Mice with the lysosomal storage disease mucopolysaccharidosis (MPS) VII, caused by a deficiency of β-glucuronidase (GUSB), have signs of disease present at birth. Bone marrow transplantation (BMT) or retroviral vector–mediated gene transfer into hematopoietic stem cells can partially correct the disease in adult mice, and BMT performed at birth results in a better clinical outcome. Thus, treatment in utero may result in further improvement. However, this must be done without cyto-ablation, and the donor cells do not have a competitive repopulating advantage over host cells. Transplantation in utero of either syngeneic fetal liver hematopoietic stem cells marked with a retroviral vector, or allogeneic donor cells that constitutively express high levels of human GUSB from a transgene, resulted in only about 0.1% engraftment in the adult. Immuno-affinity enrichment of stem and progenitor cells of 5- to 10-fold resulted in significantly higher GUSB activities at 2 months of age, but by 6 months engraftment was about 0.1%. Attempts to further increase the number of stem and progenitor cells were deleterious to the recipients. Nevertheless, GUSB expressed during the first 2 months of life in MPS VII fetuses could delay the onset of overt signs of disease. This suggests that the expression of some normal enzyme activity beginning in fetal life may offer the possibility of slowing the progression of the disease until more definitive postnatal transplantation or gene transfer to stem cells could be accomplished.
Introduction
Mucopolysaccharidosis type VII (MPS VII) is a fatal, progressive, degenerative lysosomal storage disease. It is caused by a deficiency of β-glucuronidase (GUSB; EC 3.2.1.31) activity, which is necessary for the degradation of glycosaminoglycans by cleaving glucuronide moieties.1 MPS VII is inherited as an autosomal recessive trait and has been described in humans,1 dogs,2 mice,3-5 and cats.6,7 The clinical, pathological, and genetic features of MPS VII have been extensively described in mice,3-5,8-10 and the disease is essentially the same as in human patients. Common to both animals and humans with MPS VII is the presence of histopathology in affected individuals at birth, but the disease is not usually clinically apparent until 6 months to 1 year in children and 3 to 5 weeks in mice. Since pathology is present before birth,10 this mouse model can be used to test treatment modalities during fetal life.
Treatment of most lysosomal storage diseases involves providing the normal enzyme to deficient cells for receptor-mediated endocytosis (cross-correction).11 Only a small amount of the enzyme, if continuously present, may be necessary to improve or arrest signs of disease.12 Enzyme replacement and bone marrow transplantation (BMT) studies have shown that the earlier in life treatment is initiated, the better the clinical outcome. MPS VII mice that received infusions of purified enzyme or underwent BMT as neonates had a grossly normal appearance, lived longer, and had less storage than mice transplanted as young adults.13-15 These results suggest that treatment beginning during fetal life may further decrease the severity of disease or even prevent its manifestation altogether.
Using fetal liver cells (FLCs) as donors is an approach for transplantation into the affected fetus. Graft rejection and graft-versus-host disease are avoided because of the immunotolerance and immuno-incompetence of the fetal recipient and donor cells, respectively.16 When FLCs are transplanted in utero, no cyto-ablation is used, which avoids the risks associated with transplantation conditioning. However, this results in a chimeric hematopoietic system in the recipient. Typically, only a small percentage of donor cells are present in the recipient, unless there is a developmental advantage for the donor versus the host cells.17 This reduces the amount of normal gene product that can be delivered to the diseased recipient. We have previously shown retroviral vector marking of murine FLCs using a 4-day dual-chamber cocultivation system that prevents contamination by vector producer cells.18 The transduced cells were transplanted in utero and could be detected in most tissues after birth, but long-term studies were not performed. The present study showed that the hematopoietic stem cells of the fetal liver were transduced by this procedure, by using secondary reconstitution assays in adults. However, when transduced FLCs were transplanted into fetuses, only low levels of long-term engraftment occurred, resulting in levels of GUSB expression that were too low to be therapeutic.
It may be possible to deliver higher levels of corrective enzyme by overexpressing a functional exogenous gene. Overexpression of a normal lysosomal enzyme from transgenic cells transplanted postnatally has been shown to reverse lesions in another storage disease model,19 and retroviral vectors can overexpress GUSB in transduced hematopoietic cells.12,20 21 Constitutive expression of GUSB was studied by using FLCs from an allogeneic TG mouse strain that expresses very high levels of human GUSB against the MPS VII background. The in utero transplantation properties of these allogeneic cells were compared with retroviral marking studies of syngeneic cell transplants. Since only a limited number of cells can be transplanted, we also determined whether enriching the transplant population for hematopoietic stem and progenitor cells could increase the proportion of chimerism.
Materials and methods
Animals
The MPS VII carrier strain of mouse, B6.C-H-2/bm1ByBir-gus+/mps3,was established about 10 years ago from breeding stocks obtained from E. Birkenmeier at The Jackson Laboratory (Bar Harbor, ME) and have been bred in our colony since then. These mice have a single base-pair deletion in exon 10 resulting in a frameshift and a premature stop codon.9 Translation of the resulting messenger RNA (mRNA) would produce a truncated, probably nonfunctional protein, but since the GUSB mRNA levels are reduced by more than 200-fold, probably little or no truncated protein is produced. No GUSB enzymatic activity is detectable above assay background in any tissue.3 5
We have shown that fewer than the expected 25% of affected fetuses are born from matings between B6.C-H-2bml/ByBir-gus+/mpscarrier mice.5 Because so few MPS VII fetuses were available, a system was developed using cells that constitutively express high levels of GUSB to mimic long-term–expressing retrovirally transduced cells and to examine reconstitution of FLCs after in utero transplantation. The C3H strain of mouse was chosen as a recipient strain because its members have greatly decreased tissue GUSB levels without showing clinical or pathological signs of disease.22 Mice transgenic for the human genomic GUSB DNA and mutant for murine GUSB (TG) were chosen as the donor strain, because of greatly increased GUSB activity levels and their heat-stable enzyme. The transgene contains an estimated 2 to 4 tandem repeat copies of 1.6 kilobases (kb) of the 5′ flanking sequence, the entire structural gene, and 3.8 kb of the 3′ flanking sequence of the human genomic GUSB gene.23 Thus, the TG mice produce no murine GUSB protein but maintain a normal phenotype because they produce 10 to 20 times higher than normal levels of human GUSB. They have been interbred to homozygosity and are maintained as a recombinant inbred strain.
Carrier female B6.C-H-2/bm1ByBir-gus+mpsmice (B+/mps) were bred to normal C3H males, which already have low levels of GUSB without any clinical or pathological signs of disease.22 The carrier offspring (B-C+/mps F1) were interbred to produce affected offspring (B-Cmps/mps F2), to achieve increased fitness and thus obtain breeding pairs of MPS VII intercrossed mice, as previously described.5 Mice were maintained and cared for according to the University of Pennsylvania's Guidelines for the Care and Use of Laboratory Animals, which ensured compliance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act.
FLC preparation
Fetal livers were obtained by sacrificing pregnant mice at 13.5 days gestational age, with the vaginal plug date counted as day 0.5. The liver cells were prepared in Iscove's modified Dulbecco's medium (IMDM) (GibcoBRL, Gaithersburg, MD) containing 10% fetal bovine serum (FBS) (HyClone, Logan, UT), L-glutamine, and penicillin (10 000 U/mL)–streptomycin (100 mg/mL)–fungizone (25 μg/mL) (GibcoBRL). Single liver cell suspensions were obtained by repeated trituration with successively smaller pipettes. The suspension was left to rest for 5 minutes at room temperature; the top two thirds were removed and centrifuged at 900g for 5 minutes at 4°C. The pellet was resuspended either in phosphate-buffered saline (PBS) for direct transplantation or, if the cells were to be cultured before transplantation, in IMDM containing growth factors (see below).
Retroviral vectors and transduction procedures
The human GUSB complementary DNA (cDNA) was cloned into the retroviral vector LXSN (a kind gift from A. D. Miller), designated LBGSN,45 and transferred into GP+E86 packaging cells.24 This vector was chosen because a high-titer packaging cell subclone (isolated by M. Sands) resulted in high transduction efficiency, and the vector experiments described in this paper were aimed at cell marking rather than disease correction.
FLCs were harvested as described above. The pellet was resuspended in IMDM with 10% FBS, L-glutamine, PSF (penicillin, streptomycin, fungizone), recombinant murine interleukin (IL)–3 (200 U/mL), recombinant human IL-6 (200 U/mL), and recombinant murine stem cell factor (100 ng/mL). Immediately after preparation, FLCs were cultured in T-25 flasks and incubated for 48 hours at 37°C (5% CO2 in humidified air) before transduction. All growth factors were obtained from PeproTech (Rocky Hill, NJ).
At 1 day before infection, packaging cells (LBGSN-E) were seeded into the top well of double-chambered cell-culture plates (Transwell) (Costar, Cambridge, MA) (10 cm in diameter) at 5 × 105 cells per well and incubated (24 hours, 37°C, 5% CO2 in humidified air). Thereafter, FLCs were transferred into the bottom well, which was separated from the top well by a membrane with 0.45-μm pores, preventing contamination of the FLCs with virus-producing fibroblasts but allowing diffusion of infectious viral particles. After the addition of fresh IMDM containing growth factors and polybrene at a total concentration of 8 μg/mL, both culture systems were incubated for another 48 hours at 37°C (5% CO2 in humidified air).
Enrichment for the presence of fetal hematopoietic stem and progenitor cells
Magnetic cell sorting was performed by means of MiniMACS columns according to the manufacturer's specifications (Miltenyi Biotec, Sunnyvale, CA). Briefly, FLCs were incubated with AA4.1 antibody (25 μg/mL) (from hybridoma cells, a kind gift from J. McKearn) at 4°C for 30 minutes. AA4.1 antibody was used because it had previously been shown to isolate hematopoietic cells from murine fetal livers with long-term reconstituting abilities.25 The cells were washed in a mixture of PBS, 0.5% bovine serum albumin (BSA), and 5 mM EDTA followed by incubation with goat anti–rat immunoglobulin (Ig)–G microbeads at 4°C for 15 minutes. The cells were washed again and added to the column. The positive, adherent cells were extruded from the column after the negative, nonbinding cells were allowed to flow through, and the column was washed once with the mixture of PBS, 0.5% BSA, and 5 mM EDTA. The positive cells were counted and retained for transplantation and flow cytometric analysis.
Flow cytometry
Cells were incubated with fluorescein isothiocyanate (FITC)–conjugated goat anti–rat IgG at 4°C for 15 minutes. After incubation, the samples were washed 3 times with PBS, 0.5% BSA, and 5 mM EDTA and analyzed on a flow cytometer (FACScalibur) (Becton Dickinson, Lincoln Park, NJ). Controls included unmanipulated FLCs, FLCs incubated with AA4.1 antibody, FLCs with AA4.1 antibody, and magnetic microbeads, negative fraction, wash fraction, and positive fraction of the column.
Transplantation
Adult recipients received whole-body irradiation at 9 Gy delivered from a 137Cs irradiator. Within 24 hours of irradiation, the mice received transduced 2 × 106 FLCs intravenously via the lateral tail vein.
For fetal transplants, pregnant mice of 13.5 days' gestation were anesthetized with tribromoethanol intraperitoneally (0.4 μg/g mouse). Access to the uterus was gained by a midline incision. Under the dissecting microscope, 10 μL of FLC suspension was injected with a glass micropipette (80 to 120 μm in diameter) into the placenta of each fetus. Depending on the preceding manipulation, FLCs were injected at a concentration of approximately 1 × 105 or 1 × 106 cells per 10 μL.
Preparation of tissues
At the end of each experiment, the mice were killed by deep anesthesia with tribromoethanol (20 mg/mL; 0.4 to 0.5 mL per mouse). The mice were perfused with 10 mL chilled PBS (GibcoBRL) and 10 mL chilled chloral hydrate–formalin solution (1% chloral hydrate in 20% vol/vol neutral buffered formalin in distilled water) to preserve the tissues for histology and to empty the blood vessels of enzyme-producing cells, leaving only resident enzyme-producing cells in tissues. Gross abnormalities were noted when present.
Tissues for enzymatic activity were snap-frozen in isopentane submersed in liquid nitrogen. Tissues for cryostat sectioning were embedded in OCT compound (Tissue-Tek) after freezing and were sectioned at 10 μm for GUSB expression26 and immunofluorescent staining (see below). For enzymatic and glycosaminoglycan analysis of the brain, one half of the whole brain (cerebrum, cerebellum, and hindbrain) was cut longitudinally and homogenized. For analysis of representative samples of bone and cartilage, the whole sternum was stripped of muscle tissue and homogenized. Thus, the values of brain and cartilage are not representative of specific areas of those tissues.
Enzyme assays
Cryostat sections of tissues were air-dried on glass slides and assayed for GUSB activity by means of a histochemical stain, which detects the biologically relevant function by enzymatic cleavage of a β-D-glucuronide moiety.26 When TG FLCs were transplanted into C3H mice, the endogenous C3H GUSB protein was inactivated in the tissue sections, so that only the human enzyme being produced by the TG cells was visible upon staining.27 Inactivation was performed by a modification of the naphthol-AS-BI β-D-glucuronide histochemical staining procedure. Instead of washing the tissue sections with 0.05 M sodium acetate buffer (pH 4.5), we incubated the slides in 65°C 0.05 M sodium acetate buffer for 90 minutes.
We measured GUSB activity by means of a microfluorometric assay with 4-methylumbelliferyl-β-D-glucuronide as previously described.26 Protein content of the homogenates was measured with the Biorad Protein Assay (Biorad, Hercules, CA), with BSA used as a standard.
Immunofluorescence assays
To detect the H-2 haplotypes of the B+/mps(H-2Db), C3H(H-2Dk), and TG mice(H-2Dd), bone marrow cells were harvested and the red blood cells were lysed with a commercial red blood cell lysing buffer (Sigma, St Louis, MO). The cells were washed 3 times with PBS and 5 mM EDTA, and the remaining cells were counted. Phycoerythrin-conjugated anti–H-2Dd, FITC-conjugated anti–H-2Dk, and FITC-conjugated anti–H-2Db were incubated with an aliquot of cells from each strain of mouse at 4°C for 15 minutes. The cells were washed 3 times with PBS and 5 mM EDTA and analyzed on the flow cytometer as above. All antibodies used for haplotyping were obtained from Pharmingen (San Diego, CA), and the dilutions used were adjusted separately for each experiment.
To detect TG cells in C3H tissues, direct immunofluorescence was performed by means of FITC-conjugated mouse anti–mouseH-2Dd monoclonal antibody (Pharmingen). Frozen tissue sections were fixed in 100% acetone at −20°C for 10 minutes and then washed in PBS at room temperature. The tissues were blocked with 5% mouse serum at room temperature for 1 hour. The mouse serum was aspirated, and sections were overlaid with 5 μg/mL FITC-conjugated H-2Dd at room temperature for 1 hour. The slides were washed 3 times in PBS; the excess liquid was blotted off; and a drop of Vectashield (Vector Laboratories, Burlingame, CA) was added. Finally, the tissue sections were covered with a glass coverslip and kept at 4°C in the dark until evaluation.
Glycosaminoglycan assay
Storage of glycosaminoglycans (GAGs) was quantitated as previously described,28 with the following modifications (kindly provided by E. Neufeld): 20 μL sample were mixed with 50 μL of 8 M guanidine hydrochloride and 30 μL water. The solution was left to incubate at room temperature for 5 minutes. Then 50 μL 0.054 M sulfuric acid–0.5% triton-X 100 was added, and the solution was incubated at room temperature for 10 minutes. Thereafter, 750 μL Alcian blue solution (5 mL Alcian blue stock solution [as previously described], 1 mL of 1.8 M sulfuric acid, and 2.5 mL 10% triton-X 100 diluted to 100 mL with water) was added. The samples were mixed and incubated at 4°C for 1 hour. The rest of the procedure was performed as previously described.
The urinary GAG spot test was performed by pipetting 5 μL of urine onto Whatman number 2 filter paper, letting it air-dry, and then repeating the process for a total of 10μL urine. The filter paper was soaked in 0.2% toluidin blue for 60 seconds, rinsed with water, and washed in 10% ethanol and 0.5% acetic acid for 10 minutes.
Polymerase chain reactions
Tails of fetuses or toe clips from neonatal mice were obtained for polymerase chain reaction (PCR) to determine the genetic status of each pup. Genomic GUSB DNA was amplified with the following primers: CCTGTGTCATTTGCATGTG (forward primer) and GATAACATCCACGTACCGG (reverse primer). The 95–base pair (bp) product lacks an NciI restriction site in the mutant allele; the normal allele is cleaved into a 77- and 18-bp product.26
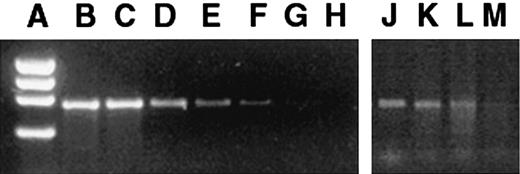
FLCs and tissues from the transplant recipients were assayed by PCR before and after transduction to determine the presence and distribution of the vector sequences. The GUSB cDNA was amplified by PCR by means of the following primers: 5′AGAATTCTGGTCATCGATGAGTGTCCC3′ (forward primer) and 5′GGCAATCCTCCAGTATCTCTCTCGC3′ (reverse primer). The PCR product specific for the GUSB cDNA is 682 bp long, which can readily be distinguished from the genomic murine GUSB PCR product (8.4 kb).21 The degree of engraftment was estimated by a PCR that has a lower limit of detection of 1 donor cell in 1 × 104 host cells (Figure1A-H). The linear range of detection was 0.01% to 10%. For tissue samples revealing PCR bands stronger than the 10% bands, the samples were diluted, and another PCR was performed until the bands were within the linear range. The estimated percentage of cells containing the proviral sequence was calculated with the use of the dilution factor and the comparison with the standard dilution of PCR bands.
Presence of retroviral vector marker sequences in bone marrow of mice 6 months after in utero transplantation of transduced FLCs.
Standard dilutions of transduced and nontransduced MPS VII cells were assayed as a standard. (A) φX174 HaeIII marker. (B) 100% transduced cells. (C) 1 in 10 dilution (D) 1 in 102 dilution. (E) 1 in 103 dilution. (F) 1 in 104 dilution. (G) Pure nontransduced cells. (H) H2O control. The PCR bands amplified from the bone marrow of 6-month-old mice that had been transplanted in utero (panels J-M) were compared with the standards. The PCR bands of panels J-L were between reference bands E and F and thus were scored as the lower value of 104, but no band was detected in panel M, which was scored negative.
Presence of retroviral vector marker sequences in bone marrow of mice 6 months after in utero transplantation of transduced FLCs.
Standard dilutions of transduced and nontransduced MPS VII cells were assayed as a standard. (A) φX174 HaeIII marker. (B) 100% transduced cells. (C) 1 in 10 dilution (D) 1 in 102 dilution. (E) 1 in 103 dilution. (F) 1 in 104 dilution. (G) Pure nontransduced cells. (H) H2O control. The PCR bands amplified from the bone marrow of 6-month-old mice that had been transplanted in utero (panels J-M) were compared with the standards. The PCR bands of panels J-L were between reference bands E and F and thus were scored as the lower value of 104, but no band was detected in panel M, which was scored negative.
Results
Cell-marking experiments with syngeneic cells
In previous experiments, we have shown transduction of FLCs in a 4-day dual-culture system that separates the retroviral vector packaging cells from the target FLCs.18 Transduced cells were detected postnatally after in utero transplantation, but only short-term studies were performed. To determine if hematopoietic stem cells from the FLCs were transduced by this method, secondary reconstitution experiments were performed with the use of lethally irradiated normal adult recipients. All of the secondary recipients had evidence of reconstitution at 6 months posttransplantation, with most organs being positive for the vector provirus sequence by PCR (Table1). The degree of engraftment was estimated by a PCR that has a lower limit of detection of 1 donor cell in 1 × 104 host cells (Figure 1A-H). For tissues that had more than 10% donor cells, the samples were diluted and assayed within the linear range (0.01% to 10%) to estimate the total proportion of donor cells. The high proportion of positive cells in hematopoietic organs indicated that most stem cells had been transduced in the initial infection.
Presence of vector proviral sequences and estimates of percentage of donor cell population in adult secondary recipients of transduced fetal liver cells
| Organs . | Group 1 secondary recipients* . | Group 2 secondary recipients* . | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 mice . | 4 mice . | 7 mice . | 5 mice . | |||||
| No. positive . | % positive cells . | No. positive . | % positive cells . | No. positive . | % positive cells . | No. positive . | % positive cells . | |
| Brain | 3 | 0.01-0.1 | 4 | 0.01-1 | 4 | 0.01-0.1 | 5 | 0.01-0.1 |
| Heart | 3 | 1-10 | 4 | 1-10 | 3 | 1-10 | 4 | 1-10 |
| Liver | 3 | 1-10 | 4 | 1-10 | 6 | 1-10 | 5 | 1-10 |
| Spleen | 3 | 50-75 | 4 | 50-100 | 5 | 50-75 | 5 | 50-100 |
| Kidney | 3 | 10-25 | 4 | 10-25 | 3 | 1-25 | 4 | 10-25 |
| BM | 3 | 75-100 | 4 | 75-100 | 7 | 75-100 | 4 | 75-100 |
| Organs . | Group 1 secondary recipients* . | Group 2 secondary recipients* . | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 mice . | 4 mice . | 7 mice . | 5 mice . | |||||
| No. positive . | % positive cells . | No. positive . | % positive cells . | No. positive . | % positive cells . | No. positive . | % positive cells . | |
| Brain | 3 | 0.01-0.1 | 4 | 0.01-1 | 4 | 0.01-0.1 | 5 | 0.01-0.1 |
| Heart | 3 | 1-10 | 4 | 1-10 | 3 | 1-10 | 4 | 1-10 |
| Liver | 3 | 1-10 | 4 | 1-10 | 6 | 1-10 | 5 | 1-10 |
| Spleen | 3 | 50-75 | 4 | 50-100 | 5 | 50-75 | 5 | 50-100 |
| Kidney | 3 | 10-25 | 4 | 10-25 | 3 | 1-25 | 4 | 10-25 |
| BM | 3 | 75-100 | 4 | 75-100 | 7 | 75-100 | 4 | 75-100 |
All primary and secondary recipient mice were lethally irradiated. The percentage of positive cells was determined by comparing polymerase chain reaction amplifications of vector sequences with controls, known percentages of transduced cells diluted in untransduced cells. Group 1 mice were transplanted with cells harvested 2 weeks after the primary transplant; group 2 mice were transplanted with cells harvested 7 weeks after the primary transplant.
We next determined the degree of chimerism and long-term engraftment of the transduced cells in the fetus, where no cyto-ablation was used. FLCs from 2 E13.5 fetuses were transduced and transplanted into 7 E13.5 recipient fetuses. Four mice were born and survived until analysis at older than 6 months of age. Most of the organs were positive for the presence of provirus (Table 2). To estimate the degree of engraftment in the tissues by PCR, a series of standard dilutions of transduced bone marrow cells in untransduced cells was made. The PCR bands were compared with the bands resulting from the tissue analyses in which the same amount of DNA was used for all of the reactions (Figure 1). In most of the tissues that tested positive, the presence of the PCR band indicated that between 0.01% and 0.1% of the cells contained the provirus. The low level of chimerism, combined with the expected down-regulation of vector expression in many cells, resulted in too few total cells expressing GUSB activity to measure above background.
Detection of retroviral vector-positive cells more than 6 months after transplantation of transduced FLCs into E13.5 MPS VII fetuses
| Organ . | PCR-positive cells, % . | |||
|---|---|---|---|---|
| Mouse 1 . | Mouse 2 . | Mouse 3 . | Mouse 4 . | |
| Blood | 0.1-1.0 | 0.01-0.1 | 0.01-0.1 | 0.01-0.1 |
| Brain | Negative | 0.01-0.1 | 1-10 | Negative |
| Liver | 0.01-0.1 | Negative | 0.1-1.0 | 0.01-0.1 |
| Spleen | 0.1-1.0 | Negative | Negative | Negative |
| Bone marrow | 0.01-0.1 | 0.01-0.1 | 0.01-0.1 | Negative |
| Organ . | PCR-positive cells, % . | |||
|---|---|---|---|---|
| Mouse 1 . | Mouse 2 . | Mouse 3 . | Mouse 4 . | |
| Blood | 0.1-1.0 | 0.01-0.1 | 0.01-0.1 | 0.01-0.1 |
| Brain | Negative | 0.01-0.1 | 1-10 | Negative |
| Liver | 0.01-0.1 | Negative | 0.1-1.0 | 0.01-0.1 |
| Spleen | 0.1-1.0 | Negative | Negative | Negative |
| Bone marrow | 0.01-0.1 | 0.01-0.1 | 0.01-0.1 | Negative |
PCR indicates polymerase chain reaction.
Transplantation of allogeneic FLCs that express GUSB constitutively at supernormal levels
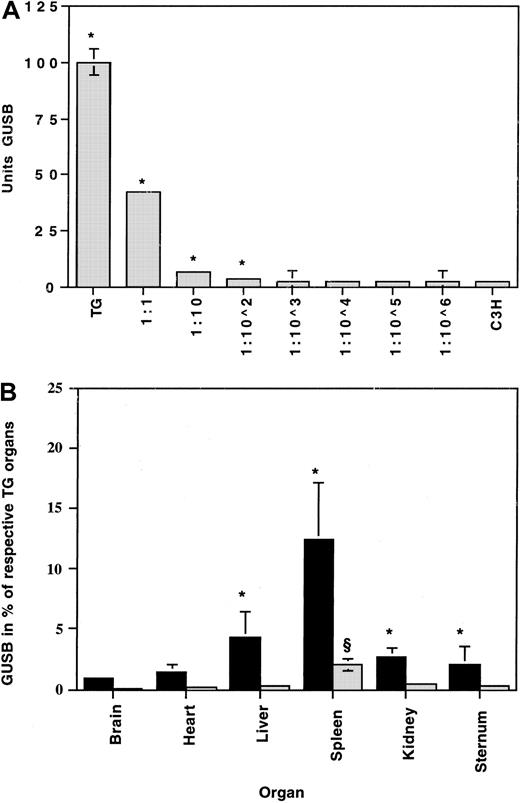
To evaluate the effects of transplanting long-term GUSB-expressing FLCs into fetuses, we used FLCs from a transgenic mouse strain (TG) that constitutively expresses high levels of human GUSB. The transgene is expressed on the MPS VII mouse background, so only human GUSB enzyme is produced.23 The recipients were C3H fetuses because this strain expresses low levels of GUSB that can be heat-inactivated relative to the human GUSB enzyme.29 To determine the number of TG cells necessary to significantly increase enzyme activity levels above the C3H background, GUSB activity was determined in a series of cell dilutions made with TG and C3H spleen cells. GUSB activity was significantly higher than in C3H cells alone at only 1 TG cell in 100 C3H cells (Figure 2A). This is consistent with the differences in GUSB activity levels between the TG and C3H cells, which is about 50-fold (Figure 2A). Similar ratios were found when different tissues from the 2 strains were compared (not shown).
Detection of GUSB activity in TG donor cells against the C3H background.
(A) Cell dilutions were prepared by mixing TG and C3H spleen cells to determine the proportion of TG donor cells needed to produce a significant difference in enzyme concentrations when compared with undiluted C3H spleen cells. Error bars (SEM) are drawn on all data points but were too small to be visible on some. *Statistically significant difference (P < .05). (B) Enzyme activity is expressed as the percentage of age-matched TG tissues (100%) above untreated age-matched baseline C3H tissues (0%). Enriched (AA4.1) (▪) and nonenriched (░) uncultured TG fetal liver cells were transplanted into C3H fetuses at 1 × 106nonenriched cells and 1 × 105 enriched cells per fetus. A 50-fold enrichment was achieved, thus resulting in a 5-fold increase in the total number of hematopoietic stem and progenitor cells transplanted. *Significant (P < .05) between the enriched and nonenriched group. §Significant (P < .05) between the nonenriched and the untreated control group.
Detection of GUSB activity in TG donor cells against the C3H background.
(A) Cell dilutions were prepared by mixing TG and C3H spleen cells to determine the proportion of TG donor cells needed to produce a significant difference in enzyme concentrations when compared with undiluted C3H spleen cells. Error bars (SEM) are drawn on all data points but were too small to be visible on some. *Statistically significant difference (P < .05). (B) Enzyme activity is expressed as the percentage of age-matched TG tissues (100%) above untreated age-matched baseline C3H tissues (0%). Enriched (AA4.1) (▪) and nonenriched (░) uncultured TG fetal liver cells were transplanted into C3H fetuses at 1 × 106nonenriched cells and 1 × 105 enriched cells per fetus. A 50-fold enrichment was achieved, thus resulting in a 5-fold increase in the total number of hematopoietic stem and progenitor cells transplanted. *Significant (P < .05) between the enriched and nonenriched group. §Significant (P < .05) between the nonenriched and the untreated control group.
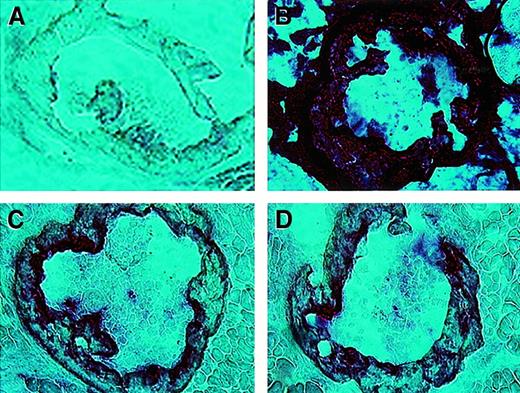
To examine early reconstitution, we used the in situ GUSB reaction26 to stain marrow-containing rib bones from C3H newborns that had been transplanted with TG FLCs at E13.5. After heat inactivation, the human GUSB reaction product resulted in a strong staining reaction (Figure 3B), which reflects the high level of activity,29 in the positive control TG mice. The C3H control mice are negative (Figure 3A). The human GUSB from the donor TG FLCs' progeny cells can be readily seen in the C3H transplant recipients (Figure 3C-D). This shows that the TG FLCs colonize the marrow within a few days after in utero transplantation.
Expression of human GUSB in bone marrow of C3H mice transplanted in utero with TG FLCs.
Sections of heat-inactivated rib bones (20 ×) from newborn mice. (A) Untreated C3H control. (B) Transgenic control. (C-D) Rib bones from 2 C3H recipients of TG FLCs 8 days after in utero transplantation of TG FLCs.
Expression of human GUSB in bone marrow of C3H mice transplanted in utero with TG FLCs.
Sections of heat-inactivated rib bones (20 ×) from newborn mice. (A) Untreated C3H control. (B) Transgenic control. (C-D) Rib bones from 2 C3H recipients of TG FLCs 8 days after in utero transplantation of TG FLCs.
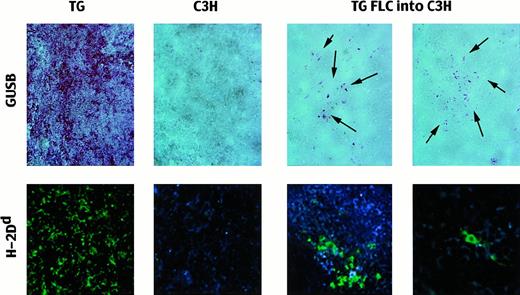
At adulthood, more than 2 months after transplantation, histochemical staining for biologically active human GUSB after heat inactivation of the endogenous murine GUSB revealed scattered positive donor cells (Figure 4). The tissues were stained with immunofluorescent antibodies to confirm the presence of TG donor cells, which differ from C3H at the major histocompatibility complex (H-2d vs H-2k) (Figure 4). Of 6 animals assayed, all 6 had donor cells in hematopoietic organs, but no donor cells were found in the brains (Table3). In most cases, the donor cells were present as singly distributed cells, but clusters of donor cells were also seen in 4 of the 6 spleens examined.
Presence of TG donor cells in adult C3H recipient spleens after in utero transplantation.
Top row: Spleens stained for the presence of biologically active GUSB (red reaction product) after the C3H GUSB was heat-inactivated. TG indicates transgenic mouse (positive control; 20 ×); B, untreated C3H mouse (negative control; 20 ×); and TG FLCs into C3H, spleens of recipients of nonenriched TG FLCs 2 months after transplantation (20 ×). The arrows point to the areas of cells expressing enzymatically active human GUSB. Bottom row: Immunofluorescent-labeled antibodies against H-2Dd to detect TG donor cells in the recipient spleens. TG indicates transgenic mouse (positive control; 20 ×); C3H, untreated C3H mouse (negative control; 20 ×); and TG FLCs into C3H, transplant recipients with clusters of positive cells (20 ×) and with single, scattered positive donor cells (40 ×).
Presence of TG donor cells in adult C3H recipient spleens after in utero transplantation.
Top row: Spleens stained for the presence of biologically active GUSB (red reaction product) after the C3H GUSB was heat-inactivated. TG indicates transgenic mouse (positive control; 20 ×); B, untreated C3H mouse (negative control; 20 ×); and TG FLCs into C3H, spleens of recipients of nonenriched TG FLCs 2 months after transplantation (20 ×). The arrows point to the areas of cells expressing enzymatically active human GUSB. Bottom row: Immunofluorescent-labeled antibodies against H-2Dd to detect TG donor cells in the recipient spleens. TG indicates transgenic mouse (positive control; 20 ×); C3H, untreated C3H mouse (negative control; 20 ×); and TG FLCs into C3H, transplant recipients with clusters of positive cells (20 ×) and with single, scattered positive donor cells (40 ×).
Detection of donor cells in adult mice after in utero transplantation of transgenic fetal liver cells
| Organs . | Distribution of positive cells . | Transplantation groups3-150 . | |||
|---|---|---|---|---|---|
| AA4.1− GF− 2 mo3-151 . | AA4.1+ GF− 2 mo3-151 . | AA4.1+ GF− > 6 mo3-151 . | AA4.1+ GF+ > 6 mo3-151 . | ||
| Spleen3-152 | Single cells | 6/6 | 22/22 | 3/3 | 15/15 |
| Clusters | 4/6 | 11/22 | 3/3 | 6/15 | |
| Bone marrow3-153 | Single cells | 4/6 | 12/21 | 1/3 | 3/15 |
| Clusters | 0/6 | 3/21 | 0/3 | 0/15 | |
| Liver3-152 | Single cells | 6/6 | 13/20 | 1/3 | 3/15 |
| Brain3-152 | Single cells | 0/6 | 0/22 | 0/3 | 0/15 |
| Organs . | Distribution of positive cells . | Transplantation groups3-150 . | |||
|---|---|---|---|---|---|
| AA4.1− GF− 2 mo3-151 . | AA4.1+ GF− 2 mo3-151 . | AA4.1+ GF− > 6 mo3-151 . | AA4.1+ GF+ > 6 mo3-151 . | ||
| Spleen3-152 | Single cells | 6/6 | 22/22 | 3/3 | 15/15 |
| Clusters | 4/6 | 11/22 | 3/3 | 6/15 | |
| Bone marrow3-153 | Single cells | 4/6 | 12/21 | 1/3 | 3/15 |
| Clusters | 0/6 | 3/21 | 0/3 | 0/15 | |
| Liver3-152 | Single cells | 6/6 | 13/20 | 1/3 | 3/15 |
| Brain3-152 | Single cells | 0/6 | 0/22 | 0/3 | 0/15 |
AA4.1+ indicates FLC column enriched for hematopoietic stem and progenitor cells before transplantation. GF+ indicates FLCs were cultured for 4 days in the presence of growth factors (IL-3, IL-6, and stem cell factor) before transplantation.
Age at analysis.
In the spleens, livers, and brains, multiple sections were assayed from each tissue to obtain a representative sampling of the whole tissue.
In bone marrow, only a small sample from one bone was assayed for each animal, and the assays do not represent the whole bone marrow. Thus, it is likely, given low total numbers of donor cells, that positive areas were missed in the negative mice.
Effect of enrichment for stem and progenitor cells before transplantation on survival
A potential approach to increase the number of donor cells in the chimeric hematopoietic system is to increase the number of stem and progenitor cells in the transplanted population. However, the number of cells that can be transplanted to an individual fetus (usually 1 × 106) is limited by both the volume of the inoculum and the density of cells that can be suspended without aggregation. When we attempted to increase the total transplant by slowly injecting 5 × 106 unenriched TG FLCs into fetuses, the dam aborted all of them within 2 days (not shown). Instead, we attempted to increase the proportion of chimerism by enriching the donor cell population for hematopoietic stem and progenitor cells using monoclonal AA4.1 antibodies.25 Flow cytometry showed that up to 90% enrichment could be achieved, with an average of 51% (Table4). In many experiments, the total number of cells available for transplantation was fewer than the 1 × 106 used with the unenriched population (Table 4). This was due to variations in the enrichment results (Table 4) and to the limited number of cells available as the starting population, since multiple-timed pregnant dams were needed to obtain enough donor cells for each timed pregnant recipient dam.
Survival of mice receiving in utero transplants of AA4.1+ enriched FLC populations ranked by number of total cells injected per fetus
| Pregnant dam . | Mice . | Transplanted cells . | Survival . | |||
|---|---|---|---|---|---|---|
| No. of injected fetuses . | Total No. of cells per fetus . | % AA4.1+ . | AA4.1+ cells per fetus . | Born . | Alive at older than 1 week . | |
| Unenriched | ||||||
| 1 | 8 | 1 × 106 | 1 | 1 × 104 | 6 | 6 |
| 2 | 5 | 1 × 106 | 1 | 1 × 104 | 5 | 5 |
| 3 | 4 | 1 × 106 | 1 | 1 × 104 | 1 | 1 |
| AA4.1+enriched | ||||||
| 4 | 9 | 1 × 106 | 75 | 7.5 × 105 | 2 | 0 |
| 5 | 6 | 1 × 106 | 75 | 7.5 × 105 | 0 | 0 |
| 6 | 2 | 5 × 105 | 90 | 4.5 × 105 | 0 | 0 |
| 7 | 7 | 5 × 105 | 14 | 7 × 104 | 4 | 4 |
| 8 | 5 | 4 × 105 | 73 | 2.9 × 105 | 0 | 0 |
| 9 | 6 | 4 × 105 | 21 | 8.4 × 104 | 3 | 1 |
| 10 | 8 | 4 × 105 | 21 | 8.4 × 104 | 4 | 2 |
| 11 | 8 | 3 × 105 | 90 | 2.7 × 105 | 0 | 0 |
| 12 | 6 | 2 × 105 | 30 | 6 × 104 | 3 | 3 |
| 13 | 7 | 2 × 105 | 17 | 3.4 × 104 | 3 | 3 |
| 14 | 8 | 2 × 105 | 17 | 3.4 × 104 | 4 | 4 |
| 15 | 3 | 2 × 105 | 67 | 1.3 × 105 | 0 | 0 |
| 16 | 4 | 1 × 105 | 80 | 8 × 104 | 2 | 2 |
| 17 | 5 | 1 × 105 | 56 | 5.6 × 104 | 5 | 5 |
| 18 | 5 | 1 × 105 | 37 | 3.7 × 104 | 5 | 5 |
| Pregnant dam . | Mice . | Transplanted cells . | Survival . | |||
|---|---|---|---|---|---|---|
| No. of injected fetuses . | Total No. of cells per fetus . | % AA4.1+ . | AA4.1+ cells per fetus . | Born . | Alive at older than 1 week . | |
| Unenriched | ||||||
| 1 | 8 | 1 × 106 | 1 | 1 × 104 | 6 | 6 |
| 2 | 5 | 1 × 106 | 1 | 1 × 104 | 5 | 5 |
| 3 | 4 | 1 × 106 | 1 | 1 × 104 | 1 | 1 |
| AA4.1+enriched | ||||||
| 4 | 9 | 1 × 106 | 75 | 7.5 × 105 | 2 | 0 |
| 5 | 6 | 1 × 106 | 75 | 7.5 × 105 | 0 | 0 |
| 6 | 2 | 5 × 105 | 90 | 4.5 × 105 | 0 | 0 |
| 7 | 7 | 5 × 105 | 14 | 7 × 104 | 4 | 4 |
| 8 | 5 | 4 × 105 | 73 | 2.9 × 105 | 0 | 0 |
| 9 | 6 | 4 × 105 | 21 | 8.4 × 104 | 3 | 1 |
| 10 | 8 | 4 × 105 | 21 | 8.4 × 104 | 4 | 2 |
| 11 | 8 | 3 × 105 | 90 | 2.7 × 105 | 0 | 0 |
| 12 | 6 | 2 × 105 | 30 | 6 × 104 | 3 | 3 |
| 13 | 7 | 2 × 105 | 17 | 3.4 × 104 | 3 | 3 |
| 14 | 8 | 2 × 105 | 17 | 3.4 × 104 | 4 | 4 |
| 15 | 3 | 2 × 105 | 67 | 1.3 × 105 | 0 | 0 |
| 16 | 4 | 1 × 105 | 80 | 8 × 104 | 2 | 2 |
| 17 | 5 | 1 × 105 | 56 | 5.6 × 104 | 5 | 5 |
| 18 | 5 | 1 × 105 | 37 | 3.7 × 104 | 5 | 5 |
In the control unenriched FLC transplants, when 1 × 106cells were injected (dams 1 to 3 in Table 4), 12 of 17 fetuses (71%) were born alive, and all of them survived to adulthood. In 2 litters that received the same total number of cells but that were enriched for stem and progenitor cells (dams 4 and 5), only 2 of 15 injected fetuses were born (13%), and both died by postnatal day 2. When a 10-fold lower total dose of enriched cells was used (dams 16 to 18), 12 of 14 injected fetuses (86%) were born, and they all survived to adulthood. When the numbers of total injected cells were between 2 and 5 × 105, a strong correlation was seen between survival and the purity of the enriched population. In 6 litters transplanted with cells enriched between 14% and 30% (dams 7, 9, 10, and 12 to 14), 21 of 42 injected fetuses (50%) were born and 17 of these survived (81% of those born). In contrast, none of 18 fetuses injected with cells that were enriched between 67% and 90% (dams 6, 8, 11, and 15) survived to birth. When survival was evaluated as a function of the number of AA4.1+ cells transplanted rather than the total cell dose, there was a clear and statistically significant difference between those that received greater versus fewer than 1 × 105 AA4.1+ cells (Table5).
Effect of the number of AA4.1+ cells on the outcome of in utero transplants
| AA4.1 cells . | No. of litters . | No. of fetuses injected . | Born . | Alive at older than 1 week . | ||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | |||
| > 1 × 105 | 6 | 33 | 2 | 6 | 0 | 0 |
| < 1 × 105 | 12 | 73 | 45 | 62 | 415-150 | 91 |
| AA4.1 cells . | No. of litters . | No. of fetuses injected . | Born . | Alive at older than 1 week . | ||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | |||
| > 1 × 105 | 6 | 33 | 2 | 6 | 0 | 0 |
| < 1 × 105 | 12 | 73 | 45 | 62 | 415-150 | 91 |
Fisher exact test indicates a significant difference between the two groups of transplant recipients (P = .0001; 3-way comparison).
Effect of enrichment for stem and progenitor cells on engraftment and enzyme expression
Among the litters that survived long enough for analysis, fetuses transplanted with the AA4.1+ enriched population of FLCs received approximately 5 times more hematopoietic stem and progenitor cells than fetuses transplanted with unenriched populations. At 2 months after transplantation, there were significant differences in the GUSB activities measured in spleen, liver, kidney, and sternum between the group of mice that received AA4.1-enriched FLCs (n = 17) and those that received unenriched cells (n = 12) (Figure 2B). An approximately 6-fold increase in GUSB activity was observed in the tissues of the recipients of enriched cells when compared with those of the unenriched cells (Figure 2B), which was proportional to the total increase in AA4.1+ cells that were transplanted. When nonenriched FLC transplants were compared with untransplanted control mice (n = 7), only the spleens had significantly elevated GUSB activities. There were no differences in enzymatic activities measured in heart and brain in either transplantation group when compared with control tissues. At 9 months after transplantation, there were no significant differences between the recipients of enriched FLCs, recipients of nonenriched FLCs, or age-matched untransplanted controls.
To determine if donor cells were present, the tissues were stained with immunofluorescent antibodies for the presence of TG-specific antigen cells (H-2Dd) (Table 3). Immunofluorescently labeled positive donor cells were detected in the spleens of all recipients, regardless of pretransplantation manipulation, and had a dendritic appearance. Positive cells, round in appearance, were found in the bone marrow of some of the short-term recipients, but not the long-term recipients. However, there were only a small number of mice in the long-term group, and only a small sample of the total marrow from each mouse was assayed. Positive sinusoidal lining cells (Kupffer cells) were detected in most of the livers. No positive cells were found in any brain sections from the transplant recipients.
Effect of culturing TG fetal liver cells with growth factors before enrichment and transplantation
The transplantation of TG FLCs into C3H mice is a model for transplantation of vector-transduced FLCs, but does not completely mimic a gene-transfer setting because C3H and TG mice are not haplo-identical. The TG FLCs were subjected to the protocol used for retroviral vector transduction18 to determine if the culture conditions would have an adverse effect on long-term engraftment. TG FLCs were cultured in the presence of IL-3, IL-6, and stem cell factor for 4 days and enriched for the AA4.1+cells before transplantation into fetuses. The mice were analyzed at more than 6 months after transplantation and were compared with those receiving enriched but uncultured cells. Positive donor cells were detected in all of the spleens from the recipients of uncultured and cultured enriched FLCs by immunofluorescence (Table 3).
Many of the donor cells were present in the form of colonies in the uncultured short- and long-term group, whereas a larger percentage of scattered donor cells than colonies was seen in the spleen sections. It was difficult to find positive cells in bone marrow, since there were few positive cells and only a small sample of the total bone marrow from each mouse was analyzed. The positive cells were mostly single scattered cells. However, colonies of cells were seen in bone marrow of the recipients of uncultured enriched FLCs that were analyzed 2 months after transplantation. More livers in the uncultured short-term group had donor cells present than in both long-term groups (uncultured and cultured). When donor cells were present, their distribution was similar regardless of the pretransplantation manipulation. All of the brains were negative for the presence of TG donor cells.
In utero transplantation of TG FLCs into MPS VII fetuses
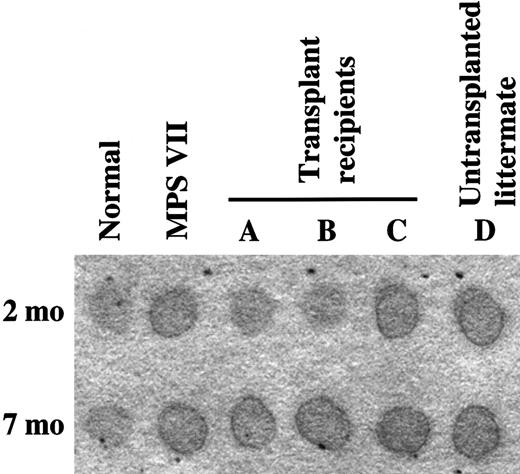
We next determined whether the low level of engraftment seen could alter the disease phenotype if the donor cells were constitutively overexpressing GUSB. We injected 3 MPS VII fetuses in a litter of 5 affecteds, which were produced from a rare affected-by-affected mating of intercrossed MPS VII mice,5 with growth factor–stimulated, AA4.1-enriched TG FLCs. There were 4 mice born. and during the first 2 months of life, 2 of them were observed to be clinically healthier than their 2 littermates as well as compared with all other untreated MPS VII intercrossed mice in our colony (Table6). These 2 mice had noticeably less facial flattening, characteristic of MPS VII owing to bone abnormalities,3 5 than their littermates. Compared with untreated MPS VII mice at the same age, they were more alert and interested in their environment; they groomed themselves; and their mobility was not impaired. Enzyme-activity assays performed on toe clips showed the presence of GUSB activity in these 2 mice (14% and 18% of normal). The 2 mice that exhibited typical MPS VII clinical behavior had no GUSB activity in toe clips (Table 6). The 2 GUSB-positive mice had urinary GAGs similar to normal controls at 2 months of age, according to a semiquantitative spot test (Figure5).
Clinical appearance and β-glucuronidase expression in mucopolysaccharidosis VII mice transplanted in utero with AA4.1-enriched, cultured transgenic fetal liver cells
| Age (days) . | Feature . | Mouse 1 . | Mouse 2 . | Mouse 3 . | Mouse 4 . |
|---|---|---|---|---|---|
| 60 | Mobility | good | good | fair-poor | fair-poor |
| Facial appearance | pointed | pointed | blunted | blunted | |
| Urinary GAGs | +/− | +/− | +++ | +++ | |
| GUSB activity (toe clip) | 14% | 18% | 0% | 0% | |
| 214 | Mobility | very poor | very poor | very poor | very poor |
| Facial appearance | blunted | blunted | blunted | blunted | |
| Urinary GAGs | +++ | +++ | +++ | +++ | |
| GUSB activity | 0% | 0% | 0% | 0% | |
| GUSB-positive cells | Spleen, Liver | spleen, liver | none | none | |
| H-2Dd-positive cells | Spleen, liver, brain | spleen, liver, brain | spleen | none |
| Age (days) . | Feature . | Mouse 1 . | Mouse 2 . | Mouse 3 . | Mouse 4 . |
|---|---|---|---|---|---|
| 60 | Mobility | good | good | fair-poor | fair-poor |
| Facial appearance | pointed | pointed | blunted | blunted | |
| Urinary GAGs | +/− | +/− | +++ | +++ | |
| GUSB activity (toe clip) | 14% | 18% | 0% | 0% | |
| 214 | Mobility | very poor | very poor | very poor | very poor |
| Facial appearance | blunted | blunted | blunted | blunted | |
| Urinary GAGs | +++ | +++ | +++ | +++ | |
| GUSB activity | 0% | 0% | 0% | 0% | |
| GUSB-positive cells | Spleen, Liver | spleen, liver | none | none | |
| H-2Dd-positive cells | Spleen, liver, brain | spleen, liver, brain | spleen | none |
GAGs indicates glycosaminoglycans; GUSB, β-glucuronidase.
Urinary GAGs.
Sharp, dark edges of the urine spots indicate the presence of increased GAGs.
Urinary GAGs.
Sharp, dark edges of the urine spots indicate the presence of increased GAGs.
By 7 months of age, however, all of the mice in this litter appeared equally affected. Postmortem analysis of spleen, bone marrow, liver, heart, kidney, and brain showed that none of the tissues had GUSB activity above background. Elevated urinary GAGs were detected in all of the affected mice, and there were no differences between the spot test results in the 2 mice that earlier had reduced urinary GAGs when compared with untreated controls (Figure 5). However, single GUSB-positive cells were detected in the spleens and livers of the 2 MPS VII mice that had had increased enzyme activity levels at 2 months of age (Table 6). Immunofluorescent staining for the presence of TG donor cells also showed positive cells in the spleens and livers, and a few positive cells were found in the meninges of these mice (Table 6). Interestingly, single positive cells were also detected in the spleen of one of the MPS VII mice that had not shown evidence of improvement (Table 6). Thus, this was the third fetus that had been transplanted in utero, but it had apparently not engrafted to the extent of its 2 littermates, which had measurable amounts of GUSB activity at 2 months of age.
Discussion
The success of postnatal BMT or gene correction of stem cells for the treatment of genetic diseases can be complicated by graft rejection, graft-versus-host disease, pretransplantation conditioning, immune responses to the transferred gene, or the severity of the disease being treated. Many of these effects may be avoided by using FLCs as the donor cell population for in utero transplantation, owing to the immuno-tolerance and immuno-incompetence of early gestational to midgestational fetal recipient and donor hematopoietic cells, respectively.16 When FLCs are transplanted in utero, no cyto-ablation is used; this avoids the risks associated with transplantation conditioning. However, this results in a chimeric hematopoietic system in the recipient. In the absence of a developmental defect, such as the W mutations in mice,17the donor cells have no selective advantage over the host cells, and the proportion of donor cells in host tissues is generally low.30-38
In previous experiments, we showed that MPS VII FLCs could be transduced with a retroviral vector under the conditions used here and that they could be detected postnatally after being transplanted in utero.18 This culture system has also been used to transduce adult bone marrow stem cells, but with a much longer incubation time (18 days).39 Our secondary reconstitution experiments in lethally irradiated adults indicate that the 4-day culture method resulted in transduction of pluripotent hematopoietic stem cells contained in the FLC population. Most of the cells in the reconstituted hematopoietic organs were provirus-positive (75% to 100% in bone marrow), indicating that most of the stem cells in the FLC population were transduced by this method. In contrast, only a small number of provirus-positive cells persisted for more than 6 months after in utero transplantation. Although there was variation among individual animals, in general, only about 0.1% of the adult hematopoietic system was composed of donor derived cells.
Since the infection method resulted in transduction of most stem cells, the low percentage of chimerism was probably an accurate reflection of the long-term survival of transplanted cells. However, these experiments would underestimate engraftment if not all stem cells were transduced. Transplantation of TG FLCs into C3H fetuses was used as a model system to examine the repopulation of donor cells where all the donor cells expressed the foreign gene. The TG cells also provided a genetic marker (differences in H-2 haplotypes between mouse strains), which allowed the donor cells to be detected in the recipient tissues. The TG donor cells produce supernormal levels of GUSB and can be distinguished from C3H tissues, which have low levels of normal GUSB enzyme activity compared with other strains.22,23,29 The transgene is constitutively expressed; thus, the cells mimic vector-transduced cells that are overexpressing the gene of interest,20 without the disadvantage of down-regulation of the vector. If only a small number of cells can be transplanted or there is a low rate of engraftment, cells overexpressing the gene of interest may be able to compensate for the difference.19,21,26 The C3H fetuses that received TG FLC cells, cultured under the same conditions used for vector transduction, had levels of chimerism (about 0.1%) at greater than 6 months of age that were similar to those of the recipients of vector-transduced syngeneic cells. Thus, the proportion of donor cells in the recipients, where the donor cells have no competitive advantage, was approximately the same for vector-transduced syngeneic and allogeneic donor cells. Similar findings have been reported for adult BMT into fetuses.38 However, the engraftment of FLCs that were not cultured was no better that when cells were cultured (Table 3).
Enriching the number of stem and progenitor cells about 5-fold in the donor inoculum significantly increased GUSB activity levels in spleen, liver, kidney, and sternum at 2 months after transplantation. Since at least 1 TG cell in 100 recipient cells was required to detect an increase in enzyme activity above the C3H background, more than 1% of the spleen was of donor origin at 2 months of age. However, by 6 months after transplantation, the recipients did not have enzyme activity levels above the C3H background, regardless of the pretransplantation manipulation of the TG FLCs. Donor cells were detected in all of the recipients by immunofluorescent staining for H-2 class I molecules, which could detect 1 TG donor cell in 1 × 103 to 1 × 104 recipient cells. Similar levels of engraftment (about 0.1%) were seen as with vector-transduced syngeneic cells. The most likely explanation for the decrease over time is that most of the donor cells were in the committed progenitor cell pool at the time of transplantation.
The number of stem and progenitor cells could be increased by enrichment, but the transplants are limited by the volume and total number of cells that can be injected. Interestingly, transplantation of more than 1 × 105 AA4.1+ FLCs per fetus resulted in perinatal loss of all of the recipients. It has been suggested elsewhere40 that fetal death may be caused by “overwhelming engraftment” if a certain number of cells transplanted in utero is exceeded. However, in our experiments, higher numbers of cells did not have as severe an adverse effect if the total number of AA4.1+ cells was lower than 1 × 105. It is possible that graft-versus-host reactions may occur even though fetal cells were used, but the fetuses that died were not available for examination. Other studies have suggested that natural killer cells or their precursors may be present by 13 days of gestation41; therefore, it is possible that the enrichment process increased their number and increased the likelihood of immune responses.
Only a limited number of MPS VII fetuses were available for transplantation owing to the necessity of using timed breedings between heterozygous carriers, not knowing which fetuses were affected at the time of transplantation, not being able to inject all fetuses, and having significant loss of affecteds from their disease before analysis. MPS VII mice are fragile and not usually able to breed, and increased neonatal losses occur in carrier matings.3,8,10In the cell-marking experiments using syngeneic transduced FLCs, the provirus was present in almost all recipient tissues examined after in utero transplantation. The rate of engraftment was low, but did not differ from the rate found in transplantation of allogeneic FLCs that received the same treatment as the retrovirally transduced FLCs. These results suggest that retroviral transduction of FLCs does not decrease their ability to engraft, but the growth factors may have increased the number of committed progenitors, possibly contributing to the low level of chimerism. It cannot be ruled out that the low rate of engraftment may also be due to immune reactions either to the allogeneic cell population or the foreign protein (GUSB) that is being produced by the proviral genome. Overall, our results do not differ significantly from those found in other animal models when FLCs were transplanted in the absence of a developmental defect,32,34,35,42 43 such as severe combined immunodeficiency or W mutations.
An increase in enzyme activity and clinical improvement was observed at 2 months of age in 2 of 3 MPS VII mice that had received enriched TG FLCs in utero. This litter occurred from a rare affected-by-affected mating and produced 5 MPS VII fetuses, 4 of which were born. These results have interesting clinical implications. Even if in utero transplantation did not lead to a complete reversal of disease or improve pathology in the long term, it did lead to improvement for a significant period of time after birth. A second transplantation or gene-transfer procedure might be performed postnatally to prevent further progression of disease. This has been shown to work in principle in MPS VII mice that received infusions of purified enzyme beginning shortly after birth followed by BMT later in life.44 Disease pathology was improved, without the side effects of irradiation seen when BMT was performed at birth.14 Introduction of the normal gene product during fetal life might also induce immunological tolerance to the foreign gene product and facilitate postnatal treatments.
The results of using TG cells that overexpress GUSB show that it is possible to correct some of the disease effects with a very low number of cells, at least in the short term. This suggests that in syngeneic transplants, therapeutic results may be improved by transducing the target cells with retroviral vectors that overexpress the gene of interest. The vector gene–transfer studies described here were used for cell marking to determine if vector infection of FLCs in the short-term dual-culture system18 resulted in the tranduction of stem cells. The results with the TG FLC transplants suggest that using heterologous FLCs engineered to overexpress a transferred gene may be feasible. An allogeneic source of donor cells may be more practical than harvesting and modifying autologous cells from a fetal patient, particularly one that may be compromised by its disease.
We thank C. Jones, M. Parente, A. Poleski, and the late J. J. Reilly for their excellent technical assistance, and C. Koch and the Department of Radiation Oncology, School of Medicine, for use of the irradiator.
Supported by a grant (DK-46637) and a fellowship (DK-09185, to M.L.C.) from the National Institute of Diabetes and Digestive and Kidney Diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John H. Wolfe, 502G Abramson Research Bldg, Children's Hospital of Philadelphia, 3400 Civic Center Blvd, Philadelphia, PA 19104; e-mail: jhwolfe@vet.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal