Recently, Lu and Andrieu1 stated that the HIV-1 protease inhibitors (PIs) indinavir and saquinavir used in concentrations at least 30-fold lower than those needed for 90% viral inhibition apparently do not influence sensitivity of peripheral blood T cells from HIV-1–infected individuals toward apoptosis induced either by T-cell receptor/CD3 ligation or by direct triggering of the CD95 receptor in vitro.1 They conclude from their data that the beneficial effect of PI treatment on clinical and immunological parameters in HIV-1–infected patients, which can be encountered even in the absence of relevant virological effects, are not related to changes in T-cell apoptosis.
In our own study in a cohort of HIV-1–infected children and adolescents, we had observed an increased sensitivity of freshly isolated T cells toward apoptosis,2 which was rapidly down-regulated following initiation of highly active antiretroviral therapy including PIs (HAART).3 Here we report that prolonged PI treatment in vivo reduced sensitivity of peripheral blood T cells toward apoptosis in vitro even when plasma viral load levels were not decreased and susceptibility for T-cell activation in these patients was not down-regulated.
Between January 1996 and January 1999, a total of 47 HIV-1–infected children and adolescents and 28 age-matched healthy controls were studied. Patients were stratified according to treatment modalities at the time of the immunological investigation (Table1). The study was conducted according to the Declaration of Helsinki and was approved by the ethical committee of the University Hospital in Heidelberg. T-cell phenotyping, plasma viral load quantification, and assessment of T-cell sensitivity toward apoptosis and activation was performed as previously described.3 Patients and controls and/or their relatives gave informed consent prior to venipuncture.
Clinical data of HIV-1–infected patients stratified according to the actual antiretroviral treatment regimen at the time of the study
| Patient group (Treatment) . | I (no therapy) . | II (2 RT inhibitors)† . | III (HAART)‡ . |
|---|---|---|---|
| N | 29 | 20 | 16 |
| Age (y) | 5 (0.5-28) | 7 (1-16) | 10 (2-21) |
| CD4 count (%) | 20 ± 2 | 23 ± 3 | 25 ± 2 |
| CD4 count (cells per μL) | 697 ± 87 | 977 ± 242 | 766 ± 143 |
| “Viral load” (log10 RNA copies per mL) | 4.54 (2.70-5.76) [n = 14]* | 4.81 (1.90-6.53) | 5.13 (1.48-5.87) |
| Duration of therapy (mo) | NA | 11 (7-20) | 10 (7-22) |
| Number of patients with “viral load” less than 3.0 log10copies per mL (%) | 2 (14) | 7 (35) | 7 (43) |
| Patient group (Treatment) . | I (no therapy) . | II (2 RT inhibitors)† . | III (HAART)‡ . |
|---|---|---|---|
| N | 29 | 20 | 16 |
| Age (y) | 5 (0.5-28) | 7 (1-16) | 10 (2-21) |
| CD4 count (%) | 20 ± 2 | 23 ± 3 | 25 ± 2 |
| CD4 count (cells per μL) | 697 ± 87 | 977 ± 242 | 766 ± 143 |
| “Viral load” (log10 RNA copies per mL) | 4.54 (2.70-5.76) [n = 14]* | 4.81 (1.90-6.53) | 5.13 (1.48-5.87) |
| Duration of therapy (mo) | NA | 11 (7-20) | 10 (7-22) |
| Number of patients with “viral load” less than 3.0 log10copies per mL (%) | 2 (14) | 7 (35) | 7 (43) |
Group I had no specific antiretroviral therapy, group II had at least 6 months of treatment with 2 nucleosidic inhibitors of HIV-1 reverse transcriptase (RT inhibitors), and group III had at least 6 months of treatment with 2 RT inhibitors and at least 1 inhibitor of HIV-1 protease (HAART). Sixteen patients were studied before and after changes in their therapeutic regimen and were included in both respective patient groups; 4 patients were studied repeatedly and were included in all 3 groups. The remaining 21 patients were studied only once. Data are given either as median values (range) or as arithmetic mean ± SEM.
AZT indicates zidovudine; d4T, stavudine; ddC, zalcitabine; ddI, didanosine; 3TC, lamivudine; IDV, indinavir; NFV, nelfinavir; SQV, saquinavir; RTV, ritonavir; and NA, not applicable.
Plasma viral load levels were not available for all patients; the actual number is given in brackets.
Specific drug combinations: 9 patients received AZT + 3TC; 8, AZT + ddI; 2, AZT + ddC; and 1, d4T + 3TC.
Specific drug combinations: 3 patients received d4T + 3TC + IDV; 4, d4T + ddI + NFV; 3, d4T + 3TC + NFV + SQV; 1, d4T + 3TC + NFV;1, AZT + 3TC + NFV; 1, AZT + ddI + IDV; 1, d4T + NFV + SQV; 1, d4T + ddI + NFV + SQV; and 1, d4T + ddI + RTV + SQV.
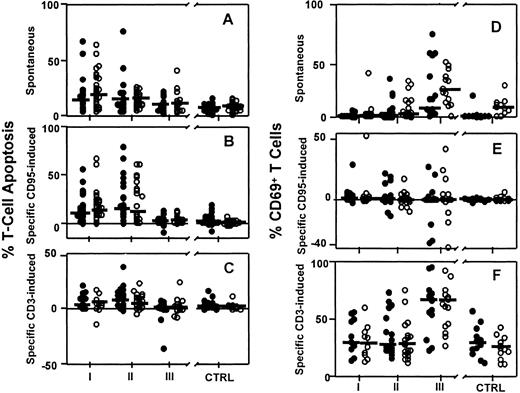
We compared T-cell apoptosis and T-cell activation (CD69 expression) in patients on HAART for more than 6 months (group III; n = 16) to patients without therapy (group I; n = 29), patients treated with HIV-1 reverse transcriptase inhibitors (RTIs) alone (group II; n = 20) and healthy controls (CTRLs; n = 28) by analysis of variance using the InStat statistical analysis software package (GraphPad Software, San Diego, CA). As shown in Figure1, the sensitivity of T cells toward anti-CD95–induced apoptosis was significantly lower in group III than in group II (Figure 1B; CD4+ T cells, 3 ± 1% versus 26 ± 5%, P < .001; CD8+ T cells, 5 ± 1% versus 24 ± 5%, P < .001) despite similar plasma viral load levels in both patient groups. Spontaneous T-cell death did not differ between these groups (Figure 1A; CD4+T cells, 10 ± 2% versus 17 ± 4%, P = NS; CD8+ T cells, 12 ± 3% versus 15 ± 2%,P = NS).
Effect of different antiretroviral treatment regimens on apoptosis and CD69 expression in CD4+ and CD8+T cell subpopulations in HIV-1–infected patients and healthy controls.
(A) Spontaneous apoptosis, (B) anti-CD95–induced apoptosis, (C) anti-CD3–induced apoptosis, (D) spontaneous CD69 expression, (E) anti-CD95–induced CD69 expression, (F) anti-CD3–induced CD69 expression. ● indicates CD4+ T cell subpopulations, and ○, CD8+ T cell subpopulations. Horizontal bars indicate the group median. Data from HIV-1–infected patients receiving either no effective antiretroviral therapy (group I, n = 26) or at least 6 months of treatment with 2 inhibitors of HIV-1 reverse transcriptase (RTIs; group II, n = 20) or 2 RTIs plus at least 1 inhibitor of HIV-1 protease (HAART; group III, n = 16) were compared to 28 age-matched healthy controls (CTRLs). The respective patient numbers for measurements of CD69 expression were group I, n = 11; group II, n = 20; group III, n = 16; and CTRL, n = 12. Methods: Freshly isolated peripheral blood mononuclear cells (PBMCs) (2 × 105 cells/well) were cultured in the presence or absence of either anti-CD3 monoclonal antibodies (moAbs; OKT3; 10 μg/mL) or anti-CD95 moAb (anti–APO-1; 10 μg/mL) and protein A (5 ng/mL) as described.3 Cells were collected after 21 ± 1 hours and stained for surface expression of CD4, CD8, and CD69 using fluorochrome-labeled moAbs (Becton Dickinson, Heidelberg, Germany), and cell death was determined in CD4+ and CD8+ T cells by flow cytometry. The percentage of specific anti-CD3– and anti-CD95–induced apoptosis was calculated by 100 × (% of experimentally induced cell death − % of spontaneous cell death)/(100 − % of spontaneous cell death). Spontaneous and experimentally induced CD69 expression on viable CD4+ and CD8+ T cells was determined as a marker for T-cell activation as described,3 specific anti-CD3– and anti-CD95–induced CD69 expression on both T-cell subpopulations was calculated using the formula for specific cell death.
Effect of different antiretroviral treatment regimens on apoptosis and CD69 expression in CD4+ and CD8+T cell subpopulations in HIV-1–infected patients and healthy controls.
(A) Spontaneous apoptosis, (B) anti-CD95–induced apoptosis, (C) anti-CD3–induced apoptosis, (D) spontaneous CD69 expression, (E) anti-CD95–induced CD69 expression, (F) anti-CD3–induced CD69 expression. ● indicates CD4+ T cell subpopulations, and ○, CD8+ T cell subpopulations. Horizontal bars indicate the group median. Data from HIV-1–infected patients receiving either no effective antiretroviral therapy (group I, n = 26) or at least 6 months of treatment with 2 inhibitors of HIV-1 reverse transcriptase (RTIs; group II, n = 20) or 2 RTIs plus at least 1 inhibitor of HIV-1 protease (HAART; group III, n = 16) were compared to 28 age-matched healthy controls (CTRLs). The respective patient numbers for measurements of CD69 expression were group I, n = 11; group II, n = 20; group III, n = 16; and CTRL, n = 12. Methods: Freshly isolated peripheral blood mononuclear cells (PBMCs) (2 × 105 cells/well) were cultured in the presence or absence of either anti-CD3 monoclonal antibodies (moAbs; OKT3; 10 μg/mL) or anti-CD95 moAb (anti–APO-1; 10 μg/mL) and protein A (5 ng/mL) as described.3 Cells were collected after 21 ± 1 hours and stained for surface expression of CD4, CD8, and CD69 using fluorochrome-labeled moAbs (Becton Dickinson, Heidelberg, Germany), and cell death was determined in CD4+ and CD8+ T cells by flow cytometry. The percentage of specific anti-CD3– and anti-CD95–induced apoptosis was calculated by 100 × (% of experimentally induced cell death − % of spontaneous cell death)/(100 − % of spontaneous cell death). Spontaneous and experimentally induced CD69 expression on viable CD4+ and CD8+ T cells was determined as a marker for T-cell activation as described,3 specific anti-CD3– and anti-CD95–induced CD69 expression on both T-cell subpopulations was calculated using the formula for specific cell death.
Although T cells from HAART-treated patients (group III) had a normal sensitivity toward anti-CD95–induced apoptosis in vitro (P = NS when compared to the control; Figure 1B), the sensitivity toward anti-CD3–induced CD69 expression in both CD4+ and CD8+ T-cell subpopulations was significantly higher in group III than in all other groups of patients and the controls (Figure 1E, P < .001). Anti-CD3–induced CD69 expression on CD4+ and CD8+ T cells tended to increase in HAART-treated patients (group III) with detectable, compared to those with undetectable, plasma viral load, although these differences were statistically not significant (CD4+ T cells, 71 ± 8% versus 51 ± 7%, P = NS; CD8+ T cells, 69 ± 8% versus 52 ± 6%,P = NS). Spontaneous CD69 expression in T cells from PI-treated patients in group III was increased compared to other treatment groups and healthy controls (28 ± 7% on CD4+and 27 ± 4% on CD8+ T cells; P < .001); see Figure 1D.
Our observations suggest that the reduced T-cell sensitivity toward CD95-induced apoptosis in vitro might partly explain the observation that an increasing number of patients with virological rebound on PI therapy maintain stable CD4 counts and do not experience clinical deterioration as fast as one could expect from the increased viral load. The CD95-resistant phenotype of peripheral blood T cells in these patients may be due to a decreased viral “fitness” or a direct inhibition of apoptosis pathways (eg, at the level of proapoptotic proteases).4 The fact that these T cells are still hypersensitive toward anti-CD3–induced activation argues against the hypothesis of Lu and Andrieu that the restoration of a normal apoptosis sensitivity is simply an epiphenomenon of the general immunological recovery in HIV-infected individuals receiving HAART.
T-cell recovery in HIV-infected patients experiencing virologic failure under highly active antiretroviral therapy
Böhler et al's comments invoke the fundamental issue: what is the interrelation between T-cell activation, anergy, and apoptosis (AAA) in HIV-infected patients treated with highly active antiretroviral therapy (HAART). We agree that this issue is important enough to deserve a deeper discussion.
Recently, we1-1 and others1,2 have reported an apparent dissociation between T-cell recovery and plasma viral-load suppression in HIV-infected patients under HAART. In addition, Böhler et al also observed a reduced sensitivity of HAART-treated patients' T cells to apoptosis that was independent from viral-load response. This dissociation implies a direct effect of HAART on patients' immune systems rather than “a decreased viral fitness” as suggested by Böhler et al, because the immune recovery in patients experiencing virologic failure could not be maintained after withdrawal of HAART (Table 1-1).
CD4+ and CD8+ T-cell counts in 5 HIV-infected patients experiencing virologic nonresponse under 3 years of HAART and during temporary withdrawal of treatment
| Patients2-1-150 . | Peripheral blood T-cell count . | Plasma viral load (log10 eq copies/mL) . | |
|---|---|---|---|
| CD4 cells/μL . | CD8 cells/μL . | ||
| Patient 88 | |||
| At entry | 36 | 425 | 4.99 |
| Month 12 | 283 | 979 | 5.04 |
| Month 36 | 380 | 1656 | 4.68 |
| Month 382-1-151 | 147 | 878 | 4.70 |
| Month 41 | 385 | 1128 | 4.26 |
| Patient 89 | |||
| At entry | 43 | 225 | 5.22 |
| Month 12 | 136 | 707 | 4.36 |
| Month 36 | 233 | 891 | 5.31 |
| Month 402-1-151 | 69 | 646 | 4.89 |
| Month 42 | 162 | 915 | 4.77 |
| Patient 90 | |||
| At entry | 81 | 615 | 4.24 |
| Month 12 | 237 | 1204 | 4.51 |
| Month 36 | 374 | 1844 | 5.23 |
| Month 392-1-151 | 144 | 943 | 5.28 |
| Month 42 | 294 | 1544 | 4.79 |
| Patient 93 | |||
| At entry | 155 | 900 | 4.79 |
| Month 12 | 274 | 1243 | 4.72 |
| Month 36 | 229 | 2564 | 4.13 |
| Month 432-1-151 | 170 | 1542 | 4.93 |
| Month 45 | 401 | 2517 | 4.28 |
| Patient 96 | |||
| At entry | 213 | 259 | 4.47 |
| Month 12 | 329 | 414 | 4.10 |
| Month 36 | 567 | 873 | 4.53 |
| Month 422-1-151 | 226 | 449 | 5.15 |
| Month 44 | 549 | 646 | 5.18 |
| Patients2-1-150 . | Peripheral blood T-cell count . | Plasma viral load (log10 eq copies/mL) . | |
|---|---|---|---|
| CD4 cells/μL . | CD8 cells/μL . | ||
| Patient 88 | |||
| At entry | 36 | 425 | 4.99 |
| Month 12 | 283 | 979 | 5.04 |
| Month 36 | 380 | 1656 | 4.68 |
| Month 382-1-151 | 147 | 878 | 4.70 |
| Month 41 | 385 | 1128 | 4.26 |
| Patient 89 | |||
| At entry | 43 | 225 | 5.22 |
| Month 12 | 136 | 707 | 4.36 |
| Month 36 | 233 | 891 | 5.31 |
| Month 402-1-151 | 69 | 646 | 4.89 |
| Month 42 | 162 | 915 | 4.77 |
| Patient 90 | |||
| At entry | 81 | 615 | 4.24 |
| Month 12 | 237 | 1204 | 4.51 |
| Month 36 | 374 | 1844 | 5.23 |
| Month 392-1-151 | 144 | 943 | 5.28 |
| Month 42 | 294 | 1544 | 4.79 |
| Patient 93 | |||
| At entry | 155 | 900 | 4.79 |
| Month 12 | 274 | 1243 | 4.72 |
| Month 36 | 229 | 2564 | 4.13 |
| Month 432-1-151 | 170 | 1542 | 4.93 |
| Month 45 | 401 | 2517 | 4.28 |
| Patient 96 | |||
| At entry | 213 | 259 | 4.47 |
| Month 12 | 329 | 414 | 4.10 |
| Month 36 | 567 | 873 | 4.53 |
| Month 422-1-151 | 226 | 449 | 5.15 |
| Month 44 | 549 | 646 | 5.18 |
HIV indicates human immunodeficiency virus; HAART, highly active antiretroviral therapy.
Patients whose characteristics and HAART regimen were reported previously.1-1
Patients' samples collected within 1-2 months after withdrawal of their HAART regimen.
In general, the immune recovery under HAART is characterized by an increase of CD4+ and CD8+ T-cell counts associated with an overall down-regulation of T-cell AAA in vivo. To learn what the effects of HAART on the AAA are, we have conducted an in vitro study to investigate the AAA kinetics of patients' peripheral blood mononuclear cells (PBMCs) following immune stimuli in the absence or the presence of HAART components (nucleoside reverse trascriptase inhibitors [NRTIs] or protease inhibitors [PIs]).1-1 Results showed that PIs (indinavir or saquinavir), even at nonantiviral doses, allowed an early (4 days after activation) reversal of T-cell anergy (ie, restoration of T-cell proliferation) but a later (14 days after activation) down-regulation of T-cell apoptosis. Therefore, we concluded that reversal of T-cell anergy should be the primary action of PIs responsible for the overall immune improvements observed in patients treated with HAART. This finding is in keeping with the increasing evidence that the progression of HIV disease is associated with an impaired T-cell proliferation/production (anergic mechanism) coupled with chronic immune activation and apoptosis (ie, reduced half-life of T cells) rather than with an increased T-cell turnover (overproduction or exhaustion hypothesis).1-6-1-10
An increased proportion of HIV-infected patients' T cells undergoing apoptosis following APO-1/Fas (CD95) ligation reflects, indeed, a subpopulation of T cells chronically activated in vivo, because purified quiescent T cells (G0) showed nearly 100% resistance to apoptosis triggered by APO-1/Fas ligation while the proportion of T cells susceptible to Fas-mediated apoptosis increased gradually after 7 days of stimulation with anti-CD3 and anti-CD28 monoclonal antibodies (Lu and Andrieu, unpublished observation, 2000). Normalized sensitivity of T cells to apoptosis in PI-treated (but not NRTI-treated) patients as reported by Böhler et al could thus be the consequence of early restoration of T-cell proliferation and subsequent down-regulation of T-cell activation by PIs.
Böhler et al's key argument challenging the normalization of T-cell proliferation/activation as the primary event involved in HAART (ie, PI)–induced immune recovery is based on their observation that CD69 expression on T cells following TCR/CD3 stimulation was significantly enhanced in HAART-treated patients. In fact, this observation supports further the PI-mediated enhancement of T-cell proliferation/differentiation following immune stimuli, because CD69 expression on T cells upon to TCR/CD3 stimulation was shown to be correlated positively with proliferation and effector function of T cells.1-11
In conclusion, anergized status of HIV-infected patients' T cells permits unresolved chronic activation and apoptosis throughout the course of infection, thus contributing to the eventual development of AIDS. PIs, even at nonantiviral concentrations, are capable of restoring T-cell proliferation and in consequence normalizing T-cell activation and apoptosis in HAART-treated patients. These findings are instrumental in improving our understanding of HIV immunopathology and in guiding (or designing) immune-based therapeutic intervention, particularly in patients experiencing viro- logic failure and/or in those suffering permanently from side effects of antiviral drugs.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal