digested with AccI andBamHI and ligated in a vector containing the wild-type GPIIb cDNA previously digested with the same enzymes. cDNAs encoding [674A]GPIIb and [687A]GPIIb mutants were prepared as above, using the overlapped pairs of primers GPIIb-A674-sense 5′-GAGACTCATCGCTAATCAGAAGAA-3′, GPIIb-A674-antisense 5′-TTCTTCTGATTAGCGATGAGTCTC-3′, GPIIb-A687-sense 5′-CAGGGTGG TGCTGGCTGAG-3′, and GPIIb-A687-antisense 5′-AGCTCAGCCAGCACCACCCT-3′, respectively. [674R]GPIIb and [687A]GPIIb cDNAs were used as templates to generate [674R/687A]GPIIb and [674A/687A]GPIIb mutant cDNAs, respectively. Nucleotide sequence analysis was performed to confirm the insertion of the amplified mutant products into the normal GPIIb and the absence of potential errors introduced by the Taq polymerase. Normal or mutated cDNAs were subcloned into the HindIII site of the pcDNA3 expression vector.
Cell culture and transfection
CHO or CHO-GPIIIa (CHO cell line stably expressing GPIIIa) cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum. Cells were transiently cotransfected by the diethyl aminoethyl-dextran method16 with different amounts of pcDNA3-GPIIIa, normal and/or mutated pcDNA3-GPIIb constructs, and/or the pMT-BiP vector. The total amount of DNA transfected was equalized with void plasmid. To determine the transfection efficiency, 2.5 μg of the pCMV, β-galactosidase plasmid was cotransfected in some experiments. Forty-eight hours after transfection, the cells were harvested, and the surface expression or total content of GPIIb-IIIa complexes was determined as follows.
Flow cytometry analysis
Transfected cells were harvested, using 0.5 mM EDTA in phosphate-buffered saline (PBS), washed with PBS, resuspended at a density of 106 cells/100 μL, and incubated for 20 minutes at 4°C with moAbs specific to GPIIb (M3) or GPIIIa (P37). Next, cells were washed and exposed to fluorescein isothiocyanate–conjugated (FITC) F(ab′)2 fragment of rabbit antimouse immunoglobulin (DAKO A/S, Denmark) at 4°C for 20 minutes, and the surface fluorescence was analyzed in a Coulter flow cytometer, model EPICS XL.
Immunoprecipitation of GPIIb-IIIa and Western analysis of BiP
Immunoprecipitation of GPIIb-IIIa from CHO cells was performed as previously described.17 18 Briefly, transfected cells were treated with lysis buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 2 mM phenylmethyl sulfonyl fluoride, 1% Triton X-100, 0.05% Tween 20, and 0.03% sodium azide) for 30 minutes at 4° C, and then precleared lysates were immunoprecipitated with either moAb M3 or moAb P37. The immunoprecipitates were then bound to protein A-Sepharose CL-4B beads, washed, and eluted by incubating 10 minutes at 100°C in 50 μL reducing loading buffer (50 mM Tris-HCl, pH 6.8, 10% glycerol, 5% β-mercaptoethanol, 4% sodium dodecyl sulfate [SDS], and 0.002% bromophenol blue). To examine the association of GPIIb and/or GPIIIa with the chaperone BiP (GRP78), immunoprecipitates obtained in the presence of 10 U/mL apyrase (Sigma, St Louis, MO) were electrophoresed in 0.1% SDS-7.5% polyacrylamide gels and transferred to nitrocellulose membranes. The membrane was incubated with a polyclonal antibody to rodent BiP and then with a secondary goat antirabbit immunoglobulin (Ig)G-peroxidase conjugated (Sigma). The membrane was developed by incubation in PBS containing 0.015% H2O2 and 0.5 mg/mL 4-chloro-1-naphtol.
Pulse-chase analysis of GPIIb-IIIa synthesis
Pulse-chase analysis of CHO cells transfected with wild-GPIIb and/or [674R]GPIIb was performed 48 hours after transfection. The cells were incubated in DMEM without methionine for 30 minutes and then pulsed for 30 minutes with [35S]methionine (200 μCi/mL). The plates were washed 3 times with PBS containing 1 mg/mL cold methionine and incubated with DMEM containing unlabeled methionine for 0, 0.5, and 2 hours. Cells were washed with PBS containing cold methionine and extracted in lysis buffer. Immunoprecipitation analysis was performed as described above. The immunoprecipitates were electrophoresed at 50 V in 0.1% SDS-7.5% polyacrylamide slab gels. The gels were vacuum dried and exposed to hypersensitive x-ray film. The immunoreactive bands were digitized with a high-resolution scanner and analyzed on a Macintosh computer, using the public domain software NIH Image (http: //rsb.info.nih.gov/nih-image).
Preparation of FITC-Fg
Human Fg was incubated with 10% celite-FITC (Calbiochem, San Diego, CA) in PBS at room temperature for 60 minutes. The pH was adjusted to 8.5 with 5% Na2CO3. The celite-FITC was separated from the labeled Fg by centrifugation, and the unreacted free FITC was cleaned from FITC-Fg by passing through a PD-10 column (Pharmacia, Uppsala, Sweden) in PBS, pH 7.4, and exhaustive dialysis at 4°C. The fluorescein-to-protein ratio was determined by measuring absorbance at 495 nm and 280 nm. Aliquots of FITC-Fg are stored at −80°C and thawed for experiments on a single day only.
Binding of Fg to activated platelets
Blood was collected in 1/10 volume of 3.8% sodium citrate, and platelet-rich plasma (PRP) was obtained by centrifugation at 180g for 20 minutes at room temperature. The PRP was then centrifuged at 800g for 10 minutes, and the platelet pellet was resuspended in Tyrode buffer, pH 7.4 (5 mM HEPES, 2 mM MgCl2, 0.3 mM NaH2PO4, 3 mM KCl, 134 mM NaCl, 12 mM NaHCO3, 0.1% glucose, 0.1% bovine serum albumin, and 1 mM Cl2Ca.) at a final concentration of 4 × 107/mL. Platelets (50 μL) were stimulated for 5 minutes at room temperature with one or more of the following activating agents: 20 mM dithiothreitol (DTT) (BRL, Life Technologies), 20 nmol/L phorbol 12-myristate 13-acetate (PMA) (Sigma), 200 μM adenosine 5′-diphosphate (Sigma), 1 mM (−)epinephrine (Sigma), 1.5 U/mL human plasma thrombin (Sigma), 100 μM thrombin receptor agonist peptide (SFLLRN), 2 μM platelet activating factor-16 (Calbiochem). To prevent thrombin-induced fibrin polymerization, GPRP peptide (Bachem, Bubendorf, Switzerland) was added to the incubation medium. Next, 15 μg FITC-Fg was added to activated platelets and incubated 15 minutes at room temperature. After washing, the platelets were resuspended in Tyrode buffer for flow cytometry analysis. We examined also the binding of FITC-labeled PAC-1, antibody specific for the active conformation of GPIIb-IIIa, in PMA-treated platelets.
Materials
Restriction enzymes were obtained from Boehringer (Mannheim, Germany). The pcDNA3 expression vector was from Invitrogen (San Diego, CA). Most other reagents were purchased from Sigma Chemical Company (St. Louis, MO), Merck (Darmstadt, Germany), or Calbiochem-Novabiochem Corporation (San Diego, CA). [35S]-methionine (SA 1000 Ci/mmol) was from Amersham Ibérica (Madrid, Spain). moAbs specific for GPIIIa (P37) and GPIIb (M3) were a gift of Drs J. González and M. V. Alvarez from the Institute Rocasolano (CSIC; Madrid, Spain). Polyclonal antirodent BiP and pMT/hamster wild BiP cDNA were a generous gift from Dr Linda Hendershot at St. Jude's Research Hospital (Memphis, TN). FITC-PAC1 was obtained from Dr J. Lopez (VA Medical Center, Houston, TX).
Results
Pulse-chase analysis of stability of [674R]GPIIb-IIIa complexes
We have reported a case of compound heterozygosity for GPIIb associated with type II GT.14 The mutation inherited from the father [IVS5(+2)CA] renders the mRNA unstable and, therefore, the only mRNA-GPIIb found in the platelets of this patient was the one encoding the mutated [C674R]GPIIb inherited from the mother. The patient showed very low platelet GPIIb-IIIa content and elevated ratio of proGPIIb to GPIIbH. Moreover, coexpression of [674R]GPIIb and GPIIIa in CHO cells led to a marked reduction in the surface exposure of GPIIb-IIIa complexes. Although the latter observation indicates that subunit dimerization is not prevented, the reason for the low rate of surface expression of GPIIb-IIIa is not apparent. In principle, it could be the result of either instability of the [674R]GPIIb-IIIa complexes and/or retardation in the intracellular transit along the secretory pathway. To investigate this point, cells transiently cotransfected with normal GPIIIa and either mutant [674R]GPIIb or normal GPIIb were pulse-labeled for 30 minutes with [35S]methionine and then chased with medium containing unlabeled methionine for 0.5 or 2 hours before GPIIb-IIIa complexes were immunoprecipitated with an anti-GPIIb moAb. As expected, reciprocal changes in the labeling of proGPIIb, and of GPIIbH and GPIIIa, as a function of the chasing time were observed in cells coexpressing normal GPIIb and GPIIIa proteins (Figure1). This observation implies stability of GPIIb-IIIa complexes and their transport into the Golgi apparatus. In cells cotransfected with GPIIIa and mutant [674R]GPIIb, proGPIIb, GPIIbH, and GPIIIa subunits were coprecipitated with anti-GPIIb at all times, but the labeling flow was considerably slower than in cells coexpressing both normal subunits. The decay of labeled proGPIIb was retarded (Figure 1), suggesting that the number of subunits entering the secretory pathway may be diminished. After 2 hours of chase, the pattern of labeling of cells expressing [674R]GPIIb was similar to that observed in the control cells at zero time. In CHO cells, the majority (99%) of GPIIb-IIIa is located on the plasma membrane.19 According to this, in a pulse-chase experiment the labeling intensities of GPIIb and GPIIIa should be proportional to the amount of GPIIb-IIIa complexes that have reached the cell surface. The reduced rate of labeling of [674R]GPIIb-IIIa complexes could be caused by impairment of the association of [674R]GPIIb with GPIIIa, by retardation of the metabolic flow along the maturation and trafficking pathway, or by the concurrent action of both mechanisms. Whatever the mechanism, it seems that the [674R]GPIIb mutation either changes the kinetics of a preexisting step or imposes a new limiting step to the normal processing pathway of GPIIb-IIIa complexes.
Pulse-chase analysis of [674C] and [674R]GPIIb-IIIa synthesis.
CHO cells, transiently transfected with cDNAs encoding GPIIIa and either normal [674C] or mutant [674R]GPIIb, were pulse-labeled with [35S]methionine for 30 minutes and then chased with medium containing unlabeled methionine. At the indicated times, the cells were lysed and immunoprecipitated with anti-GPIIb. The precipitates were analyzed by electrophoresis as described in “Materials and methods.”
Pulse-chase analysis of [674C] and [674R]GPIIb-IIIa synthesis.
CHO cells, transiently transfected with cDNAs encoding GPIIIa and either normal [674C] or mutant [674R]GPIIb, were pulse-labeled with [35S]methionine for 30 minutes and then chased with medium containing unlabeled methionine. At the indicated times, the cells were lysed and immunoprecipitated with anti-GPIIb. The precipitates were analyzed by electrophoresis as described in “Materials and methods.”
Association of abnormal GPIIb or GPIIIa subunits with the endoplasmic reticulum chaperone BiP (GRP78)
The pulse-chase analysis seems to suggest that an early step in the surface expression pathway of [674R]GPIIb-IIIa may be impaired. Since the substitution of 674C in GPIIb implies the disruption of an intramolecular disulfide bridge and subsequent conformational changes, we found of interest to analyze the interaction of normal or [674R]GPIIb with the chaperone BiP. For this purpose, total cell lysates from CHO cells coexpressing GPIIIa and either normal or mutant [674R]GPIIb, or expressing only normal GPIIb, mutant [674R]GPIIb, or GPIIIa, were immunoprecipitated with an anti-GPIIb moAb and the precipitates used for Western analysis using a polyclonal antibody against the hamster BiP chaperone. Figure2 indicates that the chaperone BiP was coprecipitated with [674R]GPIIb but not with normal GPIIb. To elucidate whether the immunoprecipitated BiP was associated with mutant monomeric pro[674R]GPIIb or it was also bound to heterodimers, we performed the same type of analysis precipitating portions of the cell lysates with an anti-GPIIIa antibody reacting with monodimeric and heterodimeric GPIIIa. Figure 3 shows that anti-GPIIIa coprecipitated BiP only in cells coexpressing [674R]GPIIb and normal GPIIIa, suggesting that, at least under our experimental conditions, BiP associated with abnormal conformations of monomeric GPIIb as well as with mutant heterodimeric complexes. The observation that BiP immunoprecipitates with a mutant form of GPIIb (E324K) (Figure 3) unable to form heterodimers with GPIIIa20 clearly indicates that the BiP binding is independent of the type of GPIIb mutation and its consequence on assembly; it also indicates that assembly is not a prerequisite for BiP binding.
Association of mutant [674R]GPIIb with the endoplasmic reticulum chaperone BiP.
CHO cells were transiently transfected with the indicated cDNAs, lysed, and immunoprecipitated with the anti-GPIIb moAb M3. The precipitates were resolved by electrophoresis, transferred to nitrocellulose membrane, and blotted with polyclonal anti-BiP antibody, as described in “Materials and methods.”
Association of mutant [674R]GPIIb with the endoplasmic reticulum chaperone BiP.
CHO cells were transiently transfected with the indicated cDNAs, lysed, and immunoprecipitated with the anti-GPIIb moAb M3. The precipitates were resolved by electrophoresis, transferred to nitrocellulose membrane, and blotted with polyclonal anti-BiP antibody, as described in “Materials and methods.”
Coimmunoprecipitation of BiP with [674R]GPIIb-IIIa complex.
CHO cells, transiently cotransfected with cDNAs encoding GPIIIa and either normal [674C]GPIIb or mutant forms of GPIIb, were immunoprecipitated with the indicated antibodies. The precipitates were analyzed by electrophoresis, transferred to nitrocellulose membrane, and blotted with polyclonal anti-BiP antibody, as described in “Materials and methods.”
Coimmunoprecipitation of BiP with [674R]GPIIb-IIIa complex.
CHO cells, transiently cotransfected with cDNAs encoding GPIIIa and either normal [674C]GPIIb or mutant forms of GPIIb, were immunoprecipitated with the indicated antibodies. The precipitates were analyzed by electrophoresis, transferred to nitrocellulose membrane, and blotted with polyclonal anti-BiP antibody, as described in “Materials and methods.”
The apparently high-affinity binding of BiP to mutated [674R]GPIIb suggested that availability of BiP could become limiting, preventing, therefore, the access of new proGPIIb peptides into the maturation pathway. To investigate this possibility, we cotransfected 2 different amounts of mutant [674R]GPIIb with normal GPIIIa and BiP expression plasmids. No significant effect of BiP was detected in the rate of surface expression of [674R]GPIIb-IIIa complexes (results not shown), making unlikely that it could have been limiting.
Mutation analysis of the 674 and 687 residues of GPIIb
The 674C of GPIIb forms an intramolecular disulfide bridge with 687C. To ascertain whether the [674R]GPIIb dysfunction was associated with the loss of the disulfide bridge, or with the specific need for a C at position 674, we analyzed the functional repercussion of loosing the 674-687 disulfide bridge by changing the Cs 674 or 687 for A. Figure 4 (upper panel) depicts the surface expression of GPIIb-IIIa in CHO-IIIa cells transfected with normal or mutated forms of GPIIb. The mutation C674R found in the proband decreased by approximately 70% the surface expression of transfected GPIIb-IIIa. The C674A or C687A mutations produced smaller (approximately 40%-50%) inhibitions. The mutation of either 674C or 687C produced similar inhibition; however, the additive result of mutating both residues suggests the appearance of effects unrelated to disulfide disruption (Figure 4). Total cell lysates from transfected cells were immunoprecipitated with anti-GPIIb, and the presence of BiP in the precipitates was analyzed by Western blotting. The results so obtained indicate (Figure 4, bottom) that BiP was tightly associated not only with natural-occurring mutations of GPIIb but also with mutated forms of GPIIb created in the laboratory.
Effects of substitutions of 674C and 687C of GPIIb in the surface exposure and BiP association of GPIIb-IIIa complexes.
CHO-IIIa cells were transfected with the cDNA encoding normal [674C]GPIIb or mutant forms of GPIIb, as indicated. In the upper panel, the surface expression of GPIIb-IIIa complex was determined by flow cytometry, using an anti-GPIIb moAb. The results are expressed as a percentage of the surface exposure of normal [674C]GPIIb-IIIa complexes. The results are the means ± SEM of at least 3 independent experiments in duplicate. In the lower panel, immunoprecipitation of the cell lysates and Western blot analysis of the precipitates were performed as described in “Materials and methods.”
Effects of substitutions of 674C and 687C of GPIIb in the surface exposure and BiP association of GPIIb-IIIa complexes.
CHO-IIIa cells were transfected with the cDNA encoding normal [674C]GPIIb or mutant forms of GPIIb, as indicated. In the upper panel, the surface expression of GPIIb-IIIa complex was determined by flow cytometry, using an anti-GPIIb moAb. The results are expressed as a percentage of the surface exposure of normal [674C]GPIIb-IIIa complexes. The results are the means ± SEM of at least 3 independent experiments in duplicate. In the lower panel, immunoprecipitation of the cell lysates and Western blot analysis of the precipitates were performed as described in “Materials and methods.”
Physiologic responsiveness of platelets carrying the [674R]GPIIb mutation
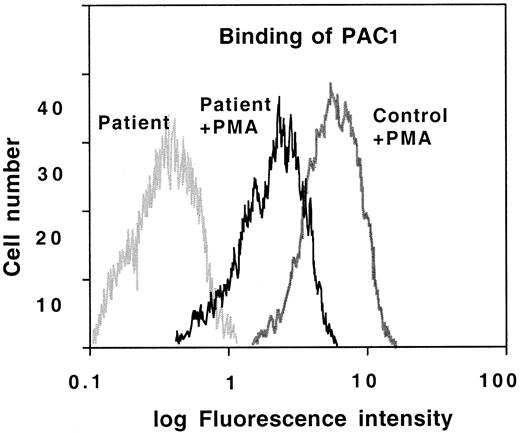
The reduced level of expression of [674R]GPIIb-IIIa justifies the thrombasthenic phenotype of the patient. However, we found of interest from the academic and prognostic standpoints to determine the ability of the mutated [674R]GPIIb-IIIa complexes to undergo an activated state in response to stimulation by agonists. Platelets from the proband and normal individuals were incubated in the presence of physiologic agonists, DTT, or PMA, and the binding of soluble FITC-Fg determined by flow cytometric analysis. The fluorescence of the proband's platelets was about 10% of the control values (results not shown). Thus, the enhanced fluorescence intensity after platelet activation was reasonably proportional to the number of surface receptors, suggesting that the mutated platelet [674R]GPIIb-IIIa complexes undergo normal in-out signaling-mediated conformational changes. This conclusion is also supported by the enhanced binding of the activation-dependent IgM PAC1 to the proband's platelets after PMA-induced postreceptor stimulation (Figure 5).
PAC1 binding to activated platelets.
Platelets from the thrombasthenic patient and normal controls were incubated with or without PMA (20 nmol/L) for 5 minutes and then incubated with the moAb FITC-PAC1 and analyzed by flow cytometry.
PAC1 binding to activated platelets.
Platelets from the thrombasthenic patient and normal controls were incubated with or without PMA (20 nmol/L) for 5 minutes and then incubated with the moAb FITC-PAC1 and analyzed by flow cytometry.
Competition between normal and [674R]GPIIb for surface expression of GPIIb-IIIa complexes
The heterozygous states for the [674R]GPIIb mutation expressed less than 50% of platelet GPIIb-IIIa of normal individuals. Since this mutated GPIIb allele yields normal amounts of full-length transcripts, we hypothesized that a reason for the decreased surface exposure of GPIIb-IIIa complexes could be a competition between the translational products of the normal and mutated alleles at some limiting step(s). To elucidate this point, we cotransfected CHO-IIIa cells with progressively increasing ratios of normal and/or mutated GPIIb cDNAs. Figure 6A depicts the surface GPIIb-IIIa content as a function of the amount of transfected expression plasmid(s). This process shows a saturation-type kinetics, and the maximal value with the mutated GPIIb plasmid was reached at much lower concentration than with normal GPIIb. Figure 6B shows the measured and estimated surface exposure of GPIIb-IIIa in cells coexpressing molar ratios of normal and mutated GPIIb from 0.2 to 5. In estimating the surface GPIIb-IIIa we assumed, according to results on panel A (Figure 6), that in this range of plasmid concentration transfection is not a limiting step and that surface expression from both cDNAs should be additive. Unexpectedly, instead of additivity, the results show a progressively increasing inhibition of surface exposure of normal GPIIb-IIIa complexes as the normal [674C]GPIIb-to-mutant [674R]GPIIb ratio decreased from 5 to 0.2 (Figure 6C). It should be remarked that cotransfection of equimolar amounts (2 μg of each expression plasmid) of normal and mutant GPIIb, presumably the expected physiologic condition, inhibited the surface exposure of GPIIb-IIIa by approximately 35%. Moreover, pulse-chase analysis of cells cotransfected with equimolar amounts of normal and mutant GPIIb clearly shows a prolonged retention of labeling in proGPIIb and decreased labeling of GPIIb-IIIa (Figure7). Densitometric analysis of data shown in Figure 7 was consistent with that obtained by flow cytometry (Figure6). The immunoprecipitation with anti-GPIIIa of heavily labeled GPIIIa in all conditions (Figure 7, bottom panel) rules out that availability of GPIIIa could have been limiting for the surface expression of GPIIb-IIIa.
Competition of normal and mutant [674R]GPIIb for surface expression of GPIIb-IIIa complexes.
(A) CHO-IIIa cells were transiently transfected with variable amounts of normal (○) or mutant (●) [674R]GPIIb cDNAs. The surface exposure of GPIIb-IIIa heterodimers was analyzed by flow cytometry with anti-GPIIb. The results are the means ± SEM of at least 3 independent experiments in duplicate. (B) CHO-IIIa cells were cotransfected with normal to mutant [674R]GPIIb molar ratios from 0.2 to 5, and the surface expression of GPIIb-IIIa complexes was determined by flow cytometry (▪). At the normal to mutant ratio of 1, 2 μg of each plasmid was cotransfected. The estimated values (■) were obtained, assuming that, according to results on panel A, the surface expression from the translational product of both cDNAs should be additive. (C) The percentage of inhibition of surface exposure of GPIIb-IIIa was calculated from the differences between the estimated and the measured values shown in panel B.
Competition of normal and mutant [674R]GPIIb for surface expression of GPIIb-IIIa complexes.
(A) CHO-IIIa cells were transiently transfected with variable amounts of normal (○) or mutant (●) [674R]GPIIb cDNAs. The surface exposure of GPIIb-IIIa heterodimers was analyzed by flow cytometry with anti-GPIIb. The results are the means ± SEM of at least 3 independent experiments in duplicate. (B) CHO-IIIa cells were cotransfected with normal to mutant [674R]GPIIb molar ratios from 0.2 to 5, and the surface expression of GPIIb-IIIa complexes was determined by flow cytometry (▪). At the normal to mutant ratio of 1, 2 μg of each plasmid was cotransfected. The estimated values (■) were obtained, assuming that, according to results on panel A, the surface expression from the translational product of both cDNAs should be additive. (C) The percentage of inhibition of surface exposure of GPIIb-IIIa was calculated from the differences between the estimated and the measured values shown in panel B.
Pulse-chase analysis of cells cotransfected with GPIIIa and either normal GPIIb, mutant [674R]GPIIb, or equimolar amounts of both GPIIb cDNAs.
CHO cells, transiently transfected with cDNAs encoding GPIIIa and either normal [674C]GPIIb, mutant [674R]GPIIb, or equimolar amounts of both cDNAs, were pulse-labeled with [35S]methionine for 30 minutes and then chased with medium containing unlabeled methionine. At the indicated times, the cells were lysed and immunoprecipitated with either anti-GPIIb or anti-GPIIIa. The precipitates were analyzed by electrophoresis as described in “Materials and methods.”
Pulse-chase analysis of cells cotransfected with GPIIIa and either normal GPIIb, mutant [674R]GPIIb, or equimolar amounts of both GPIIb cDNAs.
CHO cells, transiently transfected with cDNAs encoding GPIIIa and either normal [674C]GPIIb, mutant [674R]GPIIb, or equimolar amounts of both cDNAs, were pulse-labeled with [35S]methionine for 30 minutes and then chased with medium containing unlabeled methionine. At the indicated times, the cells were lysed and immunoprecipitated with either anti-GPIIb or anti-GPIIIa. The precipitates were analyzed by electrophoresis as described in “Materials and methods.”
Since the mutated [674R]GPIIb is bound to BiP, a limited availability of this chaperone could explain the observed competition between the normal and mutant forms of GPIIb. We investigated this possibility coexpressing BiP with variable amounts of normal or mutant GPIIb so that the mutant-to-normal GPIIb ratio varied from 1 to 3. The data in Figure 8 clearly indicate that overexpression of BiP did not overcome the inhibitory effects of the mutant [674R]GPIIb on the surface exposure of the normal subunit, but, on the contrary, a consistent inhibitory effect was observed at all mutant-to-normal GPIIb ratios analyzed. Finally, the possibility should be considered that binding of BiP to [674R]GPIIb-IIIa complexes would make limiting the availability of GPIIIa subunits. However, it is unlikely that this mechanism might be quantitatively important in view of the scarce amounts of mutant heterodimers immunoprecipitated with antibodies capable of identifying each subunit as either monomer or heterodimer.
Overexpression of BiP in CHO-IIIa cells cotransfected with normal and mutant GPIIb.
CHO-IIIa cells were cotransfected with different proportions of normal and mutant [674R]GPIIb cDNAs, as described in Figure 6, and 4μg of the BiP cDNA or the void plasmid. Forty-eight hours after transfection, the surface exposure of GPIIb-IIIa was analyzed by flow cytometry with the anti-GPIIb moAb M3. The results are the means ± SEM of at least 3 independent experiments in duplicate.
Overexpression of BiP in CHO-IIIa cells cotransfected with normal and mutant GPIIb.
CHO-IIIa cells were cotransfected with different proportions of normal and mutant [674R]GPIIb cDNAs, as described in Figure 6, and 4μg of the BiP cDNA or the void plasmid. Forty-eight hours after transfection, the surface exposure of GPIIb-IIIa was analyzed by flow cytometry with the anti-GPIIb moAb M3. The results are the means ± SEM of at least 3 independent experiments in duplicate.
Discussion
Role of the 674-687 disulfide bond of GPIIb on the biological function of the GPIIb-IIIa complex
The C674R GPIIb mutation disrupts the intrachain 674-687 disulfide bond. This mutation has been found in a thrombasthenic phenotype, heterozygote compound for GPIIb, whose platelet content of GPIIb-IIIa was about 10% of the normal control individuals and that only platelet mRNA-GPIIb was the one encoding [674R]GPIIb. Coexpression of normal GPIIIa and [674R]GPIIb in CHO cells confirmed the reduced rate of surface expression of [674R]GPIIb-IIIa complexes. However, from this type of experiment it cannot be ascertained whether the loss of the 674-687 disulfide bond impairs the function of the cell surface receptors. The platelets from the proband respond to a battery of activating agents by increasing the binding of soluble Fg with intensity proportional to the number of exposed receptors. This observation clearly indicates that loss of the 647-687 intrachain disulfide bond does not compromise the agonist-mediated responsiveness of the exposed [674R]GPIIb-IIIa complexes. Thus, considering that the [674R]GPIIb allele is transcribed at normal rates and this mutation does not preclude a normal receptor responsiveness, the knowledge of the limiting step(s) in the expression of mutant heterodimers could eventually help to define a therapeutic target(s).
In secreted proteins, disulfide bonds are important for stabilization of the tertiary structure as well as for their assembly into multimeric complexes.21 Disruption of intrachain disulfide bonds in GPIIb or GPIIIa produces variable degrees of functional impairment. Deletion of 106A-111Q, including 107C, disrupts the 107-130 intrachain disulfide bond in GPIIb,22 preventing heterodimerization of GPIIb-IIIa.23 Similar results were observed with the 129S-161S deletion comprising the 130C and 146C residues.24 In GPIIIa, disruption of disulfide bonds by the C374Y and C560F mutations are associated with type II thrombasthenia,25,26 and C542R and C457Y substitutions are involved in type I GT.27,28 However, removal of C655 GPIIIa by site-directed mutagenesis does not affect the GPIIb-IIIa.29 The Cs 674 and 687 in GPIIb are conserved in all the αIIbβ3 integrins of all the species studied so far (human, dog, pig, rabbit, and rodents). In general, the conservation of Cs in alpha subunits of integrins runs in parallel to their degree of homology with GPIIb.30 However, it is worth noting that Cs 674 and 687 are conserved in the alpha subunit of Arg-Gly-Asp (RGD)–reactive integrins (αIIbβ3, α5β1, and αvβ3) that undergo a process of cleavage and lack an I domain. In cleaved but non–RGD-reactive integrins, these Cs are conserved in α7 and α8. These observations suggest that the 674-687 disulfide bridge in GPIIb might play an important role in determining the optimal functional folding. The functional preservation of the [674R]GPIIb-IIIa complexes supports the idea that disruption of the 674-687 disulfide bond in GPIIb impairs primarily the assembly and/or intracellular trafficking of heterodimers.
The nascent polypeptide chains would tend to aggregate in the high-protein–containing intracellular medium unless it was impeded by the assistance of molecular chaperones. The chaperones are important in polypeptide folding, protein assembly, and intracellular translocation.31-33 They bind to hydrophobic domains on the surface of unfolded polypeptides. As the disulfide bonds are formed and the protein folding proceeds, most likely, different chaperones act sequentially until a competent native protein is secreted.34-36 The BiP is a molecular chaperone that targets irreversibly misfolded proteins for their degradation in the endoplasmic reticulum (ER). It is generally accepted that misfolded proteins are never secreted and remain sequestered in the ER until their degradation is completed. On these grounds, we found of interest to investigate the interaction of [674R]GPIIb with BiP and whether this chaperone was involved in determining the slow rate of surface exposure of [674R]GPIIb-IIIa complexes. In agreement with a previous observation on the mutagenesis of G273 of GPIIb,37 BiP bound mutated forms of residues 674 or 687 of GPIIb but not to normal [674C]GPIIb. It should be remarked that, although some of the introduced mutations improved the exposure of GPIIb-IIIa, none of them approached values above 50% of the normal rates. Thus, it seems reasonable to conclude that, at least under our experimental conditions, a relationship exists between the association of GPIIb with BiP and reduced surface expression of GPIIb-IIIa. It is generally accepted that the fate of misfolded proteins is their complete degradation in the ER. However, in our case, in agreement with an observation made on some mutated forms of the human chorionic gonadotropin-β subunit,38 at least part of the mutant [674R]GPIIb protein succeeded in progressing along the secretory pathway to reach the cell surface. The association of BiP with heterodimeric complexes supports the relationship between binding of BiP and the reduced rate of expression of [674R]GPIIb-IIIa. The tight binding of BiP might impose a major restraint on the progress of protein complexes along the secretory pathway.
It is worth noting that replacement of 674C for R appeared to have a more deleterious effect in terms of surface expression than A, which might be due to constraints imposed by the side-chain on the BiP binding. It should also be mentioned that, although mutation of either 674C or 687C produced similar effects, the additive effect of both mutations suggests their effect may not be due only to disulfide disruption. A likely interpretation for this observation is an altered polypeptide conformation independent from disulfide disruption. Alternatively, the nonmutated C may engage in disulfide bridging with another C, sufficient to provide a conformation improving the trafficking or retaining BiP less efficiently.
Competition between normal and mutant [674R]GPIIb subunits
The heterozygous states for mutations in GPIIb or GPIIIa are known to contain subnormal amounts of platelet GPIIb-IIIa, although near normal amounts have also been reported.18 In principle, the finding of subnormal platelet GPIIb-IIIa content suggests that transcripts from a single allele are not sufficient to maintain normal translational rates and, therefore, assembly and surface exposure. The amount of mutated platelet mRNA-GPIIb in the heterozygote state for [674R]GPIIb was within the expected normal range; nevertheless, the amount of platelet GPIIb-IIIa was approximately 30% of the normal values or, what is the same, about half of the estimated content, assuming that the contribution of both, normal (50%) and mutant allele (10%), was additive. The lack of additivity implies that some type of negative interaction must be taking place between the mutant and normal subunits. Under our experimental conditions, coexpression of normal GPIIIa and progressively increasing ratios of mutant to normal GPIIb led to progressive inhibition of the surface expression of GPIIb-IIIa in CHO cells. The physiologic significance of this finding is supported by the fact that transfection of CHO cells with GPIIIa and equimolar amounts of normal and mutant GPIIb inhibited the exposure of normal GPIIb-IIIa to a similar extent to that observed in the platelets of the heterozygote individual. The inhibitory effect of a mutant protein on the wild-type gene product is known as “dominant-negative” effect.39,40 Dominant-negative effect of mutated proteins seems to be involved in the etiopathogenesis of several human diseases, such as the thyroid hormone resistance syndrome41,42 and those originated by mutant p53 tumor suppressor proteins43and mutations in the tyrosine kinase domain of the insulin receptor.44
We have reported competition between other mutated forms of GPIIb and normal subunits.19 The competition between normal and mutant subunits may be exerted in different ways, depending for instance on whether they form homodimers or heterodimers that may compete for the binding of other subunits or ligands. On the basis of the strong binding of BiP to monomeric as well as to heterodimeric mutant [674R]GPIIb-IIIa complexes, we investigated whether the availability of BiP for the assisted folding of normal subunits could have been limiting. This possibility can be ruled out since coexpression of BiP enhanced rather than diminished the inhibitory effects of mutant [674R]GPIIb. The increased availability of BiP could result in increasing steady-state levels of [674R]GPIIb-BiP complexes. Thus, the inhibitory effect of overexpressing BiP adds further support to the postulate that the mutant [674R]GPIIb-BiP complex perturbs the secretory pathway of normal subunits by competing at some limiting step.
To conclude, the lack of the 674-687 disulfide bond in [674R]GPIIb does not preclude a normal agonist-induced binding of Fg or of the activation-dependent IgM PAC1 by [674R]GPIIb-IIIa receptors. BiP forms very stable complexes with either pro[674R]GPIIb and [674R]GPIIb-IIIa heterodimers but not with normal GPIIb or GPIIb-IIIa. Mutation analysis of residue 674 of GPIIb indicates that noncharged or no-polar residues improved the functional performance of the subunit. However, none of the analyzed mutants reached the normal values when coexpressed with normal GPIIIa, and all of them were tightly bound to BiP. There is a dose-dependent competition between normal and [674R]GPIIb-IIIa complexes for cell surface expression, and this effect is enhanced by coexpression of BiP. These observations suggest that the tight binding of BiP to [674R]GPIIb may be related to the slow rate of progression of [674R]GPIIb-IIIa along the secretory pathway and the competition between normal and mutant heterodimers.
Antibodies P37 and M3 directed against GPIIIa and GPIIb, respectively, were a gift of Drs J. González and M. V. Alvarez from the Instituto Rocasolano of the Spanish Council of Research (CSIC). We are greatly indebted to Dr Linda Hendershot at St Jude Children's Research Hospital, Memphis, TN, for the generous gift of antibody against rodent BiP and the BiP expression plasmid.
Supported in part by grants from the Dirección General de Investigación Cientı́fica y Técnica (DGICYT PB97-1240 and DGICYT PM97-0016), Fondo de Investigaciones Sanitarias (96/2014), and Comunidad Autónoma de Madrid 08.4/0031/1998, and by a grant-in-aid from the Agencia Española de Cooperación Internacional (AECI, n/ref 99CN0009). E.G.A.-S. received a fellowship from the Fundación Areces.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Roberto Parrilla, Centro de Investigaciones Biológicas (CSIC), Velázquez 144, Madrid-28006, Spain; e-mail: rparrilla@cib.csic.es.

![Fig. 1. Pulse-chase analysis of [674C] and [674R]GPIIb-IIIa synthesis. / CHO cells, transiently transfected with cDNAs encoding GPIIIa and either normal [674C] or mutant [674R]GPIIb, were pulse-labeled with [35S]methionine for 30 minutes and then chased with medium containing unlabeled methionine. At the indicated times, the cells were lysed and immunoprecipitated with anti-GPIIb. The precipitates were analyzed by electrophoresis as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2640/6/m_h80910985001.jpeg?Expires=1769348091&Signature=u5P~ouXTuFOV5XQMlSZ9hvCQkA9-GX0UFezLHhHdPtBrMuwpqA~2azVd~3VjyWFgE9pvgaV7jPpUrRBRM25qv3w3Oe8r6cSxGOetsTkEIxKVtjLCizlm2s8S1aETLz4l3PY0nbZ3PjMbnHiY2xF1ZXpWsE~UCQAAS0iWKmPjG4nWx4J9OPGAc9UGS8GPglgvxuVs47QTAhnIOt11n-anayl4m80XkIMikwd6c-ySl-mSstqtGk36jjLQehbag4fZWEs-9-MoN3DH0jqnydWG5vG7gX4vaoDXfZmeVm2DIhiDWBa8MRpHDShay4gkRYAtq1qSsAFMbMAnGM7xXk1bKA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Association of mutant [674R]GPIIb with the endoplasmic reticulum chaperone BiP. / CHO cells were transiently transfected with the indicated cDNAs, lysed, and immunoprecipitated with the anti-GPIIb moAb M3. The precipitates were resolved by electrophoresis, transferred to nitrocellulose membrane, and blotted with polyclonal anti-BiP antibody, as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2640/6/m_h80910985002.jpeg?Expires=1769348091&Signature=1p09Sbp~XDXtPhJFFayi9qyUUxJ35pCh2lUY3ULXeVe1gftRRzCpArER-dvvBLrZqTAJ1bph6K24QOfve~Udjw-ZkCb1jeG9SHn4p~ecNPPyF4AI9u97zNdEP3DO3l3cYW6jRWBYPWiuxfrZVqHrKQXsmang7rgzxcKb6feoELyYrY-V8fUxPpgrJsKTRE28Z57UZY1g26wGt-YRQavvarGyYxuNRx5G1TL17QrZBh9biXvPBvYkbeBMOLPTlscKEp6bMW2hpEWos~hI5ZU1sW-fT~gOtN390L4Zdc~l2kPwdiFFV2y5usG5U46k06tcyEXrE3v5w5tZRNhDOnX~9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Coimmunoprecipitation of BiP with [674R]GPIIb-IIIa complex. / CHO cells, transiently cotransfected with cDNAs encoding GPIIIa and either normal [674C]GPIIb or mutant forms of GPIIb, were immunoprecipitated with the indicated antibodies. The precipitates were analyzed by electrophoresis, transferred to nitrocellulose membrane, and blotted with polyclonal anti-BiP antibody, as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2640/6/m_h80910985003.jpeg?Expires=1769348091&Signature=BrvXQbXwPcWXqmLSsp5CwLmH33HownRY7rqQwoj27TJAGreDI3icQczBBRv5rY9-b17YK2gvPkbQNfyzJ~etK4gBFgAD8xt553xo-PDXhmmNyV4hhRpsecdrTqjpqBrr6XtSVeHgplG8nOrgdoJbyV-ifOyJCs0AuV~xpnWMApwEWdaxpkoo~9rcnKVzfYQlskKuo0AU-kwN1I7HCEXCCF6cNOgG3Ey~0FraXK7JZ2W1kVm9VX6N6lgPwpmzNEXUBxvsfkwNjKCJYGAeXj3zCV8sNs2Ou0fLzMbbTswSvPwJuinIYx1RXsw6hfXx3UX59ss2JNzhSKq-bp9kenmMCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effects of substitutions of 674C and 687C of GPIIb in the surface exposure and BiP association of GPIIb-IIIa complexes. / CHO-IIIa cells were transfected with the cDNA encoding normal [674C]GPIIb or mutant forms of GPIIb, as indicated. In the upper panel, the surface expression of GPIIb-IIIa complex was determined by flow cytometry, using an anti-GPIIb moAb. The results are expressed as a percentage of the surface exposure of normal [674C]GPIIb-IIIa complexes. The results are the means ± SEM of at least 3 independent experiments in duplicate. In the lower panel, immunoprecipitation of the cell lysates and Western blot analysis of the precipitates were performed as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2640/6/m_h80910985004.jpeg?Expires=1769348091&Signature=T9BDXZBpb9T7onEYQhmPXsE7hmuhRgFw9FzNUAziekJqDtHfIpPxB-EgpPV0AQAMRDBMASSAlWqUMOWRoiq4yHHg3gVLQuT~f-CcLduPQPOibyq3sBRlX5d2cUgFBkXOu52qwUzK~4yXWJAd7c5JAbh8FEz8u0KLrtiUQjzNMpfWLcXbpVPTtngG81zg8EWANi7lhh50YIB7y6pWVHs1-5cD8Jyf2xNT2f3rwZV4HyNvpnUP6roinTN6uv75gCPz8R39DZ2JTNthWo8kljquxmnstcfXyW~sKR8mPvXzTh7jXhZJNG90Z-6~fN6DCC-97cIa~7zsibZ8TS49dutovw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Competition of normal and mutant [674R]GPIIb for surface expression of GPIIb-IIIa complexes. / (A) CHO-IIIa cells were transiently transfected with variable amounts of normal (○) or mutant (●) [674R]GPIIb cDNAs. The surface exposure of GPIIb-IIIa heterodimers was analyzed by flow cytometry with anti-GPIIb. The results are the means ± SEM of at least 3 independent experiments in duplicate. (B) CHO-IIIa cells were cotransfected with normal to mutant [674R]GPIIb molar ratios from 0.2 to 5, and the surface expression of GPIIb-IIIa complexes was determined by flow cytometry (▪). At the normal to mutant ratio of 1, 2 μg of each plasmid was cotransfected. The estimated values (■) were obtained, assuming that, according to results on panel A, the surface expression from the translational product of both cDNAs should be additive. (C) The percentage of inhibition of surface exposure of GPIIb-IIIa was calculated from the differences between the estimated and the measured values shown in panel B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2640/6/m_h80910985006.jpeg?Expires=1769348091&Signature=i5HjPmRtRDv1aKjvMPH1vKMoUfkt8fUyoyFbgtcB~FNszNL2N2EcGJlngKfbtuCwqJ69460wvOnv5bR1DIrVZUMMOX4mC8COvN~OzwYVhDHbC-AzgD2ce0kdffgbw0itsjN0ekJOspvMw9aekE64JljvnfN9Nogy34-~lgq8DbNrxsT~VW5VOcJuH0ahPYmF-Koigsypc8CPTOCgvwvZe1vq2Zh1zZgQ550DcOxXmWM4Bl~J-5FP~-jUMupBZL7dVg4t3BqVlqKp5mAJgY3Bnljfwv4mOOLhtHYSkRS9AMO1GW9CffN74~44NjScJdXwAxPl~1rKukC9Q8fWPuU4Sg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Pulse-chase analysis of cells cotransfected with GPIIIa and either normal GPIIb, mutant [674R]GPIIb, or equimolar amounts of both GPIIb cDNAs. / CHO cells, transiently transfected with cDNAs encoding GPIIIa and either normal [674C]GPIIb, mutant [674R]GPIIb, or equimolar amounts of both cDNAs, were pulse-labeled with [35S]methionine for 30 minutes and then chased with medium containing unlabeled methionine. At the indicated times, the cells were lysed and immunoprecipitated with either anti-GPIIb or anti-GPIIIa. The precipitates were analyzed by electrophoresis as described in “Materials and methods.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2640/6/m_h80910985007.jpeg?Expires=1769348091&Signature=QsI5J4byg4XQHgSP8Q3T77xj8uH~NnBkYJ5G5vR8-6pcrlQBhccdtb7U6zsJzRyOsU-4yF-EXp7M5rJlgkCLgmgcWJiTdG5zaEid6PyWvg0vc~WMcBeqBPDyoOqCldsYInTStAe61DnmEZdR~e0kfNFaX4hHAqiNI5Tfn6-cR1qgr-6rti06pzhqga6HuidtaKug23iuRZXBFagYPhfpQOrmZCvtOGnrQATUux7AYwT1OyPOM6~92DQfnOXnU3KhBj~EKr4WCrq4WrM~adKlgK48A94~in5CqyBltrl1dcAatvyEITKWsfguanFIuyhalDKlOOWOK3jGBkcrzt8Pug__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. Overexpression of BiP in CHO-IIIa cells cotransfected with normal and mutant GPIIb. / CHO-IIIa cells were cotransfected with different proportions of normal and mutant [674R]GPIIb cDNAs, as described in Figure 6, and 4μg of the BiP cDNA or the void plasmid. Forty-eight hours after transfection, the surface exposure of GPIIb-IIIa was analyzed by flow cytometry with the anti-GPIIb moAb M3. The results are the means ± SEM of at least 3 independent experiments in duplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/9/10.1182_blood.v97.9.2640/6/m_h80910985008.jpeg?Expires=1769348091&Signature=kj4lQvvR14h1851ulT7e-ayRI5RVB04BHQhk2dL86o8epb1JcqryptgdaXdqnZ0etA7K70zWcXp27~upIV-l25VSgk6Ond5Po8W0bihroOmyGZvSPtDHFvuWpBTK~MWnxWW8bKDhl-c3eWevxXZAhkVRUbw8fjs8tqPk0T4Najaz-BhAAa6dorHQb4bvf7mexY~c9hXyFTDmIE54vjwlsCNU5BrVBmH4ycvQkuIivHZqQwtJ-RRMESRYdJCEszdbwpVuvKqE9-YfmY0SstGXLKjmXAyX1MwfVO0CQeVrlXcGmavT7rjFix0e8Vkhu04qarTnMXFZYjVqiXcpM8qSAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal