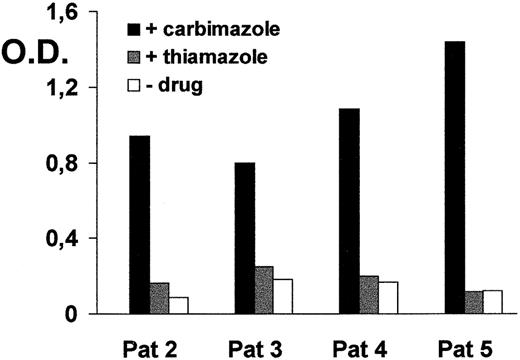
Drug-induced immune thrombocytopenia (DITP) is a sometimes severe complication of drug treatment. Recently, we described 5 patients who presented with relatively mild thrombocytopenia after treatment with the antithyroid drug carbimazole (1-carbethoxy-3-methyl-2-thioimidazole).1 Serologic and immunochemical analysis revealed drug-dependent antibodies (DDAbs) against the platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31). Because the chemical analog of carbimazole, thiamazole (3-methyl-2-thioimidazole), is widely used for the treatment of hyperthyroidism in the US, we asked the question whether carbimazole DDAbs cross-reacted with thiamazole. Sera from 4 patients of our initial study (patients 2-5; patient 1 died and no further serum was available) were analyzed by enzyme immunoassay with intact platelets in the presence and in the absence of 1 mg/mL carbimazole or thiamazole, as previously described.1 As shown in Figure1, all carbimazole DDAbs failed to react with platelets in the presence of thiamazole. Analysis of DDAbs in the glycoprotein-specific immunoassay (MAIPA) revealed positive reactions with PECAM-1 in the presence of carbimazole but not with thiamazole (data not shown). Specific interaction between carbimazole and the platelet membrane has to be assumed because only a minor chemical modification of carbimazole led to destruction of the carbimazole DDAb reactivity. This has already been suggested by our finding that the second extracellular loop of PECAM-1 was crucial for epitope formation.1 In addition, the carbethoxy group of carbimazole at position C-1 seems to be important for the immune response and subsequent thrombocytopenia in the patient after drug treatment. Carbimazole is a prodrug that is rapidly and totally converted to thiamazole in the body by cleavage of the carbethoxy group.2 This phenomenon could contribute to the clinical presentation of mild thrombocytopenia in our patients. Due to the high specificity of DDAbs, binding and platelet destruction could only occur during the phase immediately after carbimazole administration. After metabolism to thiamazole, no further platelet destruction takes place. This phenomenon might represent an interesting counterpart of the situation observed in patients in whom metabolite-specific DDAbs induce a prolonged effect in relation to drug excretion.3 We conclude that both the chemical structure and the metabolism of the drug may have a major influence on the clinical presentation of DITP, particularly on the degree of thrombocytopenia. Further observations will be required to define whether thiamazole itself is able to raise a drug-dependent immune response against platelets.
Carbimazole-dependent antibodies in enzyme immunoassay.
Binding of carbimazole-dependent antibodies in sera from 4 patients in the presence of carbimazole (1 mg/mL), thiamazole (1 mg/mL), and without either drug. O.D., optical density.
Carbimazole-dependent antibodies in enzyme immunoassay.
Binding of carbimazole-dependent antibodies in sera from 4 patients in the presence of carbimazole (1 mg/mL), thiamazole (1 mg/mL), and without either drug. O.D., optical density.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal